Abstract
Cancer is being re-interpreted in light of recent discoveries related to the histone code and the dynamic nature of epigenetic regulation and control of gene programs during development, as well as insights gained from whole cancer genome sequencing. Somatic mutations or deregulated expression of chromatin modifying enzymes are being identified at high frequency. Nowhere is this more relevant than in pediatric embryonal solid tumors wherein a picture is emerging which shows that classic genetic alterations associated with these tumors ultimately converge on the epigenome to dysregulate developmental programs. In this review we relate how alterations in components of the transcriptional machinery and chromatin modifier genes contribute to the initiation and progression of pediatric solid tumors. We also will discuss how dramatic progress in our understanding of the fundamental mechanisms that contribute to epigenetic deregulation in cancer is providing novel avenues for targeted cancer therapy.
Introduction
Cancer is a developmental disease and the hijacking of biologic processes that are central to normal embryonic development is an essential feature of human malignancy. Nowhere is this more apparent than in pediatric tumors where disruptions to normal development are believed to underlie the genesis of many if not all childhood tumors (1). Normal mammalian development is a precisely orchestrated process that results in the creation of hundreds of differentiated cell types from a single pluripotent stem cell. This process of progressive lineage specification and cellular differentiation is dependent on epigenetic regulation, which directs heritable changes in gene expression independently of DNA sequence changes (2–4). In eukaryotic cells DNA is wrapped around core histone proteins and packaged into compact chromatin structures termed nucleosomes (Fig. 1). Epigenetic regulation of gene expression is predominantly controlled by covalent modifications of histones, changes to nucleosome conformation and position (nucleosome remodeling), and DNA methylation (Fig. 1, 2). In this manuscript we will review normal epigenetic regulation and discuss how disruptions to the epigenetic machinery contribute to the initiation and progression of pediatric solid tumors. In addition, we will discuss how advanced understanding of epigenetic regulatory mechanisms is providing novel avenues for targeted cancer therapy.
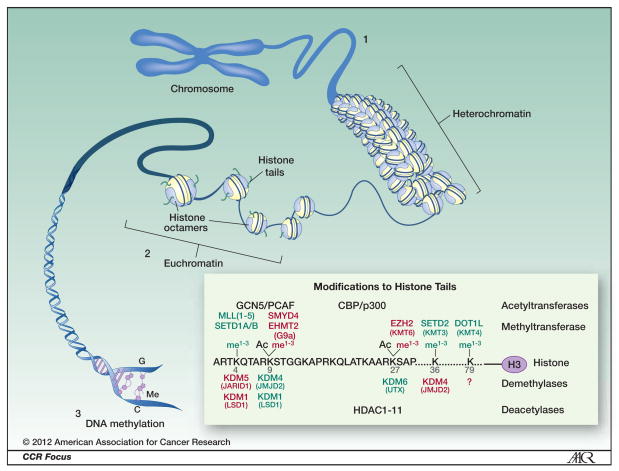
Figure 1. Higher order complexity of DNA.
To achieve required nuclear compactness, eukaryotic DNA is wrapped around core histone proteins (histone octamers) and packaged into compact chromatin structures termed nucleosomes. Epigenetic regulation of gene expression is predominantly controlled by covalent modifications to histones (on histone tails). These post-translational modifications signal the recruitment of protein complexes that: 1) more tightly package the nucleosomes causing condensed chromatin referred to as heterochromatin. Heterochromatin is devoid of gene transcription; 2) remodel the nucleosomes leading to more loosely or irregularly spaced nucleosomes referred to as euchromatin. Regulated gene transcription takes place in euchromatin regions; and 3) recruit proteins responsible for DNA methylation. Insert Box: Modifications to Histone Tails. In particular, methylation of lysine residues 9 and 27 on histone 3 (H3K9me2, H3K9me3 and H3K27me3) and ubiquitination of histone 2A (H2AUb) are associated with a more compact heterochromatin structure and gene silencing (6). The activity of methyltransferases is countered by proteins with demethylase activity. Lysines may also be acetylated by acetyltransferases including GCN5/PCAF or CBP/p300 and typically acetylated lysines favor gene transcription. A series of histone deacetylases (HDAC1–11) deacetylated lysines and increased activity or levels of these proteins is associated with gene silencing. Proteins involved in these processes are explained in more detail in Figure 2. Histone modifications associated with silencing and proteins mediating them are denoted in red while those associated with gene activation are denoted in green.
Figure 2. Protein modifications and complexes that regulate higher order chromatin conformation.
A. PcG-protein complexes. The PRC2 protein EZH2 is the key effector of PRC2 action, catalyzing trimethylation of H3K27 (H3K27me3) (7, 8). Histone deacetylases (HDACs) also bind the PRC2 complex decreasing acetylation of H3K27 and favoring its methylation and inhibiting gene transcription. In contrast, inhibitors of histone deacetylases (HDACi) such as Vorinistat or Romidepsin would be expected to counteract this activity resulting in increased acetylation at these loci, favoring gene expression. For example, at steady-state EZH2 mediates increased H3K27me3 at the CASZ1 tumor suppressor gene and loss of gene transcription in neuroblastoma and Romidepsin (depsipeptide) treatment leads to increased H3K27Ac and increased gene transcription at this locus (57). PRC2 is targeted to DNA by JARID2, which binds GC containing DNA regions. PRC1 in turn mono-ubiquitinates H2A, a task that is achieved by the PRC1 protein BMI-1 in cooperation with the E3 ubiquitin ligase RING1B (7, 8). In contrast, acetylation of lysine residues on histones 3 and 4 (H3K, H4K) and methylation of H3K residue 4 (H3K4me3) promote an open euchromatin state and active transcription.
B. Nucleosome remodeling The ATP-dependent chromatin remodeling complexes are a family of proteins SWI/SNF, ISWI, NURD/MI-2/CHD and INO80 characterized by common DExx and HELICc domains. These chromatin-remodeling complexes use energy-dependent mechanisms to move the DNA around the histone octamer and also alter histone octamer composition. Most significantly, the SWI/SNF complex has been shown to play essential roles in regulating nucleosome remodeling, contributing to both the activation (left panel denoted in green) and repression (right panel denoted in red) of gene expression programs in a context-dependent manner (17). During lineage-specific differentiation the SWI/SNF complex cooperates with transcription factors to promote activation of differentiation genes and silencing of proliferation genes.
C. DNA methylation. Methylation of CpG islands located in proximity to transcription initiation is associated with heritable silencing (denoted in red) of gene expression (gene off) and is responsible for physiologic processes that depend on permanent gene silencing such as X-chromosome inactivation and gene imprinting (11). DNA methylation may be mediated by transcription factor or PcG –mediated recruitment of DNMTs to specific gene loci.
D. DNA demethylation. More recent studies have identified DNA demethylases (TET1,2) which hydroxylate 5-Methylcytosine(5mC) in CpG dinucleotides to 5-Hydroxymethylcytosine(5-hC). Evidence proposes that TET enzymes are capable of iterative oxidation on substrates to 5-formylmethylcytosine (5-fC) or 5-carboxymethylcytosine(5-caC) (23, 24). This leads to substrates upon which base excision repair mechanisms mediated by thymine-DNA glycosylase (TDG) excise a modified C and replace with an unmodified C, allowing for rapid re-activation (gene ON) of previously silenced genes (24).
Epigenetic Regulation in Normal Development
Histone Modifications
Histone modifications, including acetylation, methylation, and ubiquitination, lead to changes in chromatin structure that determine the accessibility of DNA to transcription factors (5, 6) (Fig. 1). Histone acetylation and methylation are dynamically regulated by the competing actions of histone acetyltransferases (HAT) and histone deacetylases (HDAC) and by histone methyltransferases (HMT) and histone demethylases, respectively (6). Repressive histone modifications are largely driven by the action of polycomb group (PcG) proteins (7, 8). PcG proteins function as multi-protein complexes, PRC1 and PRC2, which act cooperatively to silence transcription, mainly by trimethylating lysine residue 27 on histone 3 (H3K27me3). In stem cells, PcG proteins maintain self-renewal and pluripotency by repressing differentiation genes (2, 8). PcG proteins also interact with DNA methyltransferases (DNMTs) to induce permanent transcriptional silencing by enabling DNA methylation (9, 10) (Fig 2A,C). In many cancers, PcG genes and proteins, especially BMI-1 and EZH2, are aberrantly over-expressed resulting in persistent activation of stem cell programs and repression of cellular differentiation (7, 11) (Fig. 3).
Figure 3. Chromatin Regulation of Developmental Gene Expression and Oncogenic Dysregulation.
A. Genome wide analyses of chromatin marks indicate that in embryonic stem cells expression of lineage specific transcriptional programs is suppressed while pluripotency genes such as OCT4, MYC, SOX2 and BMI-1 are expressed (2–4). Key developmental genes appear to be enriched for H3K4me3 gene activation and H3K27me3 gene suppression modifications at their enhancers or promoters. These “bivalent” marks are thought to identify key developmental genes “poised” to be dynamically regulated upon appropriate activation of lineage specific pathways (15). In a stem cell these genes may be viewed as being “reversibly silenced”. During normal differentiation, lineage specific programs silenced in the stem cell are activated while pluripotency genes are silenced. During cell specification, non-specified lineage gene programs are suppressed.
B. Oncogenic alterations may occur at any point during stem cell differentiation leading to blocks in developmental programs. Moreover, failure to suppress alternate lineage programs could lead to tumors with mixed lineage phenotypes. In models of EFT initiation, ectopic expression of EWS-FLI1 in mesenchymal (MSC) or neural crest (NCSC) stem cells modulates the expression of numerous transcripts and proteins involved in epigenetic regulation (shown in red; functional significance of proteins in box – BMI-1, EZH2, NKX2.2 – has been experimentally validated – See Refs. 33, 35, 37, and 39). Over-expression of DNA methyltransferases (DNMTs) in these cells is also observed and contributes to preferential silencing of transcription factors that normally instruct terminal differentiation of neuro-mesenchymal cells (E.R. Lawlor Lab, unpublished data). The precise molecular mechanisms that dictate persistent PcG- and DNA methylation-mediated repression of these developmental pathways remain to be elucidated but may involve EWS-FLI1-mediated deregulation of non-coding RNAs (miRNAs and lncRNAs).
Transcriptionally active chromatin is acetylated and methylated at H3K4 (5) (Fig. 1 & 3). H3K4 methylation is supported by trithorax group proteins with histone methyltransferase activity that are encoded by mixed lineage leukemia (MLL) genes (12). Alterations in H3K4 methylation lead to differentiation defects and rearrangements of MLL1 and mutations in MLL2 and MLL3 are common in high-risk leukemias of childhood (13) and pediatric medulloblastomas (14), respectively. Importantly, in embryonic stem cells, repressed genes that are involved in early lineage commitment maintain both repressive (H3K27me3) and activating (H3K4me3) histone modifications (15). These bivalent genes are considered to be poised for rapid activation in response to appropriate differentiation signals (15) and they are frequently aberrantly irreversibly silenced in cancer (Fig. 3) (16).
Nucleosome remodeling
Changes in nucleosome position along the DNA strand and conformational changes to DNA-histone interactions influence the accessibility of transcription factors to DNA. Such nucleosome remodeling is critical for normal differentiation and is controlled by ATP-dependent, multi-protein chromatin-remodeling complexes, in particular SWI/SNF (5, 17) (Fig. 2B). In addition, SWI/SNF contributes to the epigenetic regulation of gene expression by competing with and antagonizing PcG protein function (18). The core subunit of SWI/SNF, SNF5 (also known as SMARCB1, INI1, and BAF47) is an established tumor suppressor that is mutated in aggressive malignant rhabdoid tumors in children (19). Mutations of other members of the SWI/SNF complex are also prominent in several aggressive adult malignancies (reviewed in (17)).
DNA Methylation
DNA methylation at gene promoters is associated with gene silencing and, during differentiation, cellular reprogramming or tumorigenesis the pattern of global DNA methylation undergoes significant changes (20–22) (Fig. 2C). In normal differentiated tissues DNA methylation is quite stable and is characterized by methylated intergenic regions and unmethylated gene promoters (22). By contrast, in cancer large regions of methylation are lost from intergenic regions and gene promoters are aberrantly hypermethylated (22). In addition, tissue specific differentially methylated regions (DMRs) that are highly stable in normal tissues are highly unstable in malignant tissues indicating epigenetic instability (22). Although DNA methylation was long considered to be irreversible, the recent discovery of TET family proteins has elucidated a physiologic mechanism of DNA demethylation (23). Through their action as 5-methylcytosine dioxygenases, TET enzymes convert 5’-methylcytosine residues on methylated DNA to 5’-hydroxymethylcytosine (24)(Fig. 2D). Importantly, mutations in TET-encoding genes and genes that alter TET function are common in human myeloid malignancies (25, 26) and gliomas (27), respectively, and are associated with abnormal DNA methylation phenotypes. Hypermethylated genes in cancer are highly enriched for bivalent genes as well as other genes that are subject to PcG-mediated silencing in stem cells (16, 28). These findings together suggest a developmental model of cancer initiation in which tumor-initiating cells become locked in an undifferentiated state as a result of aberrant DNA methylation at PcG-marked loci (Fig. 3B).
Epigenetic Deregulation in Pediatric Solid Tumors
Whether a tumor arises from an embryonic stem cell, multi-potent progenitor or the de-differentiation of a more specialized post-natal cell, a unifying model is that genetic alterations associated with tumorigenesis lead to initiation of malignant transformation as a result of epigenetic deregulation. Recent and emerging data generate compelling support for the critical contribution of this model to the origin and progression of pediatric solid tumors, in particular to Ewing sarcoma, neuroblastoma and brain tumors.
Ewing sarcoma
Ewing sarcoma family tumors (EFT) are characterized by an undifferentiated histology, a highly metastatic phenotype and the presence of chromosomal translocations that result in the creation of fusion oncogenes (29). In 85% of cases the resulting chimeric protein is a fusion between EWS and FLI1 (30). EWS-FLI1 functions as aberrant transcription factor and is believed to be an initiating tumorigenic event. The very primitive neuroectodermal histology of EFT and their diverse presentation in bones and soft tissues suggest an early stem or progenitor cell of origin. Indeed, recent experimental evidence supports this hypothesis and bone progenitors, mesenchymal (MSC) and neural crest (NCSC) stem cells are now all implicated as potential cells of origin (31–35).
Initial investigations into EWS-FLI1 function focused largely on identifying genes that were induced by the fusion. In the course of these studies several promising candidates were identified and genes such as NKX2.2 (36), NR0B1 (DAX1) (37) and EZH2 (38) are now well-established direct transcriptional targets of EWS-FLI1. However, EWS-FLI1 represses as many genes as it induces (34, 39, 40) and the mechanisms by which EWS-FLI1 represses transcription and the contribution of gene repression to the EFT phenotype have become the focus of intense investigation.
A significant portion of EWS-FLI1-repressed targets are down regulated by the activity of its target gene NKX2.2, which functions to repress transcription by recruiting TLE transcriptional co-repressors and HDACs to target gene promoters (39). Similarly, EWS-FLI1-mediated induction of EZH2 blocks differentiation of MSC and leads to the repression of genes involved in neuroectodermal and endothelial differentiation (38, 41). In NCSC, EZH2 and BMI-1 are both induced by EWS-FLI1, however, unlike EZH2, the mechanism of BMI-1 induction is likely to be indirect (34). This up regulation of BMI-1 leads to repression of p16 and maintenance of stemness, supporting the hypothesis that epigenetic silencing of p16 may be a key step in EFT initiation (34) (Fig. 3B). Sustained expression of EWS-FLI1 in NCSC results in progressive down-regulation of transcription factors that are normally required for neuroectodermal and skeletal differentiation ((34) and C von Levetzow & ER Lawlor, unpublished work). In established EFT tumorigenicity is dependent on continued over-expression of EZH2 and BMI-1 (32, 38, 42, 43). To exploit this, cell therapy with cytotoxic T-cells directed against EZH2 was recently proposed as a novel approach to EFT therapy (44) demonstrating that the abnormal cancer epigenome could be a source of novel antigenic targets for immunotherapy (45).
Finally, regulation of PcG protein expression and function is mediated by a complex network of miRNAs (46) and by long non-coding RNAs (ncRNAs) (7). EWS-FLI1 modulates the expression of both miRNAs (47) and lncRNAs (48) so it will be interesting to discover whether EWS-FLI1-mediated deregulation of ncRNA transcripts contributes to PcG protein dysfunction. In addition, the contribution of altered DNA promoter methylation to EFT pathogenesis is beginning to come into focus. Global analysis of DNA promoter methylation has revealed that the CpG islands of PcG target genes are aberrantly hypermethylated in EFT and the promoters of transcription factors involved in neural differentiation become progressively methylated over time in EWS-FLI1-expressing NCSC (C von Levetzow and ER Lawlor, unpublished work). Thus, EWS-FLI1 drives EFT pathogenesis by invoking global deregulation of the epigenome through diverse mechanisms (Fig 3B).
Progression of established EFT depends on continued expression of EWS-FLI1 (36). Inhibition of tumor growth following EWS-FLI1 inhibition is associated with re-expression of numerous repressed target genes suggesting that the oncogenic phenotype is dependent, at least in part, on maintaining epigenetic repression (36). In support of this, targeted knockdown of BMI-1 in EFT leads to reduced tumorigenicity and induction of differentiation genes (Fig. 4A)(42). In addition, exposure of EFT cells to HDAC inhibitors (see Table 1) restores expression of numerous EWS-FLI1-repressed genes and also inhibits growth and tumorigenicity (39, 49). Combining HDAC inhbition with decitabine, an inhibitor of DNA methylation, potentiates the growth inhibitory effects of either agent alone, supporting a role for combination approaches to epigenetic therapy (50). Direct pharmacologic targeting of transcription factors continues to be an immense therapeutic challenge. In EFT targeting the epigenome may be an effective way to circumvent the oncogenic activity of EWS-FLI1 without having to directly target the oncogene itself.
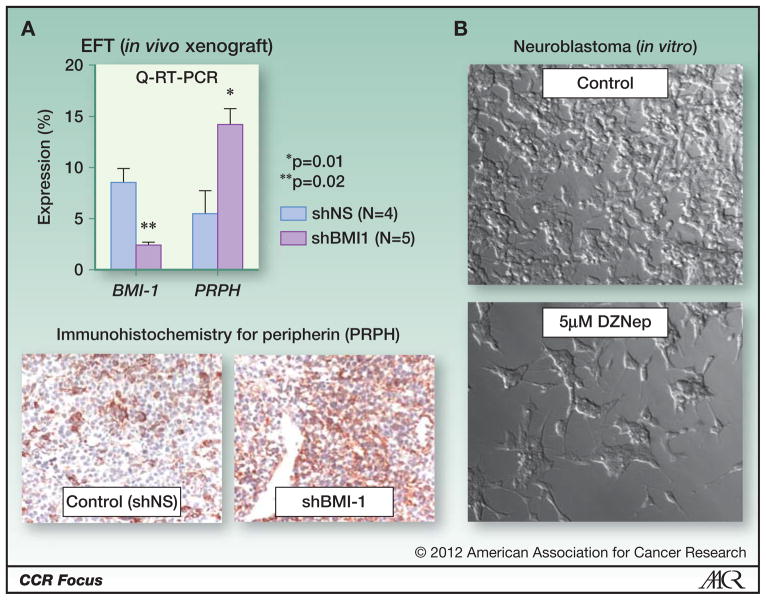
Figure 4. Inhibition of polycomb proteins promotes tumor cell differentiation.
A. BMI-1 knockdown (shBMI1) EFT cells have reduced tumorigenic capacity in immune deficient mice (42). As shown here, TC-71 xenografts established from stably transduced BMI-1 knockdown cells express higher levels of peripherin, a cytoskeletal marker of differentiated peripheral neurons, than tumors established from non-silenced control cells (shNS). Top panel: Q-RT-PCR - expression of PRPH is normalized relative to expression of GAPDH (Taqman assays from Applied Biosystems). Bottom panel: Peripherin immunostaining of fresh-frozen sections (peripherin antibody 1:100 dilution; Cat# AB1530, Millipore, Temecula, CA.).
B. Deazaneplanocin A (DZnep) inhibits EZH2 as well as histone methylation more broadly and induces apoptosis or differentiation in pre-clinical models of a number of tumors (102, 103). Treatment of the NB cell line SKN-BE2 for 4-days with DZnep inhibits growth and induces morphologic differentiation.
Table 1.
Epigenetic Modifiers-Current Drugs & Agents in Development
| CLASS OF AGENT | TARGET | STATUS | TUMOR | REF |
|---|---|---|---|---|
| HDAC inhibitors1 | ||||
| Vorinistat (SAHA) (Zolinza®) | HDAC1–3,6 | FDA2 Ped.Phase I |
Cutaneous T-cell lymphoma Relapsed solid tumors |
(86, 95, 96) |
| Romidepsin(FK228) (Istodax®) | HDAC1–3,8 Weak-HDAC6,4 |
FDA2 Ped.Phase I |
Cutaneous, Peripheral T-cell lymphoma Relapsed solid tumors |
(86) (97) |
| Entinostat(SNDX-275), Panobinostat(LBH589) Belinostat(PDX101) |
HDAC1 HDAC1–4 HDAC1–4 eakHDAC8 |
Phase II Phase I Phase I |
Breast Lymphoma Relapsed solid tumors |
(86) |
| Valproic Acid3 | HDAC1–3, weakHDAC8 | Ped.Phase I, | Refractory solid or CNS tumors | (86, 98) |
| PCI-34051 | HDAC8 | Pre-clinical | (99) | |
| Modifiers of Acetylated Histones | ||||
| JQ1 | KAC | Pre-clinical | Lymphoma, MYC-driven tumors | (75, 100) |
| HMT Inhibitors | ||||
| EZH2 (SET domain) inhibitors (Epizyme, Inc.) | EZH2 | Pre-clinical | EZH2 mutated lymphomas | (101) |
| 3-Deazaneplanocin A, DZNep | EZH2 | Pre-clinical | (102, 103) | |
| EPZ004777 | DOT1L | Pre-clinical | MLL-rearranged AML & ALL | (104) |
| Histone Demethylase Inhibitors | ||||
| Monamine Oxidase Inhibitors: Parglyine, clorgyline, tranylcypromine (Parnate®)3 | LSD1 | Pre-clinical FDA3 |
(61) | |
| Polyamine analogs3 Bisguanidines, biguanides | LSD1 | Pre-clinical | (105) | |
| DNA Methylation inhibitors | ||||
| Decitibine (Dacogen®) Azacytidine (Vidaza®) |
DNMT | FDA2 | MDS, CML | (86) |
| Zebularine | DNMT | Clinical trials | MDS, CML | (86) |
Target specificity for particular HDACs based on functional data (See Ref (95)).
FDA approved for a cancer therapy indication.
FDA approved for non-cancer indications
Neuroblastoma
Neuroblastomas (NB) are neural crest-derived tumors, expressing genes characteristic of sympathoadrenal cell lineages. Mutations in PHOX2B, a major regulator of sympathoadrenal development, have been identified in 6% of familial NB and also more rarely in NB cell lines (51). In the 1980s, histological classification of NB into subsets based on the extent of differentiation was prognostically relevant (52). Functional studies contributed to the concept that NB cells corresponded to different stages of sympathoadrenal development, retained potential to differentiate into a number of neural crest cell lineages and thus represented a multi-potent sympathoadrenal progenitor cell with distinct tumorigenic potential (53, 54). Consistent with these concepts, aggressive NB tumors are characterized by increased expression of cell cycle genes (55), while ganglioneuroma and ganglioneuroblastoma express genes associated with neural development (56).
EZH2 is elevated in poor prognosis, undifferentiated NB and is associated with enrichment of H3K27me3 at the promoters of tumor suppressor genes such as CASZ1, RUNX3, NGFR (p75) and NTRK1 (TrkA) (57). What leads to elevated EZH2 levels is still unknown, but a number of genetic alterations at the EZH2 locus may contribute: 7q35–36 is amplified in 10–15% of tumors and over 50% of NB tumors have gains of the entire Chr7 or 7q (58). BMI-1 is a direct transcriptional target of MYCN (59) and is also over-expressed by and essential for tumorigenicity of NB (60). In addition, the lysine specific demethylase, LSD1 is over-expressed in poorly differentiated NB (61). In embryonic stem cells, LSD1 preferentially demethylates H3K4me3 thus favoring PcG-associated H3K27me3 silencing at bivalent loci and maintenance of stemness. This physiologic function of LSD1 and EZH2 over-expression most likely contribute to the poor prognosis that is associated with LSD1 over-expressing NB compared to more differentiated forms of the tumor (61).
The relationship between PcG protein dysregulation and DNA methylation in NB tumors is tenuous but intriguing. The CHD5 tumor suppressor gene, part of a family of chromatin remodeling proteins, is in a chromosomal region that is subject to loss of heterozygosity (LOH) in a number of tumors including NB (62). CHD5 contains a bivalent chromatin mark in stem cells (15) and in NB cells with LOH the remaining allele is silenced via DNA methylation (63). Examination of genome-wide expression microarray data from primary NB tumors (R2 database-http://hgserver1.amc.nl/cgi-bin/r2/main.cgi) reveals an inverse correlation between EZH2 and CHD5 expression (r=−0.314, p=2.6 × 10−3) and a direct correlation between EZH2 and DNMT1 (r=0.679, p=3.5 × 10−13). Thus, over-expression of PcG proteins in NB may promote recruitment of DNMTs and induce permanent gene silencing of key developmental loci. It is not clear whether silencing of a single gene or an entire pathway is needed for NB initiation or progression but a number of genes, including a commonly hypermethylated gene in cancer (HIC1), putative tumor suppressor genes (RASSF1A, PRKCDBP), differentiation genes (HOXA9) and apoptosis genes (CASP8, APAF1, TMS1) have been consistently identified (62, 64–67). In addition, emerging data from unbiased, global approaches have confirmed established loci and identified novel DNA methylated regions (telomeric silencing of 1p, 3p, 11q, and 19p) and a “methylator” phenotype that is associated with a worse clinical outcome (65, 67, 68).
While MYCN has been associated with activating histone chromatin modifications (69), there is also an intriguing association of MYCN amplification with DNA methylation. This was first noted with CASP8 (70) and has now been associated with additional loci (66, 67). In NB cell lines genome-wide MYCN DNA binding is significantly associated with the binding of MeCP2, a CpG methyl binding protein (71). At gene promoters transcription is relatively high when MYCN is bound alone, intermediate when both MYCN and MeCP2 are present and low with only MeCP2. This suggests that MYCN may serve as an initial but transient focus to recruit components required to mediate DNA methylation (71).
There is abundant evidence to support the use of epigenetic modifiers in NB therapy. First, the ability of retinoids to restore differentiation indicates that despite the genetic alterations associated with high-risk tumors the regulatory signaling pathways controlling growth and differentiation are intact but dysregulated. Indeed, it is becoming increasingly evident that retinoids function to relieve epigenetic suppression. NB cells treated with retinoids have decreases in steady-state levels of BMI-1 (60), EZH2 (DY Oh and CJ Thiele unpublished work) and LSD1 (61) and a recent genome-wide study indicates that retinoids reverse the methylation status of hundreds of gene promoters (72). Second, HDAC inhibitors, either alone or in combination with cis-retinoic acid, inhibit NB growth in vitro and in vivo (73, 74). Third, reactivation of caspase 8 in drug-resistant NB cells by exposure to DNA methylation inhibitors restores sensitivity to standard cytotoxic agents (70). Fourth, inhibition of EZH2 by DZNep leads to re-expression EZH2 silenced tumor suppressor genes and results in decreased growth and differentiation of NB cells (Fig. 4B) (57). Finally, the most aggressive NB are driven by aberrant activation of MYCN. Recent studies have shown that JQ1, a novel small molecule inhibitor of the chromatin modifying co-factor BRD4, dramatically inhibits proliferation and promotes terminal differentiation of acute myelogenous leukemia cells and it achieves this, in part, by suppressing MYC function (75). Whether epigenetic therapy with JQ1 can be used to block the oncogenic activity of MYCN in NB remains to be determined but provides an exciting new opportunity for investigation.
Brain Tumors
Epigenetic deregulation is emerging as a fundamental process underlying the pathogenesis of pediatric brain tumors. Hypermethylation of the RASSF1A tumor suppressor gene has been identified in nearly 90% of medulloblastomas and ependymomas (76) and upregulation of PcG proteins, in particular BMI-1 and EZH2, is common and is associated with worse clinical outcomes (77, 78). Intriguingly, recent next generation sequencing studies of pediatric medulloblastomas have discovered that, although tremendous genetic diversity exists between individual tumors, there is a marked over-representation of disruptions in genes that encode for chromatin modifiers and epigenetic regulators (14, 79). Specifically, deletions in individual genes that encode H3K9 methytransferases (i.e. EHMT1, SMYD4) were detected in 2% of cases in a series of 212 tumors while H3K9 demethylase genes were selectively amplified in another 2% (79). In total, mutations, deletions or amplifications in genes that converge on modulating H3K9 methylation were detected in 19% of tumors examined in this study and absence of nuclear staining for methylated H3K9 was confirmed in 41% of cases in an independent cohort (79). Dimethylation of H3K9 is required for silencing of proliferative genes and successful terminal differentiation of progenitor cells in the external granule layer (79). Mutations that disrupt H3K9 methylation likely contribute to malignant transformation by blocking normal differentiation (79). In a second study, inactivating mutations of histone methyltransferases MLL2 and MLL3 were detected in 16% of primary and mutations in SWI/SNF components SMARCA4 and ARID1A in another 4% (14). Thus, mutations in epigenetic regulators figure prominently in pediatric medulloblastoma and cumulatively these lesions contribute to tumor initiation and progression by repressing normal developmental pathways whilst promoting maintenance of a more stem-like state.
Next generation sequencing studies of gliomas have also uncovered a basis for epigenetic deregulation with the discovery that 70–80% of grade II and III astrocytomas harbor a mutation in either the IDH1 or IDH2, isocitrate dehydrogenase genes (80). IDHs produce α-ketoglutarate, a necessary co-substrate for histone demethylases and TET family proteins (25). Mutated IDH proteins produce D-2-hydroxyglutarate which acts as a competitive inhibitor of α-ketoglutarate and thereby inhibits histone and DNA demethylation (81). Thus, IDH-mutated gliomas are epigenomically abnormal and this contributes to an abnormal DNA methylator phenotype (82). Importantly, however, mutations in IDH genes are associated with younger age and an improved prognosis indicating that epigenetically driven tumors may be less aggressive and more responsive to therapy than tumors that are characterized by genetic instability, such as those that occur in older patients (25).
Other embryonic solid tumors
Genome-wide studies of Wilms’ tumor chromatin and DNA methylation have been extremely informative and have identified global epigenetic aberrations beyond the well-established hyper-methylation of H19 that contributes to loss of imprinting and over-expression of IGF2 (83). In particular, the chromatin landscape of Wilms’ tumors has been shown to be highly related to embryonic stem cells and is associated with upregulation of PcG activity, abnormal retention of bivalent marks and silencing of genes that direct early renal differentiation (84). Although the precise cell of origin of retinoblastoma tumors remains controversial their clinical presentation before birth and in early infancy leaves no doubt as to their embryonic origin. Interestingly, a recent study found that developmental pathways that are normally expressed in a mutually exclusive fashion during normal retinal cell development are abnormally co-expressed in single retinoblastoma cells (85). Although the mechanisms underlying this observation remain to be elucidated, it is highly probable that disruption of normal epigenetic regulation within developing retinal cells contributes to this oncogenic developmental abnormality.
Therapeutic Opportunities and Challenges
FDA approved use of epigenetic modifiers is currently limited to two classes of agents (Table 1). Specifically, HDAC inhibitors (vorinostat and romedepsin) and DNA methylation inhibitors (5-azacytidine and deoxyazacytidine (decitabine)) are approved for use in the treatment of cutaneous T cell lymphomas and myelodysplastic syndrome, respectively, and function to reactivate aberrantly silenced genes (86). These agents, as well as other classes of HDAC inhibitors (e.g. valproate) are now being evaluated in early phase clinical trials in adult solid tumors, in particular for patients with relapsed and metastatic sarcomas (www.cancer.gov/clinicaltrials). Pediatric Phase I studies for romidepsin, vorinistat and valproic Acid have been completed and report that each is well tolerated and result in increased acetylated-H3 histones in peripheral blood lymphocytes, a surrogate marker of drug target activity (see Table 1). No objective responses were seen with the single agents but a complete response in one neuroblastoma patient when combined with 13-cis retinoic acid on the Vorinistat Phase I study encouraged the testing of combination trials. Currently the NANT (New Approaches to Neuroblastoma Therapy) consortium is evaluating the toxicity of vorinostat in combination with 13-cis-retinoic acid (www.cancer.gov/clinicaltrials; NCT01208454) or 131I-MIBG (Meta-Iodo-Benzyl-Guanidine) therapy (NCT01019850) (87). A Phase I/II study is also underway evaluating vorinostat in combination with etoposide in pediatric patients with relapsed and refractory sarcomas as well as other solid tumors (NCT01294670). Unfortunately, a recent Children’s Oncology Group study found that dose-limiting hematologic toxicities were experienced by children with relapsed solid tumors who were treated with low-dose decitabine in combination with cytotoxic chemotherapy (88). It remains to be determined whether drug toxicity and response rates to HDAC and DNA methylation inhibitors are strictly determined by the somatic tumor genotype or whether heritable germ-line factors may also contribute to outcome (89). Thus, there is still much work to be done to determine the optimal dose and schedule regimens of currently available drugs, especially when considering heavily pre-treated patients. Strong consideration should be given to testing epigenetic modifiers as components of initial therapy in newly diagnosed patients with high-risk disease.
Current HDAC and DNA methylation inhibitors are very non-specific in their action, invoking genome-wide effects and inducing cell death and differentiation through multiple different molecular mechanisms (90). Approved HDAC inhibitors broadly inhibit the activity of multiple different HDACs (Table 1) and it is the hope that as new more target-specific agents are developed there will be fewer dose-limiting toxicities. Improved understanding of the biology of epigenetic regulation has also contributed to the development of new classes of agents that have been designed to specifically target the cancer epigenome (Table 1). In addition, as discussed above with respect to retinoids in NB, an improved understanding of developmental biology is uncovering epigenetic mechanisms of action for established drugs. This raises the possibility that in the future other drugs may also be identified for repurposing as epigenetic modifiers.
Early failures with HDAC and DNA methylation inhibitors in clinical studies of adult solid tumors dampened initial enthusiasm for their potential as effective therapeutic agents. However, an improved understanding of mechanisms of drug action and of tumor biology led to changes to drug dose and delivery schedules that have proved beneficial (86). It is now apparent that epigenetic modifiers exert their biologic effects at doses far below the maximally tolerated doses that are identified in classic Phase I trials. In addition, single agent therapy with DNMT inhibitors may lead to only transient re-expression of silenced genes as a result of continued histone deacetylation of the repressed loci (91). Therefore, chronic low doses of epigenetic modifiers – DNMT inhibitors together with HDAC inhibitors –in combination with standard cytotoxic therapy may be required to normalize the epigenetic landscape of tumor cells and induce sustainable clinical remissions (86, 91). Indeed, a recent clinical trial of low-dose combination epigenetic therapy was successful at inducing clinical responses in heavily pre-treated patients with non small cell lung cancer (92). However, it is noteworthy that responses occurred gradually over several months. This observation is consistent with studies of epigenetic modifiers in animal models, which are demonstrating that the therapeutic efficacy of these agents may be better determined by evaluating tumor initiating potential rather than tumor shrinkage (93). Thus, standard RECIST criteria for evaluation of treatment response may not be the best initial measure of epigenetic drug efficacy. Ideally, biomarkers of drug efficacy should be used to ascertain that the agents are having the predicted biologic effect and it will be imperative that tissue be available for pathologic assessment of response whenever new epigenetically targeted agents are introduced into the clinical setting.
Summary
Genetic mutations that result in alterations in DNA sequence have classically been considered drivers of tumorigenicity. The recent explosion of knowledge surrounding stem cell and developmental biology combined with the advent of next-generation sequencing technologies has led to the realization that disruptions to the epigenome are at least equal contributors to the pathogenesis of human cancer (86). Thus, one might consider that tolerance and propagation of genetic lesions that initiate malignant transformation are dependent on epigenetic plasticity in the target cell (94). Nowhere is this model more supported than in the diverse array of pediatric solid tumors that, in comparison to adult malignancies, arise as a consequence of relatively few genetic mutations and progress to invasive, drug resistant and metastatic phenotypes despite the persistence of relatively stable genomes (14, 94). Although epigenetic therapies have not yet been extensively tested in children the essential contribution of epigenetic deregulation to pediatric solid tumors provides compelling rationale for their use. Continued elucidation of the contribution of epigenetic deregulation to the pathogenesis of these tumors will provide critical insights into the role of epigenomic instability as a driving force behind the malignant phenotype and will instruct the translation of this knowledge into effective epigenetically targeted therapies.
Acknowledgments
The authors acknowledge members of their respective labs for helpful discussion and data generation. We apologize to our respected colleagues whose work we were unable to cite due to space constraints. ER Lawlor is supported by a Stand Up to Cancer Innovative Research Grant, a program of the Entertainment Industry Foundation (AACR-SU2C-IRB-1309) and the Russell G. Adderley Endowment, Department of Pediatrics, University of Michigan.
Grant support: AACR-SU2C-IRG-1309, R01-CA134604 (ERL); Center for Cancer Research in the National Cancer Institute, the Intramural Research Program of the NIH (CJT).
Contributor Information
Elizabeth R. Lawlor, Email: elawlor@umich.edu.
Carol J. Thiele, Email: ct47a@nih.gov.
References
- 1.Maris JM, Denny CT. Focus on embryonal malignancies. Cancer Cell. 2002;2:447–50. doi: 10.1016/s1535-6108(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454:766–70. doi: 10.1038/nature07107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–19. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–95. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nat Rev Cancer. 2009;9:773–84. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- 8.Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008;20:201–7. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 10.Negishi M, Saraya A, Miyagi S, Nagao K, Inagaki Y, Nishikawa M, et al. Bmi1 cooperates with Dnmt1-associated protein 1 in gene silencing. Biochem Biophys Res Commun. 2007;353:992–8. doi: 10.1016/j.bbrc.2006.12.166. [DOI] [PubMed] [Google Scholar]
- 11.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove MS, Patel A. Mixed lineage leukemia: a structure-function perspective of the MLL1 protein. FEBS J. 2010;277:1832–42. doi: 10.1111/j.1742-4658.2010.07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loh ML, Mullighan CG. Advances in the genetics of high-risk childhood B-progenitor acute lymphoblastic leukemia and juvenile myelomonocytic leukemia—implications for therapy. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1936. [DOI] [PubMed] [Google Scholar]
- 14.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2010;331:435–9. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Ohm JE, Baylin SB. Stem cell chromatin patterns: an instructive mechanism for DNA hypermethylation? Cell Cycle. 2007;6:1040–3. doi: 10.4161/cc.6.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–92. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 18.Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18:316–28. doi: 10.1016/j.ccr.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–6. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 20.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–86. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43:768–75. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–72. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–3. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta R, Webb-Myers R, Flanagan S, Buckland ME. Isocitrate dehydrogenase mutations in diffuse gliomas: clinical and aetiological implications. J Clin Pathol. 2011;64:835–44. doi: 10.1136/jclinpath-2011-200227. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114:144–7. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–8. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 29.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–92. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 30.Kovar H. Downstream EWS/FLI1 - upstream Ewing's sarcoma. Genome Med. 2010;2:8. doi: 10.1186/gm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riggi N, Cironi L, Provero P, Suva ML, Kaloulis K, Garcia-Echeverria C, et al. Development of Ewing's sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res. 2005;65:11459–68. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 32.Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, et al. EWS-FLI-1 expression triggers a Ewing's sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res. 2008;68:2176–85. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 33.Riggi N, Suva ML, De Vito C, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–32. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Levetzow C, Jiang X, Gwye Y, von Levetzow G, Hung L, Cooper A, et al. Modeling initiation of ewing sarcoma in human neural crest cells. PLoS One. 2011;6:e19305. doi: 10.1371/journal.pone.0019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillero-Trejo Y, Eliazer S, Xiang L, Richardson JA, Ilaria RL., Jr Expression of the EWS/FLI-1 oncogene in murine primary bone-derived cells Results in EWS/FLI-1-dependent, ewing sarcoma-like tumors. Cancer Res. 2005;65:8698–705. doi: 10.1158/0008-5472.CAN-05-1704. [DOI] [PubMed] [Google Scholar]
- 36.Smith R, Owen LA, Trem DJ, Wong JS, Whangbo JS, Golub TR, et al. Expression profiling of EWS/FLI identifies NKX2. 2 as a critical target gene in Ewing's sarcoma. Cancer Cell. 2006;9:405–16. doi: 10.1016/j.ccr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Kinsey M, Smith R, Lessnick SL. NR0B1 is required for the oncogenic phenotype mediated by EWS/FLI in Ewing's sarcoma. Mol Cancer Res. 2006;4:851–9. doi: 10.1158/1541-7786.MCR-06-0090. [DOI] [PubMed] [Google Scholar]
- 38.Richter GH, Plehm S, Fasan A, Rossler S, Unland R, Bennani-Baiti IM, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A. 2009;106:5324–9. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2. 2 during oncogenic transformation in Ewing's sarcoma. PLoS ONE. 2008;3:e1965. doi: 10.1371/journal.pone.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kauer M, Ban J, Kofler R, Walker B, Davis S, Meltzer P, et al. A molecular function map of Ewing's sarcoma. PLoS One. 2009;4:e5415. doi: 10.1371/journal.pone.0005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdach S, Plehm S, Unland R, Dirksen U, Borkhardt A, Staege MS, et al. Epigenetic maintenance of stemness and malignancy in peripheral neuroectodermal tumors by EZH2. Cell Cycle. 2009;8:1991–6. doi: 10.4161/cc.8.13.8929. [DOI] [PubMed] [Google Scholar]
- 42.Douglas D, Hsu JH, Hung L, Cooper A, Abdueva D, van Doorninck J, et al. BMI-1 promotes Ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res. 2008;68:6507–15. doi: 10.1158/0008-5472.CAN-07-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu JH, Lawlor ER. BMI-1 suppresses contact inhibition and stabilizes YAP in Ewing sarcoma. Oncogene. 2011;30:2077–85. doi: 10.1038/onc.2010.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiel U, Pirson S, Muller-Spahn C, Conrad H, Busch DH, Bernhard H, et al. Specific recognition and inhibition of Ewing tumour growth by antigen-specific allo-restricted cytotoxic T cells. Br J Cancer. 2011;104:948–56. doi: 10.1038/bjc.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DW, Barrett D, Mackall C, Orentas R, Grupp SA. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–99. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ban J, Jug G, Mestdagh P, Schwentner R, Kauer M, Aryee DN, et al. Hsa-mir-145 is the top EWS-FLI1-repressed microRNA involved in a positive feedback loop in Ewing's sarcoma. Oncogene. 2011;30:2173–80. doi: 10.1038/onc.2010.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajaj SV, Wai DH, Buckley JD, Kapranov P, Lawlor ER, Triche TJ. A large non-coding RNA that is characteristic of Ewing sarcoma family of tumors. Annual Meeting of the American Association for Cancer Research; 2011; Orlando, Fl. 2011. [Google Scholar]
- 49.Jaboin J, Wild J, Hamidi H, Khanna C, Kim CJ, Robey R, et al. MS-27–275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002;62:6108–15. [PubMed] [Google Scholar]
- 50.Hurtubise A, Bernstein ML, Momparler RL. Preclinical evaluation of the antineoplastic action of 5-aza-2'-deoxycytidine and different histone deacetylase inhibitors on human Ewing's sarcoma cells. Cancer Cell Int. 2008;8:16. doi: 10.1186/1475-2867-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raabe EH, Laudenslager M, Winter C, Wasserman N, Cole K, LaQuaglia M, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27:469–76. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- 52.Shimada H, Chatten J, Newton WA, Jr, Sachs N, Hamoudi AB, Chiba T, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–16. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 53.Cooper MJ, Hutchins GM, Cohen PS, Helman LJ, Mennie RJ, Israel MA. Human neuroblastoma tumor cell lines correspond to the arrested differentiation of chromaffin adrenal medullary neuroblasts. Cell Growth Differ. 1990;1:149–59. [PubMed] [Google Scholar]
- 54.Gaetano C, Matsumoto K, Thiele CJ. In vitro activation of distinct molecular and cellular phenotypes after induction of differentiation in a human neuroblastoma cell line. Cancer Res. 1992;52:4402–7. [PubMed] [Google Scholar]
- 55.Krasnoselsky AL, Whiteford CC, Wei JS, Bilke S, Westermann F, Chen QR, et al. Altered expression of cell cycle genes distinguishes aggressive neuroblastoma. Oncogene. 2005;24:1533–41. doi: 10.1038/sj.onc.1208341. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Diskin S, Rappaport E, Attiyeh E, Mosse Y, Shue D, et al. Integrative genomics identifies distinct molecular classes of neuroblastoma and shows that multiple genes are targeted by regional alterations in DNA copy number. Cancer Res. 2006;66:6050–62. doi: 10.1158/0008-5472.CAN-05-4618. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Liu Z, Woo CW, Li Z, Wang L, Wei JS, et al. EZH2 Mediates Epigenetic Silencing of Neuroblastoma Suppressor Genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72:315–24. doi: 10.1158/0008-5472.CAN-11-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stallings RL, Howard J, Dunlop A, Mullarkey M, McDermott M, Breatnach F, et al. Are gains of chromosomal regions 7q and 11p important abnormalities in neuroblastoma? Cancer Genet Cytogenet. 2003;140:133–7. doi: 10.1016/s0165-4608(02)00681-7. [DOI] [PubMed] [Google Scholar]
- 59.Ochiai H, Takenobu H, Nakagawa A, Yamaguchi Y, Kimura M, Ohira M, et al. Bmi1 is a MYCN target gene that regulates tumorigenesis through repression of KIF1Bbeta and TSLC1 in neuroblastoma. Oncogene. 2010;29:2681–90. doi: 10.1038/onc.2010.22. [DOI] [PubMed] [Google Scholar]
- 60.Cui H, Ma J, Ding J, Li T, Alam G, Ding HF. Bmi-1 regulates the differentiation and clonogenic self-renewal of I-type neuroblastoma cells in a concentration-dependent manner. J Biol Chem. 2006;281:34696–704. doi: 10.1074/jbc.M604009200. [DOI] [PubMed] [Google Scholar]
- 61.Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res. 2009;69:2065–71. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 62.Caren H, Djos A, Nethander M, Sjoberg RM, Kogner P, Enstrom C, et al. Identification of epigenetically regulated genes that predict patient outcome in neuroblastoma. BMC Cancer. 2011;11:66. doi: 10.1186/1471-2407-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujita T, Igarashi J, Okawa ER, Gotoh T, Manne J, Kolla V, et al. CHD5, a tumor suppressor gene deleted from 1p36. 31 in neuroblastomas. J Natl Cancer Inst. 2008;100:940–9. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alaminos M, Davalos V, Cheung NK, Gerald WL, Esteller M. Clustering of gene hypermethylation associated with clinical risk groups in neuroblastoma. J Natl Cancer Inst. 2004;96:1208–19. doi: 10.1093/jnci/djh224. [DOI] [PubMed] [Google Scholar]
- 65.Yang Q, Kiernan CM, Tian Y, Salwen HR, Chlenski A, Brumback BA, et al. Methylation of CASP8, DCR2, and HIN-1 in neuroblastoma is associated with poor outcome. Clin Cancer Res. 2007;13:3191–7. doi: 10.1158/1078-0432.CCR-06-2846. [DOI] [PubMed] [Google Scholar]
- 66.Hoebeeck J, Michels E, Pattyn F, Combaret V, Vermeulen J, Yigit N, et al. Aberrant methylation of candidate tumor suppressor genes in neuroblastoma. Cancer Lett. 2009;273:336–46. doi: 10.1016/j.canlet.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 67.Grau E, Martinez F, Orellana C, Canete A, Yanez Y, Oltra S, et al. Hypermethylation of apoptotic genes as independent prognostic factor in neuroblastoma disease. Mol Carcinog. 2011;50:153–62. doi: 10.1002/mc.20700. [DOI] [PubMed] [Google Scholar]
- 68.Buckley PG, Das S, Bryan K, Watters KM, Alcock L, Koster J, et al. Genome-wide DNA methylation analysis of neuroblastic tumors reveals clinically relevant epigenetic events and large-scale epigenomic alterations localized to telomeric regions. Int J Cancer. 2011;128:2296–305. doi: 10.1002/ijc.25584. [DOI] [PubMed] [Google Scholar]
- 69.Cotterman R, Jin VX, Krig SR, Lemen JM, Wey A, Farnham PJ, et al. N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor. Cancer Res. 2008;68:9654–62. doi: 10.1158/0008-5472.CAN-08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–35. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 71.Murphy DM, Buckley PG, Bryan K, Das S, Alcock L, Foley NH, et al. Global MYCN transcription factor binding analysis in neuroblastoma reveals association with distinct E-box motifs and regions of DNA hypermethylation. PLoS One. 2009;4:e8154. doi: 10.1371/journal.pone.0008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Angrisano T, Sacchetti S, Natale F, Cerrato A, Pero R, Keller S, et al. Chromatin and DNA methylation dynamics during retinoic acid-induced RET gene transcriptional activation in neuroblastoma cells. Nucleic Acids Res. 2011;39:1993–2006. doi: 10.1093/nar/gkq864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coffey DC, Kutko MC, Glick RD, Butler LM, Heller G, Rifkind RA, et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 2001;61:3591–4. [PubMed] [Google Scholar]
- 74.De los Santos M, Zambrano A, Aranda A. Combined effects of retinoic acid and histone deacetylase inhibitors on human neuroblastoma SH-SY5Y cells. Mol Cancer Ther. 2007;6:1425–32. doi: 10.1158/1535-7163.MCT-06-0623. [DOI] [PubMed] [Google Scholar]
- 75.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Bont JM, Packer RJ, Michiels EM, den Boer ML, Pieters R. Biological background of pediatric medulloblastoma and ependymoma: a review from a translational research perspective. Neuro Oncol. 2008;10:1040–60. doi: 10.1215/15228517-2008-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol Cancer. 2010;9:265. doi: 10.1186/1476-4598-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zakrzewska M, Zakrzewski K, Gresner SM, Piaskowski S, Zalewska-Szewczyk B, Liberski PP. Polycomb genes expression as a predictor of poor clinical outcome in children with medulloblastoma. Childs Nerv Syst. 2011;27:79–86. doi: 10.1007/s00381-010-1260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–22. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fukuzawa R, Reeve AE. Molecular pathology and epidemiology of nephrogenic rests and Wilms tumors. J Pediatr Hematol Oncol. 2007;29:589–94. doi: 10.1097/01.mph.0000212981.67114.ec. [DOI] [PubMed] [Google Scholar]
- 84.Aiden AP, Rivera MN, Rheinbay E, Ku M, Coffman EJ, Truong TT, et al. Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network. Cell Stem Cell. 2010;6:591–602. doi: 10.1016/j.stem.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McEvoy J, Flores-Otero J, Zhang J, Nemeth K, Brennan R, Bradley C, et al. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell. 2011;20:260–75. doi: 10.1016/j.ccr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–34. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.George RE, Lahti JM, Adamson PC, Zhu K, Finkelstein D, Ingle AM, et al. Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children's Oncology Group study. Pediatr Blood Cancer. 2010;55:629–38. doi: 10.1002/pbc.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinto N, Cohn SL, Dolan ME. Using germline genomics to individualize pediatric cancer treatments. Clin Cancer Res. 2012;18:xx–xx. doi: 10.1158/1078-0432.CCR-11-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piekarz RL, Bates SE. Epigenetic modifiers: basic understanding and clinical development. Clin Cancer Res. 2009;15:3918–26. doi: 10.1158/1078-0432.CCR-08-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mossman D, Scott RJ. Long term transcriptional reactivation of epigenetically silenced genes in colorectal cancer cells requires DNA hypomethylation and histone acetylation. PLoS One. 2011;6:e23127. doi: 10.1371/journal.pone.0023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Juergens RA, Wrangle J, Vendetti FP, Murphy SC, Zhao M, Coleman B, et al. Combination Epigenetic Therapy Has Efficacy in Patients with Refractory Advanced Non-Small Cell Lung Cancer. Cancer Discovery. 2011;1:598–607. doi: 10.1158/2159-8290.CD-11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, et al. Transient Low Doses of DNA Demethylating Agents Exert Durable Anti-tumor Effects on Hematological and Epithelial Tumor Cells. Cancer Cell. 2012 doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKenna ES, Roberts CW. Epigenetics and cancer without genomic instability. Cell Cycle. 2009;8:23–6. doi: 10.4161/cc.8.1.7290. [DOI] [PubMed] [Google Scholar]
- 95.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–43. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fouladi M, Park JR, Stewart CF, Gilbertson RJ, Schaiquevich P, Sun J, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J Clin Oncol. 2010;28:3623–9. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fouladi M, Furman WL, Chin T, Freeman BB, 3rd, Dudkin L, Stewart CF, et al. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children's Oncology Group report. J Clin Oncol. 2006;24:3678–85. doi: 10.1200/JCO.2006.06.4964. [DOI] [PubMed] [Google Scholar]
- 98.Su JM, Li XN, Thompson P, Ou CN, Ingle AM, Russell H, et al. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a children's oncology group report. Clin Cancer Res. 2011;17:589–97. doi: 10.1158/1078-0432.CCR-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balasubramanian S, Ramos J, Luo W, Sirisawad M, Verner E, Buggy JJ. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia. 2008;22:1026–34. doi: 10.1038/leu.2008.9. [DOI] [PubMed] [Google Scholar]
- 100.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–17. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Copeland R. Protein methyltransferases as a target class for cancer drug discovery. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 April 2–6; Orlando, FLA: AACR, Philadelphia. 2011. [Google Scholar]
- 102.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–63. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8:1579–88. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, et al. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci U S A. 2007;104:8023–8. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]