Abstract
This comprehensive meta-analysis was applied to case-control studies of the association between pancreatitis and PRSS1 gene to assess the joint evidence for the association, the influence of individual studies, and evidence for publication bias. PubMed, EMBASE, and Cochrane Library were searched in order to identify longitudinal studies evaluating pancreatitis disease and PRSS1 gene. Odds ratios (ORs) were pooled using a random-effects model. For the case-control studies, the authors found 1) support for the association between total pancreatitis and PRSS1 gene, both totally analyzed and subdivided analyzed {total: [OR:10.799, 95%CI:(5.489-21.242), p<0.000]; Europe: [OR:9.795, 95%CI:(2.923-32.819), p<0.000]; Asia: [OR:11.994, 95%CI:(5.156-27.898), p<0.000]}. 2) no evidence showed that this association was accounted for by any one study, and 3) no evidence showed any publication bias exist. In conclusion, PRSS1 gene was significantly associated with total pancreatitis disease, both totally and separately.
Keywords: PRSS1, pancreatitis, meta-analysis
Introduction
The pancreas is a gland organ in the digestive system which has both endocrine and exocrine functions. It produces several important hormones such as insulin, glucagon, and somatostatin. The organ secrets pancreatic juice containing digestive enzymes which pass to the small intestine. Both pancreatic enzymes and hormones are needed to keep the body working correctly. Pancreatitis is a kind of inflammation of the pancreas that can occur in two very different forms: acute and chronic. Acute pancreatitis (AP) is a sudden inflammation that occurs over a short period of time, in which premature activation of zymogens in pancreatic acinar cells leads to autodigestion and induces the pathologic process such as edema, hemorrhage, and even necrosis 1, 2. It is a common pancreatic disease that follows a variable clinical course and shows different pathological pancreatic pattern, ranging from Mild AP, with simple edema of parenchyma and mild pancreatic pain, to Severe AP, with parenchyma necrosis, abscesses, pseudocysts and severe pancreatic pain, fever, up to multisystem organ failure and death. Chronic pancreatitis is a persistent inflammation of the pancreas that results in irreversible morphological changes and impairment of both exocrine and endocrine functions. It is characterized by the presence of chronic inflammatory cells within the pancreas, progressive fibrosis, sclerosis and parenchymal atrophy. The main symptoms of CP are malnutrition, steatorrhea and abdominal pain, since exocrine and endocrine insufficiency 3-5. According to the initiating causes, it could be subtyped to Alcoholic chronic pancreatitis, Hereditary chronic pancreatitis, Cystic fibrosis-associated chronic pancreatitis, Tropical pancreatitis, Autoimmune chronic pancreatitis, Biliary-associated chronic pancreatitis, Idiopathic chronic pancreatitis and some other forms 6, 7.
Both AP and CP are complex diseases caused by a number of etiological factors 8, 9. During the last several decades, more and more studies have indicated that genetic risk factors contribute to the pathogenesis of pancreatitis 10, 11. Genetic studies have demonstrated that the p.N34S variant of secretory pancreatic trypsin inhibitor 1 (SPINK1) was associated with AP 12. Other genes such as TNF-α and iNOS also have been indicated as candidate genes for AP by some groups, but results in different studies are inconsistent. A number of genetic risk factors were identified in CP. In particular, mutations of the cationic trypsinogen (PRSS1), chymotrypsinogen C (CTRC), the cystic fibrosis transmembrane conductance regulator (CFTR) an SPINK1, have been found to be associated with both the hereditary and the idiopathic form of CP 13-17.
PRSS1 locates in the short arm of chromosome 7 and encodes cationic trypsinogen, which is the most abundant isoform of trypsinogen in human pancreatic juice. In 1996, Whitcomb et al first demonstrated that mutations in PRSS1 gene were associated with hereditary pancreatitis 18. Since then, numerous mutations in this gene have been identified in families with hereditary pancreatitis or sporadic cases [http://www.uni-leipzig.de/pancreasmutation/]. Several groups also found mutations in the patients with acute pancreatitis. All the studies indicated that mutations of PRSS1 are associated with acute and chronic pancreatitis. However, the published genetic association results are inconclusive 19-25. This inconsistency may be due to inadequate statistical power, racial and ethnic differences, and publication bias. For all the reasons above, we conducted this comprehensive meta-analysis, which is a powerful tool for summarizing the results from different studies by producing a single estimate of the major effect with enhanced precision.
Materials and methods
Identification of eligible studies
We have performed an exhaustive search for studies that examined the association of the PRSS1 mutations with pancreatitis. A search of the literature was made using Medline citations to identify available articles in which PRSS1 mutations were determined in pancreatitis patients and controls through May. 2012. References in the Medline-cited studies were reviewed to identify additional reports not indexed by Medline. The following key words and subject terms were searched: 'PRSS1', 'cationic trypsinogen', 'candidate gene', 'association study', 'polymorphism' and 'pancreatitis'. We have only used data from the full-published paper, not from any meeting or conference abstract.
Quality assessments
All the included studies satisfied the following criteria: they (1) were association studies between the PRSS1 gene and pancreatitis (both AP and CP); (2) used disease-free people as controls; (3) were independent studies and the subject groups investigated did not overlap with each other; (4) were published in peer-reviewed journals and were indexed by PubMed or cited by articles indexed by PubMed. Authors were contacted where clarification was required.
Data extraction
The following information was independently extracted from the identified studies by two participants in the meta-analysis: first author, journal, year of publication, ethnicity of the study population, the number of cases and controls or OR, country in which the study was conducted and confirmation of diagnosis. The results were compared and any disagreement was discussed and resolved by consensus.
Statistical analysis
The effects model using the DerSimonian and Laird method was employed, and the estimate of heterogeneity was determined using the Mantel-Haenszel model. The effect size was represented by an odds ratio (OR) with 95% confidence interval (CI). Sensitivity analysis was conducted by removing each study and analyzing the others to ensure no single study was totally responsible for overall results. The significance level was set at 0.05, and all P values were two-tailed.
The comprehensive meta-analysis was performed using Comprehensive Meta-Analysis software (Version 2.2.046, BIOSTAT, Englewood, NJ, USA).
Results
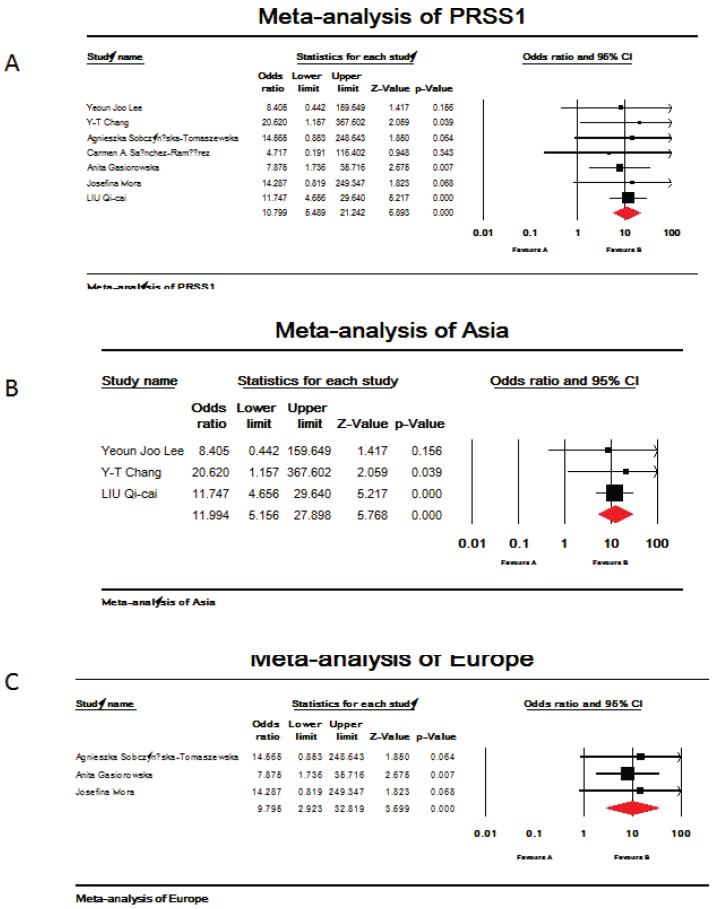
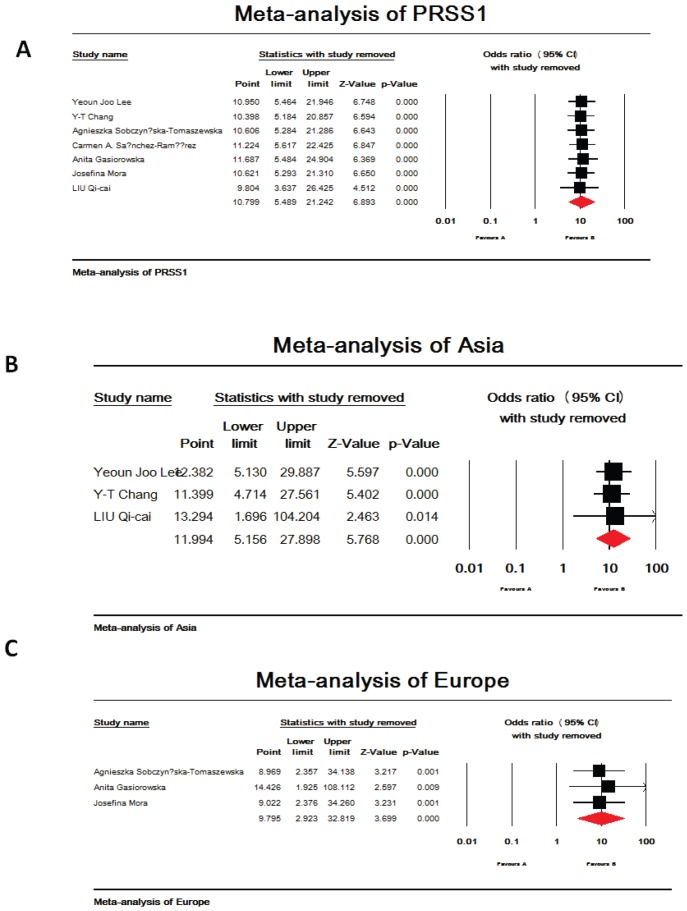
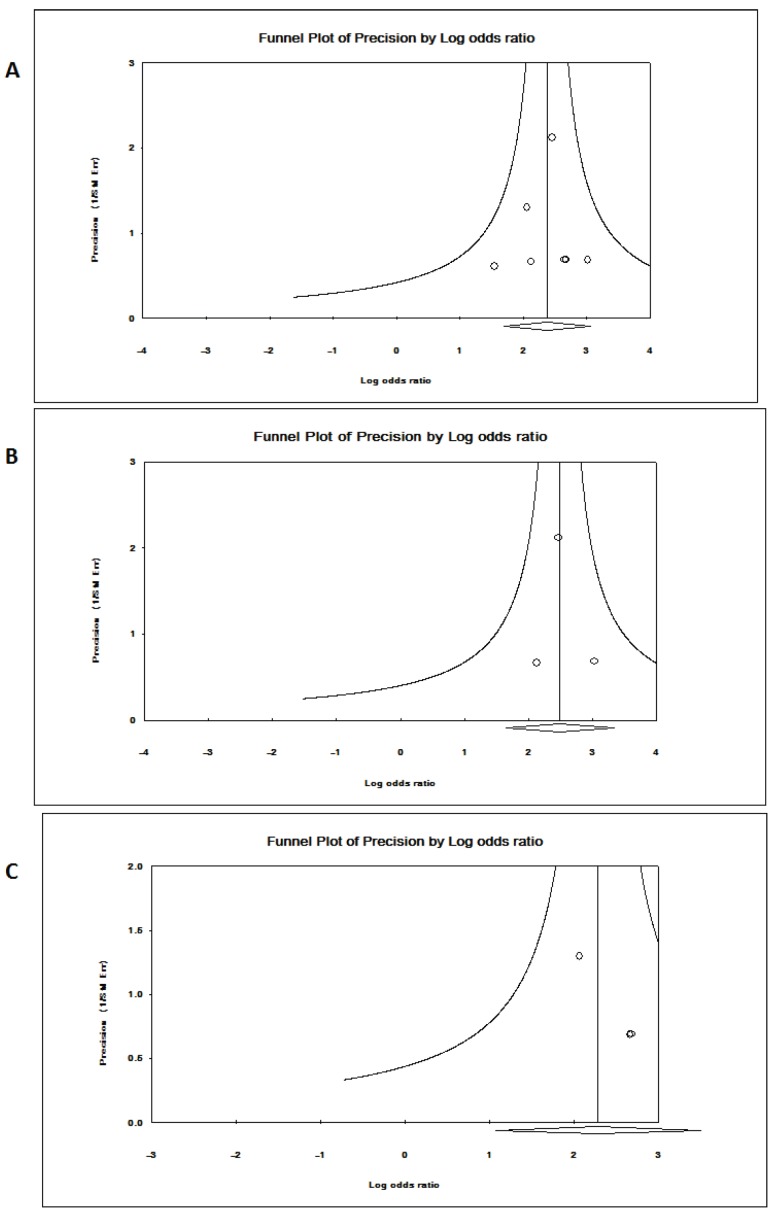
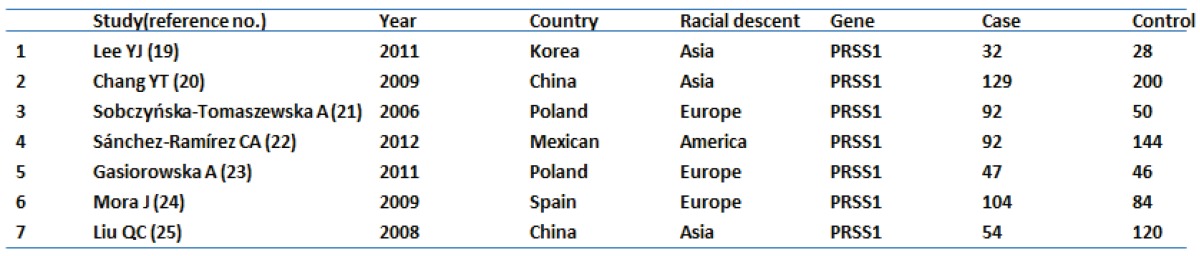
Seven references were included in this research 19-25. Among them, studies in reference 21 and 22 analyzed PRSS1 mutations in both acute and chronic pancreatitis, others focused on the chronic pancreatitis. The total analysis showed that PRSS1 gene was significantly associated with total pancreatitis {OR: 10.799, 95%CI: (5.489-21.242), p<0.000} (Figure 1-A). According to the different races, the total group was subdivided into European subgroup and Asian subgroup. In the European subgroup, PRSS1 was still significantly associated with the disease (both AP and CP) {OR: 9.795, 95%CI: (2.923-32.819), p<0.000} (Figure 1-C). When analyzed the Asian subgroup, the results showed that this group people, the same as the European subgroup, was significantly associated with pancreatitis (both AP and CP) {OR: 11.994, 95%CI: (5.156-27.898), p<0.000} (Figure 1-B). The sensitivity analysis, both in the total group and the subdivided subgroups, showed that when any one study was removed, the results still showed significant (Figure 3-A,B,C). This indicated that no heterogeneity existed in the populations. The Egger's funnel plots of publication bias analysis was shown in Figure 2-A,B,C.
Figure 1.
Meta-analysis of mutations in PRSS1 gene and total pancreatitis. (A,B,C). A. Analysis of PRSS1 gene and total pancreatitis. The overall OR is shown. The OR of each study is marked with a black square. The overall OR is indicated by red diamond. B. Asia subgroup analysis of PRSS1 gene and total pancreatitis. The overall OR is shown. The OR of each study is marked with a black square. The overall OR is indicated by red diamond. C. Europe subgroup analysis of PRSS1 gene and total pancreatitis. The overall OR is shown. The OR of each study is marked with a black square. The overall OR is indicated by red diamond.
Figure 3.
The sensitivity analysis of PRSS1 and total pancreatitis. (A,B,C). A. Total analysis of the sensitivity analysis. When any one of the studies was removed, the heterogeneity of the population was not changed. B. Asia subgroup analysis of the sensitivity analysis. When any one of the studies was removed, the heterogeneity of the population was not changed. C. Europe subgroup analysis of the sensitivity analysis. When any one of the studies was removed, the heterogeneity of the population was not changed.
Figure 2.
Egger's funnel plots of publication bias analysis PRSS1 gene and total pancreatitis. (A,B,C). A. Analysis for the PRSS1 and total pancreatitis. The larger the deviation from the funnel curve of each study, the more pronounced the asymmetry. Results from small studies will scatter widely at the bottom of the graph, with the spread narrowing among larger studies. B. Asia subgroup analysis for the PRSS1 and total pancreatitis. The larger the deviation from the funnel curve of each study, the more pronounced the asymmetry. Results from small studies will scatter widely at the bottom of the graph, with the spread narrowing among larger studies. C. Europe subgroup analysis for the PRSS1 and total pancreatitis. The larger the deviation from the funnel curve of each study, the more pronounced the asymmetry. Results from small studies will scatter widely at the bottom of the graph, with the spread narrowing among larger studies.
Discussion
As heterogeneous diseases, pancreatitis could be triggered by a variety of factors. More than 80 percent of acute pancreatitis is caused by gallstones and alcohol abuse. Other causes include medications, infections, trauma, metabolic disorders, and surgery. In about 10% to 15% of people with acute pancreatitis, the cause is unknown. Prolonged alcohol use is the leading cause of chronic pancreatitis. Other causes include metabolic, anatomical, obstructive, and autoimmune etiological factors. However, the mechanisms responsible for the development of pancreatitis have not yet been fully elucidated. Over the past several decades, genetic studies have provided insight into components of the pathogenic mechanisms leading to acute or chronic pancreatitis, and have identified several firmly established susceptibility genes such as PRSS1, SPNIK1, CTRC and CFTR 26. Among them, the PRSS1 gene, which located within 7q35, is the first gene found to be associated with hereditary pancreatitis. Patients suffering from CP caused by PRSS1 gene mutation have a significant life time risk for pancreatic cancer. This is the reason of genetic, clinical and imaging studies of these patients. So, it becomes more and more important to do more work on this subject. In order to explore the relationship between pancreatitis and PRSS1 gene, we conducted this comprehensive meta-analysis. The lack of concordance across many of single case-control studies reflects limitation in the studies, such as small sample sizes, ethnic difference and research methodology. Meta-analysis is a powerful tool for summarizing the results from different studies by producing a single estimate of the major effect with enhanced precision. It can overcome the problem of small sample size and inadequate statistical power of genetic studies of complex traits, and provide more reliable results than a single case-control study.
The human pancreas produces the digestive pro-enzyme trypsinogen in three highly similar isoforms: cationic trypsinogen (PRSS1), anionic trypsinogen (PRSS2), and mesotrypsinogen (PRSS3). Normally, about 2/3 of total trypsinogen in the pancreatic juice are cationic trypsinogen 27-29. Therefore, genetic defects or specific polymorphisms in PRSS1 may influence the susceptibility and severity of pancreatitis. Since 1996, over 25 mutations in this gene have been identified to be associated with pancreas. The most common mutations are R122H and N29I, which have been frequently found in hereditary pancreatitis families (http://www.uni-leipzig.de/pancreasmutation/). Moreover, the PRSS1 mutations can also be found in a variable incidence in idiopathic chronic pancreatitis 19-25. Several biochemical studies demonstrated that most of these mutations led to enhanced trypsinogen auto-activation and/or increased trypsin stability 30-36. Then the pancreatic protease anti-protease equilibrium may be disturbed. And this imbalance may initiate the subsequent auto-digestion and pancreatitis.
Considering the results of genetic association studies for PRSS1 and pancreatitis are inconsistent, we conducted this comprehensive meta-analysis. The current comprehensive study pooled larger sample sizes analyzing them both together and separately. The design of systematic methods and analytical approaches as well as tests of heterogeneity and sensitivity analyses has produced more significant results. The results, both in the total group and in the subgroups, showed that the gene was significantly associated with pancreatitis, and there was no heterogeneity existed in the populations. In conclusion, this meta-analysis supports significant association of marker in the PRSS1 gene with pancreatitis.
Table 1.
Characteristics of individual studies included in meta-analysis.
Acknowledgments
This work is supported by grants from the National Natural Science Foundation of China (31000408). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributions
J. Liu designed the study. J. Liu and HX. Zhang performed the statistical analysis and drafted the manuscript. All authors critically revised the manuscript and gave final approval of the article for submission.
References
- 1.Yin YW, Hu AM, Sun QQ. et al. Association between tumor necrosis factor-alpha gene -308A/G polymorphism and the risk of acute pancreatitis: A meta-analysis. J Surg Res. 2012;178:409–14. doi: 10.1016/j.jss.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XP, Zhang J, Ma ML. et al. Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9:83–7. [PubMed] [Google Scholar]
- 3.Witt H, Apte MV, Keim V. et al. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Braganza JM, Lee SH, McCloy RF. et al. Chronic pancreatitis. Lancet. 2011;377:1184–97. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Santamaria DM, Taxonera C, Giner M. Update on pathogenesis and clinical management of acute pancreatitis. World J Gastrointest Pathophysiol. 2012;3:60–70. doi: 10.4291/wjgp.v3.i3.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramythiotis D, Kleeff J, Schmidt J. et al. Detection of oncogenes in chronic pancreatitis. HPB (Oxford) 2003;5:214–25. doi: 10.1080/13651820310017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derikx MH, Drenth JP. Genetic factors in chronic pancreatitis; implications for diagnosis, management and prognosis. Best Pract Res Clin Gastroenterol. 2010;24:251–70. doi: 10.1016/j.bpg.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Thrower E, Husain S, Gorelick F. Molecular basis for pancreatitis. Curr Opin Gastroenterol. 2008;24:580–5. doi: 10.1097/MOG.0b013e32830b10e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uomo G, Manes G. Risk factors of chronic pancreatitis. Dig Dis. 2007;25:282–4. doi: 10.1159/000103903. [DOI] [PubMed] [Google Scholar]
- 10.LaRusch J, Whitcomb DC. Genetics of pancreatitis. Curr Opin Gastroenterol. 2011;27:467–74. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–24. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 12.O'Reilly DA, Witt H, Rahman SH. et al. The SPINK1 N34S variant is associated with acute pancreatitis. Eur J Gastroenterol Hepatol. 2008;20:726–31. doi: 10.1097/MEG.0b013e3282f5728c. [DOI] [PubMed] [Google Scholar]
- 13.Keim V. Role of genetic disorders in acute recurrent pancreatitis. World J Gastroenterol. 2008;14:1011–5. doi: 10.3748/wjg.14.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creighton J, Lyall R, Wilson DI. et al. Mutations of the cationic trypsinogen gene in patients with chronic pancreatitis. Lancet. 1999;354:42–3. doi: 10.1016/S0140-6736(99)01814-0. [DOI] [PubMed] [Google Scholar]
- 15.Monaghan KG, Jackson CE, KuKuruga DL. et al. Mutation analysis of the cystic fibrosis and cationic trypsinogen genes in patients with alcohol-related pancreatitis. Am J Med Genet. 2000;94:120–4. doi: 10.1002/1096-8628(20000911)94:2<120::aid-ajmg4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.O Reilly DA, Yang BM, Creighton JE. et al. Mutations of the cationic trypsinogen gene in hereditary and non-hereditary pancreatitis. Digestion. 2001;64:54–60. doi: 10.1159/000048839. [DOI] [PubMed] [Google Scholar]
- 17.Rosendahl J, Witt H, Szmola R. et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2007;40:78–82. doi: 10.1038/ng.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitcomb DC, Gorry MC, Preston RA. et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Kim KM, Choi JH. et al. High incidence of PRSS1 and SPINK1 mutations in Korean children with acute recurrent and chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2011;52:478–81. doi: 10.1097/MPG.0b013e31820e2126. [DOI] [PubMed] [Google Scholar]
- 20.Chang YT, Wei SC, L PC. et al. Association and differential role of PRSS1 and SPINK1 mutation in early-onset and late-onset idiopathic chronic pancreatitis in Chinese subjects. Gut. 2009;58:885. doi: 10.1136/gut.2007.129916. [DOI] [PubMed] [Google Scholar]
- 21.Sobczynska-Tomaszewska A, Bak D, Oralewska B. et al. Analysis of CFTR, SPINK1, PRSS1 and AAT mutations in children with acute or chronic pancreatitis. J Pediatr Gastroenterol Nutr. 2006;43:299–306. doi: 10.1097/01.mpg.0000232570.48773.df. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Ramirez CA, Flores-Martinez SE, Garcia-Zapien AG. et al. Screening of R122H and N29I Mutations in the PRSS1 Gene and N34S Mutation in the SPINK1 Gene in Mexican Pediatric Patients With Acute and Recurrent Pancreatitis. Pancreas. 2012;41:707–11. doi: 10.1097/MPA.0b013e31823cd873. [DOI] [PubMed] [Google Scholar]
- 23.Gasiorowska A, Talar-Wojnarowska R, Czupryniak L. et al. The prevalence of cationic trypsinogen (PRSS1) and serine protease inhibitor, Kazal type 1 (SPINK1) gene mutations in Polish patients with alcoholic and idiopathic chronic pancreatitis. Dig Dis Sci. 2011;56:894–901. doi: 10.1007/s10620-010-1349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mora J, Comas L, Ripoll E. et al. Genetic mutations in a Spanish population with chronic pancreatitis. Pancreatology. 2009;9:644–51. doi: 10.1159/000181177. [DOI] [PubMed] [Google Scholar]
- 25.Liu QC, Gao F, Ou QS. et al. Novel mutation and polymorphism of PRSS1 gene in the Chinese patients with hereditary pancreatitis and chronic pancreatitis. Chin Med J (Engl) 2008;121:108–11. [PubMed] [Google Scholar]
- 26.Chen JM, Férec C (2012) Genetics, pathogenesis of chronic pancreatitis. The 2012 update. Clin Res Hepatol Gastroenterol. 2012;36:334–40. doi: 10.1016/j.clinre.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Teich N, Rosendahl J, Tóth M. et al. Mutations of human cationic trypsinogen (PRSS1) and chronic pancreatitis. Human Mutation. 2006;27:721–30. doi: 10.1002/humu.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guy O, Lombardo D, Bartelt DC. et al. Two human trypsinogens. Purification, molecular properties, and N-terminal sequences. Biochemistry. 1978;17:1669–75. doi: 10.1021/bi00602a014. [DOI] [PubMed] [Google Scholar]
- 29.Rinderknecht H, Renner IG, Carmack C. Trypsinogen variants in pancreatic juice of healthy volunteers, chronic alcoholics, and patients with pancreatitis and cancer of the pancreas. Gut. 1979;20:886–91. doi: 10.1136/gut.20.10.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JM, Kukor Z, Le Marechal C. et al. Evolution of trypsinogen activation peptides. Mol Biol Evol. 2003;20:1767–77. doi: 10.1093/molbev/msg183. [DOI] [PubMed] [Google Scholar]
- 31.Teich N, Ockenga J, Hoffmeister A. et al. Chronic pancreatitis associated with an activation peptide mutation that facilitates trypsin activation. Gastroenterology. 2000;119:461–5. doi: 10.1053/gast.2000.9312. [DOI] [PubMed] [Google Scholar]
- 32.Ferec C, Raguenes O, Salomon R. et al. Mutations in the cationic trypsinogen gene and evidence for genetic heterogeneity in hereditary pancreatitis. J Med Genet. 1999;36:228–32. [PMC free article] [PubMed] [Google Scholar]
- 33.Pfutzer R, Myers E, Applebaum-Shapiro S. et al. Novel cationic trypsinogen (PRSS1) N29T and R122C mutations cause autosomal dominant hereditary pancreatitis. Gut. 2002;50:271–2. doi: 10.1136/gut.50.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahin-Toth M, Toth M. Gain-of-function mutations associated with hereditary pancreatitis enhance autoactivation of human cationic trypsinogen. Biochem Biophys Res Commun. 2000;278:286–9. doi: 10.1006/bbrc.2000.3797. [DOI] [PubMed] [Google Scholar]
- 35.Le Marechal C, Chen JM, Quere I. et al. Discrimination of three mutational events that result in a disruption of the R122 primary autolysis site of the human cationic trypsinogen (PRSS1) by denaturing high performance liquid chromatography. BMC Genet. 2001;2:19. doi: 10.1186/1471-2156-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon P, Weiss FU, Sahin-Toth M. et al. Hereditary pancreatitis caused by a novel PRSS1 mutation (Arg-122 --> Cys) that alters autoactivation and autodegradation of cationic trypsinogen. J Biol Chem. 2002;277:5404–10. doi: 10.1074/jbc.M108073200. [DOI] [PubMed] [Google Scholar]