Abstract
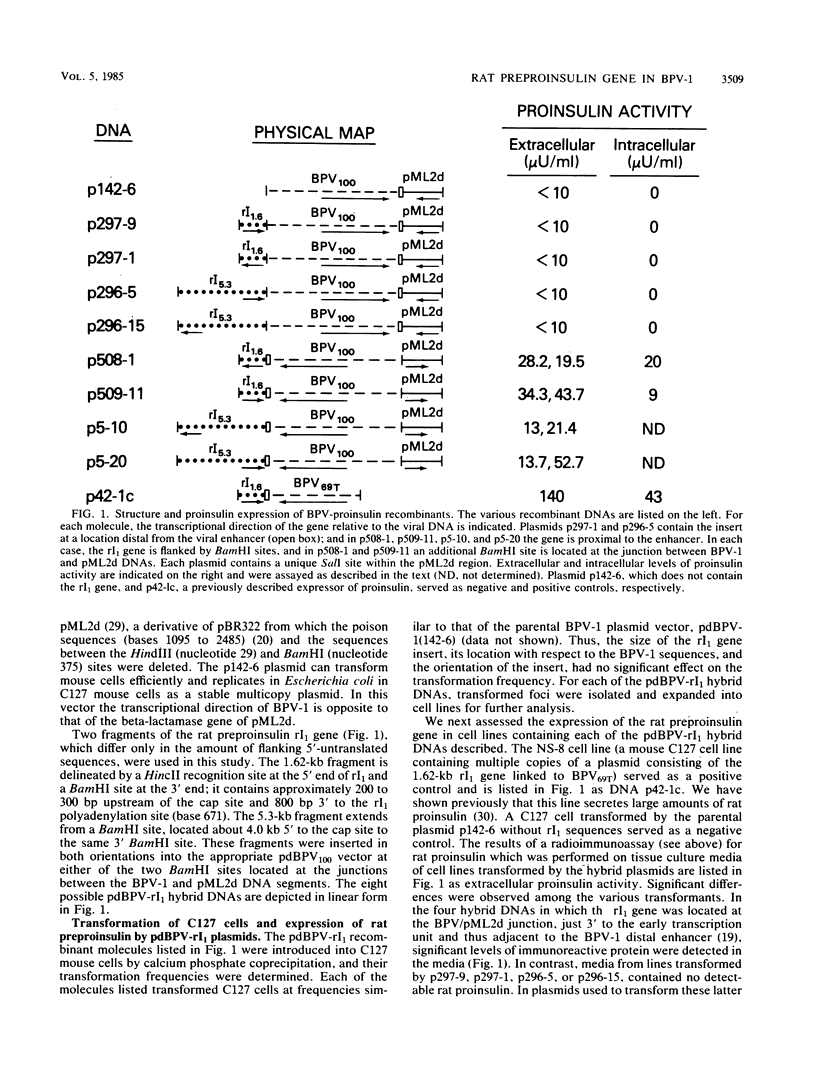
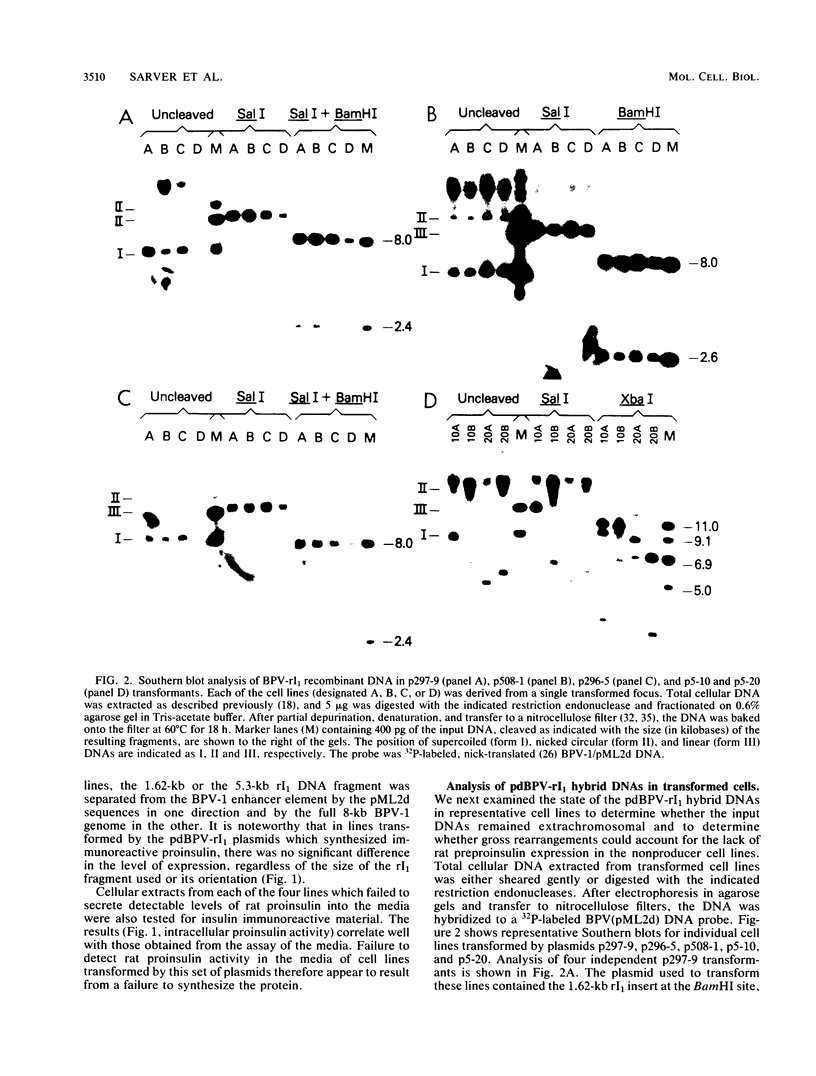
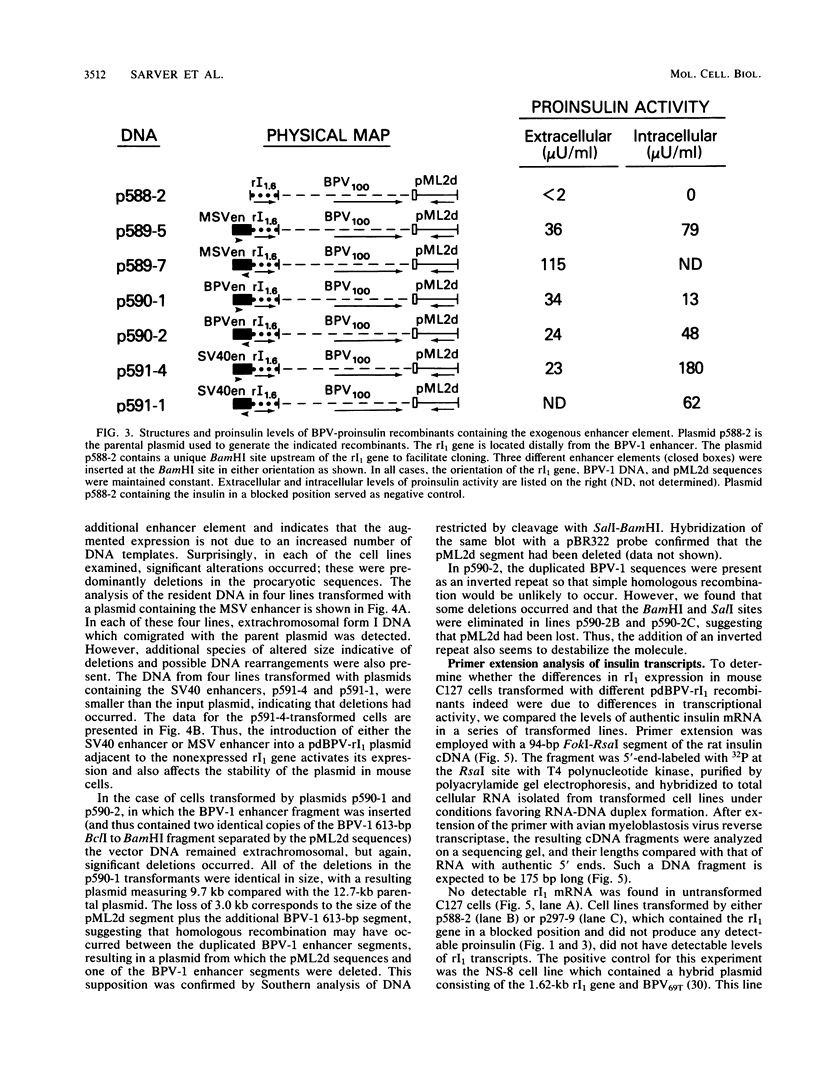
The effect of position in a bovine papillomavirus type 1 (BPV-1) vector on foreign gene expression was assessed with the rat preproinsulin (rI1) gene. The rI1 gene was inserted at each of the BPV-1/pML2d junctions in either transcriptional orientation in derivatives of the pdBPV-1(142-6) vector which consists of the BamHI linear genome of BPV-1 DNA cloned into pML2d. Transformed lines of C127 cells were established and assayed for rI1 gene expression. Cells containing the rI1 gene at the 3' end of the BPV-1 transforming region expressed rat proinsulin, whereas cells with the gene at the 5' end of the nontransforming region did not. Variability in the plasmid copy number or in the extent of DNA rearrangement could not account for this difference. We conclude that the expression of the rat preproinsulin gene (which is normally tissue specific for pancreatic islet cells) in C127 cells depends on the transcriptional activation afforded by viral enhancer sequences located at the 3' end of the transforming region. Intervening BPV-1 or pML2d sequences appear to block this enhancer-mediated gene activation. In agreement with enhancer-dependent activation, a rat preproinsulin gene located in a blocked position (i.e., not adjacent to the BPV-1 enhancer) could be activated by the insertion of a DNA fragment containing the simian virus 40, Moloney murine sarcoma virus, or BPV-1 enhancer element adjacent to the rI1 gene. Thus, a gene which is normally not expressed in a particular cell may be activated when placed adjacent to a viral enhancer in a BPV-1 vector.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Cold-sensitive regulatory mutants of simian virus 40. J Mol Biol. 1980 Jun 15;140(1):129–142. doi: 10.1016/0022-2836(80)90359-9. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Episkopou V., Murphy A. J., Efstratiadis A. Cell-specified expression of a selectable hybrid gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4657–4661. doi: 10.1073/pnas.81.15.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Karin M., Cathala G., Nguyen-Huu M. C. Expression and regulation of a human metallothionein gene carried on an autonomously replicating shuttle vector. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4040–4044. doi: 10.1073/pnas.80.13.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Levinson B. B., Goodman H. M. A plasmid that replicates in both mouse and E. coli cells. J Mol Appl Genet. 1982;1(6):527–538. [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Berg L., Weiher H., Botchan M. Bovine papilloma virus contains an activator of gene expression at the distal end of the early transcription unit. Mol Cell Biol. 1983 Jun;3(6):1108–1122. doi: 10.1128/mcb.3.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Mitrani-Rosenbaum S., Maroteaux L., Mory Y., Revel M., Howley P. M. Inducible expression of the human interferon beta 1 gene linked to a bovine papilloma virus DNA vector and maintained extrachromosomally in mouse cells. Mol Cell Biol. 1983 Feb;3(2):233–240. doi: 10.1128/mcb.3.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M. C., Richard-Foy H., Wolford R. G., Berard D. S., Hager G. L. Glucocorticoid regulation of transcription at an amplified, episomal promoter. Mol Cell Biol. 1983 Nov;3(11):2045–2057. doi: 10.1128/mcb.3.11.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlakis G. N., Hamer D. H. Regulation of a metallothionein-growth hormone hybrid gene in bovine papilloma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(2):397–401. doi: 10.1073/pnas.80.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. Rapid assay for extrachromosomal homologous recombination in monkey cells. Mol Cell Biol. 1985 Mar;5(3):529–537. doi: 10.1128/mcb.5.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. The minimum amount of homology required for homologous recombination in mammalian cells. Mol Cell Biol. 1984 Nov;4(11):2253–2258. doi: 10.1128/mcb.4.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Augereau P., Chambon P. The SV40 72 bp repeat preferentially potentiates transcription starting from proximal natural or substitute promoter elements. Cell. 1983 Feb;32(2):503–514. doi: 10.1016/0092-8674(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Zinn K., Mellon P., Ptashne M., Maniatis T. Regulated expression of an extrachromosomal human beta-interferon gene in mouse cells. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4897–4901. doi: 10.1073/pnas.79.16.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]