To the Editor:
Prior studies have shown that 25% to 50% of clinical trials are never published.1–4 However, among those published, we know little about the length of time required for publication in the peer-reviewed biomedical literature after study completion. Ioannidis previously demonstrated that a sample of randomized phase 2 and 3 trials conducted between 1986 and 1996 required nearly 2.5 years for publication,5 while our more recent study of National Institutes of Health (NIH)-funded trials found that average time to publication was almost two years.4 We sought to determine time to publication for a recent and representative sample of trials published in 2009.
METHODS
We examined clinical trials published during the 2009 calendar year that were indexed via MEDLINE and linked to a ClinicalTrials.gov identifier (NCT Number).6 We excluded articles that were not the first publication reporting on the clinical trial by reviewing article text for citations to and searching MEDLINE for earlier trial publications. We imputed completion dates based on the subject enrollment dates and the longest period of follow-up required for any outcome measure reported in the article. For trials without enrollment dates, we used the completion date provided on ClinicalTrials.gov; this date was not used uniformly because our prior work demonstrated its unreliability.3 We determined publication dates using MEDLINE, using on-line publication dates for articles made available ahead of print. Because ClinicalTrials.gov reports completion dates in month/year format, publication dates were similarly recorded for purposes of calculating time to publication.
We obtained additional information through the National Library of Medicine, using the ClinicalTrials.gov identifier, including data elements reporting lead sponsor, study design, and condition studied (Online Table). We obtained journal impact factors from Web of Knowledge (Thomson Reuters, New York, NY). We used Kruskal-Wallis tests to compare median times to publication between trial characteristic categories using a type I error rate of 0.003 to account for multiple comparisons (n=16). Statistical analysis was performed using JMP 7.0.1 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 1336 clinical trials published during the 2009 calendar year, indexed via MEDLINE and linked to a ClinicalTrials.gov identifier. The majority was funded by non-profit organizations and enrolled more than 100 subjects (Online Table). Nearly half were published in journals with an impact factor less than five.
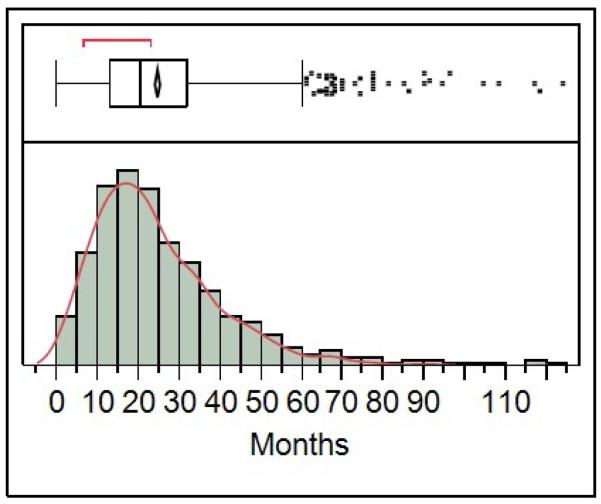
Median time to publication was 21 months with an interquartile range of 13–32 months (Figure) and modest differences across types of trials (Online Table). For instance, median time to publication was longer among trials funded by industry when compared with trials funded by government and non-profit organizations (24 versus 20 months; p<0.001), but was shorter among trials enrolling 1000 subjects or more when compared with trials enrolling 100 subjects or fewer and trials enrolling between 100 and 1000 subjects (18, 20 and 23 months, respectively; p<0.001) and was shorter among trials published in journals with an impact factor greater than 10 when compared with trials published in journals with an impact factor less than 10 (17 versus 23 months; p<0.001).
Figure.
Time to publication after completion among clinical trials registered in ClinicalTrials.gov and published in the biomedical literature (cited in MEDLINE).
DISCUSSION
We found, on average, nearly two years passed between completion and publication of clinical trials, across all trial funders. Moreover, given our study was necessarily limited to examining time to publication among completed trials that were eventually published, this estimate is conservative. First, we studied trials registered in and linked to ClinicalTrials.gov, a select group of studies. Because of policies in place as of 2005, many may have been registered to ensure compliance with International Committee of Medical Journal Editors requirements for publication7 and thus more likely to publish in a timely manner. Second, we only studied trials that were published (and indexed via MEDLINE and linked to ClinicalTrials.gov identifiers). Between 50% and 70% of studies registered in ClinicalTrials.gov are eventually published many years after trial completion;3,4 30% to 50% are never published.
Differences across trial types were generally modest and indicate that timely dissemination of research needs to be uniformly prioritized to enhance science. Many steps must be successfully taken between trial completion and publication, including data management, statistical analysis, and writing, along with processes out of investigators' control, such as peer review. The substantial variation in publication time, from months to years, suggests that there may be opportunities to improve the speed and efficiency with which investigators publish completed studies.
We cannot rule out that study findings may have been disseminated through means other than publication, including scientific meeting presentations. However, with the exception of public results reporting, these alternative dissemination strategies lead to limited public awareness of the research. Given the time required to publish results from these clinical trials, our findings support current federal initiatives requiring results reporting of clinical studies within 12 months of trial completion8 to ensure the timely dissemination of clinical science.
Supplementary Material
Acknowledgment
Data access and responsibility: All authors had full access to all the data in the study and Dr. Ross takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/support and role of the sponsor: The analyses on which this publication is based were performed under Contract No. HHSN276201000788P, entitled “Evaluating Time-to-Publication Following Completion of Clinical Trials and Related Factors,” funded by the National Library of Medicine, Department of Health and Human Services. The ideas and opinions expressed are the authors'. The content of this publication does not necessarily reflect the views or policies of the U.S. National Institutes of Health, Public Health Service, or Department of Health and Human Services. The authors assume full responsibility for the accuracy and completeness of the ideas presented. Drs. Ross and Krumholz receive support from Medtronic, Inc. and from the Centers of Medicare and Medicaid Services (CMS) to develop and maintain performance measures that are used for public reporting. Dr. Ross is supported by the National Institute on Aging (K08 AG032886) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. Drs. Zarin and Tse are supported by the Intramural Research Program of the National Institutes of Health, National Library of Medicine. Dr. Krumholz is supported by a National Heart Lung Blood Institute Cardiovascular Outcomes Center Award (1U01HL105270-02).
Footnotes
Conflicts of interest: Dr. Ross reports that he is a member of a scientific advisory board for FAIR Health, Inc. Dr. Krumholz reports that he chairs a scientific advisory board for UnitedHealthcare. Drs. Zarin and Tse report that they are Director and Program Analyst, respectively, for ClinicalTrials.gov.
Author contributions: Drs. Ross, Tse, Zarin, and Krumholz were responsible for the conception and design of this work. Drs. Ross, Lampropulos, Mocanu, and Tse were responsible for acquisition of data. Dr. Ross drafted the manuscript, conducted the statistical analysis, and provided supervision. Dr. Krumholz obtained funding. All authors participated in the analysis and interpretation of the data and critically revised the manuscript for important intellectual content.
REFERENCES
- 1.Dwan K, Altman DG, Arnaiz JA, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE. 2008;3(8):e3081. doi: 10.1371/journal.pone.0003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee K, Bacchetti P, Sim I. Publication of Clinical Trials Supporting Successful New Drug Applications: A Literature Analysis. PLoS Med. 2008 Sep 23;5(9):e191. doi: 10.1371/journal.pmed.0050191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009 Sep;6(9):e1000144. doi: 10.1371/journal.pmed.1000144. ClinicalTrials.Gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. doi: 10.1136/bmj.d7292. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998 Jan 28;279(4):281–286. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 6.U.S. National Institutes of Health [Accessed February 5, 2012]; ClinicalTrials.gov. 2009; Available at: http://www.clinicaltrials.gov/.
- 7.DeAngelis CD, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004 Sep 15;292(11):1363–1364. doi: 10.1001/jama.292.11.1363. [DOI] [PubMed] [Google Scholar]
- 8.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. N Engl J Med. 2011 Mar 3;364(9):852–860. doi: 10.1056/NEJMsa1012065. ClinicalTrials.gov [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.