Abstract
There is increasing interest in the development of reporter agents to reveal enzyme activity in vivo using small animal imaging. We have previously demonstrated the feasibility of detecting lacZ gene activity using the commercially available 3,4-cyclohexenoesculetin-β-D-galactopyranoside (S-Gal™) as a 1H MRI reporter. Specifically, β-galactosidase (β-gal) releases the aglycone, which forms an MR contrast-inducing paramagnetic precipitate in the presence of Fe3+. Contrast was primarily T2-weighted signal loss, but T1 effects were also observed. Since T1-contrast generally provides signal enhancement as opposed to loss, it appeared attractive to explore whether analogues could be generated with enhanced characteristics. We now report the design and successful synthesis of novel analogues together with characterization of 1H MRI contrast based on both T1 and T2 response to β-gal activity in vitro for the lead agent.
Keywords: NMR, enzyme, hydrolases, reporter molecules, relaxivity
Introduction
Given the importance of reporter genes in various applications ranging from molecular biology to clinical trials, the development of non-invasive techniques to assay gene expression in vivo is becoming increasingly significant[1,2]. Traditionally, the lacZ gene encoding β-galactosidase (β-gal) was the most popular reporter including assays of clonal insertion, transcriptional activation, protein expression, and protein interaction[3,4]. The broad specificity of β-gal activity allows diverse molecular structures for substrates and successful detection techniques included colorimetric[5,6], fluorescence[7-9], bioluminescence[10], chemiluminescence[11-13], as well as radiotracers for positron emission tomography (PET)[14] or single-photon emission computed tomography (SPECT)[15] and probes for 1H magnetic resonance imaging (MRI)[16-20] and 19F-NMR approaches[21-26]. Many approaches have been demonstrated for in vitro detection, but few have been applied in vivo to date[8,13,17,18] and these often required direct injection into the tissue of interest[24,25,27]. While we focus on the detection of transgene activity in stably transfected human tumor cells, it is important to note that expression may also arise in normal tissues following exposure to stress such as radiation or doxorubicin induced senescence activated β-galactosidase[28, 29]. Moreover, epithelial exposure of lactase (the human analog of β-galactosidase) has been associated with metaplasia in developing esophageal cancer[12].
The pioneering study of Moats et al. demonstrated T1-weighted MRI contrast based on β-gal activated unmasking of a gadolinium ligand[16] and this was later applied to tracing the developing cell lineages in frog embryos following direct intra cellular injection of substrate[17]. We recently demonstrated the feasibility of detecting β-gal activity in vitro in cultured cancer cells and in vivo in mice with human breast tumor MCF7 xenografts using 3,4-cyclohexenoesculetin-β-D-galactopyranoside (S-Gal™). Specifically, β-gal cleaves S-Gal™ to release the cyclohexenoesculetin aglycone, which forms a paramagnetic precipitate in the presence of Fe3+ generating T2*-weighted 1H MRI contrast [20]. This approach was also used to detect genetically engineered β-gal expressing bone marrow cells by MRI in vivo following labeling in vitro [30]. Both studies exploited T2*-weighted signal loss to identify β-gal activity. We had noticed that there was additionally T1 relaxivity, but the high T2 relaxivity (up to 100 s-1 for 15 mM S-Gal™) tended to mask T1-effects. These results prompted us to examine whether molecular modifications could provide T1-activity without the high T2 relaxivity.
β-galactosidase catalyses the hydrolysis of β-D-galactopyranosides by cleavage of the C-O bond between D-galactose and the aglycone. MRI detection of β-gal based on S-Gal™ depends on contrast produced by the formation of a complex between the 3,4-cyclohexenoesculetin aglycone and Fe3+ ions[20,31]. Schwert,[32] Davies,[33] and Raymond et al.[34] have described the design and evaluation of series of siderophores that contain catechol binding groups (catecholate ligands) to coordinate Fe3+. Thus, we considered analogous dihydroxy coumarin-based catecholate aglycones. Coumarins are reported to have numerous therapeutic applications including antibacterial, anti-inflammatory and anti-coagulant as well as photochemotherapy and anti-HIV therapy [35]. Therefore, structure-activity relationships and synthetic procedures have been widely examined. Inspired by these studies, we designed 4 analogs based on the structure of 3,4-cyclohexenoesculetin (1, aglycone of S-Gal™): 7,8-dihydroxy-3,4-cyclohexenocoumarin (2), 6,7-dihydroxy-4-methyl-coumarin (3), 7,8-dihydroxy-4-methylcoumarin (4), and 7,8-dihydroxy-6-methoxycoumarin (5) (Figure 1).
Figure 1. Comparison of MRI Contrast for aglycone ligands 1-5 with Fe3+.
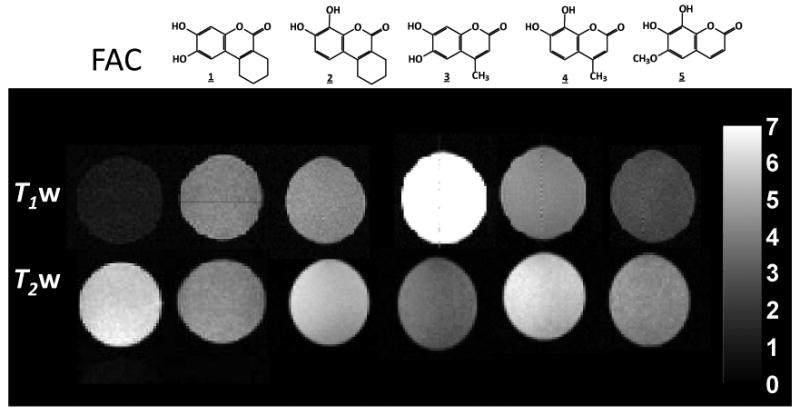
200 MHz 1H MRI of vials of ferric ammonium citrate (FAC) (3.0 mM) in PBS/DMSO (V/V′ 1:1) alone (leftmost) or mixed with ligands shown above (1-5; 9.0 mM). Upper row of images: T1-weighted 1H MRI with TR = 300 ms, TE = 20 ms, 1.5 mm slice with, 128 × 128 resolution over 50 × 50 mm2. Lower row Corresponding T2-weighted 1H MRI with TR = 2000 ms, TE= 80 ms.
We now report the design, synthesis, and evaluation of these novel analogs of S-Gal™, and in vitro assessment of their hydrolytic kinetics. MRI contrast with respect to lacZ-transfected human MCF7 breast and PC3 prostate cancer cells is presented for the most promising agent.
Results and Discussion
Aglycone synthesis
noting the variety of strategies for synthesizing coumarins[32], we chose the Pechmann reaction, coupling the two components (phenol and β-ketoester) with ZrCl4 (10 mol%) as catalyst[36]. We started the synthesis by subjecting pyrogallol or 1,2,4-benzenetriol to the Pechmann reaction with ethyl cyclohexanone-2-carboxylate or ethyl acetoacetate for coumarins 1∼4, while 6-methoxy-7,8-dihydroxycoumarin 5, was purchased commercially. The reactions were performed at 80 °C in toluene, to give 1∼4 in high yields (92-95%) within 30 minutes. After confirming the structure of coumarins 1∼4, we evaluated the T1- and T2-weighted MR image contrast of their Fe3+-complexes. Each showed substantial T1-weighted contrast (Figure 1), suggesting potential as Fe-based 1H MRI lacZ gene reporters. Greatest T1 response was observed for 6,7-dihydroxy-4-methylcoumarin(3)/Fe3+, which also showed the greatest T2-weighted MRI contrast.
Mono β-D-galactopyranosides
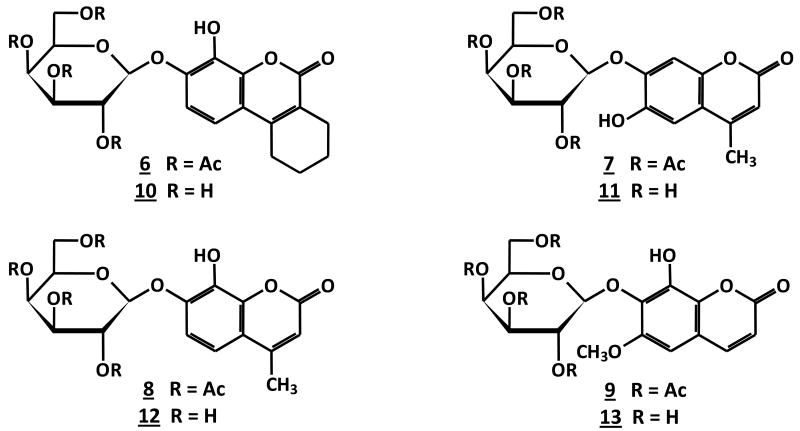
To generate β-gal reporters a β-D-galactopyranosyl group was added to the coumarins forming β-D-galactopyranosides (Figure 2). Each coumarin 2∼5 has two hydroxyl groups located at the 6,7- or 7,8-positions, which were expected to show differences in reactivity and hence an opportunity for regioselective synthesis. Indeed, straightforward regioselective mono-glycopyranosylation [37] and etherification [38] have been reported at 7-hydroxyl group of 3,4-cyclohexenoesculetin 1. 13C and 1H NMR chemical shifts of coumarin derivatives (δC-7 > δC-5 > δC-6 > δC-8)[39] also suggested that the relative electron deficiency of C-7, C-6, and C-8 would result in relative reactivity: 7-hydroxyl > 6-hydroxyl > 8-hydroxyl. Hydroxyl pKa values in coumarins 1∼5 corresponding to their positions (Table 1) suggested that phase-transfer-catalysis at pH = 8∼9 could provide regio- and stereoselective synthesis of β-D-galactopyranosides, as we also exploited previously for 19F-NMR β-gal reporters [40, 41]. Reaction of 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide with equimolar coumarin (2∼5) at room temperature catalyzed by tetrabutylammonium bromide (TBAB) in a dichloromethane-aqueous biphasic system (pH 8∼9) under N2 afforded 7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-3,4-cyclohexenocoumarin (6), 7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-6-hydroxy-4-methylcoumarin (7), 7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-4-methylcoumarin (8) and 7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-6-methoxycoumarin (9) in moderate yields (72∼88%) (Figure 2). Nuclear Overhauser enhancements (NOE) showed that the mono β-D-galactopyranosylations occurred at the O-7 positions, as predicted. Subsequent deacetylation with NH3/MeOH from 0°C to room temperature gave the free mono galactopyranosides 10∼13 (Figure 3) in nearly quantitative yields. The anomeric β-D-configuration of compounds 10∼13 in the 4C1 chair conformation was confirmed by the 1H-NMR chemical shifts (δh 4.75∼5.03 ppm) of the anomeric protons and the J1,2 (J ∼8 Hz), and J2,3 (J ∼10 Hz) coupling constants. The anomeric carbon resonances appeared at δC-1′ 100.85∼105.53 ppm in accordance with the β-D-configuration[40,42].
Figure 2. General reaction scheme.
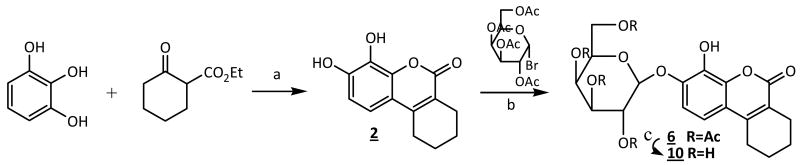
(a) pyrogallol (5 mmol), ethyl cyclohexanone-2-carboxylate (5 mmol), ZrCl4 (0.5 mmol), toluene (20 mL), 80 °C, N2, 20 min, 93%(→2); (b) 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide (2.5 mmol), 2 (2.5 mmol), TBAB (0.5 mmol), CH2Cl2-H2O (60 mL), pH 8∼9, rt °C, N2, 3∼4 hr, 88%(→6); (c) 0.5M NH3-MeOH, 0°C→r.t., 24 hr, quantitative yields.
Table 1. The hydroxyl pKa values of coumarins 1-5.
| Coumarins | pKa(OH-7) | pKa(OH-6) | pKa(OH-8) |
|---|---|---|---|
| 3,4-cyclohexenoesculetin 1 | 11.84 | 8.74 | --- |
| 7,8-dihydroxy-3,4-cyclohexenocoumarin 2 | 11.48 | --- | 7.95 |
| 6,7-dihydroxy-4-methylcoumarin 3 * | 10.28 | 8.52 | --- |
| 7,8-dihydroxy-4-methylcoumarin 4 * | 10.35 | --- | 8.00 |
| 7,8-dihydroxy-6-methoxycoumarin 5 | 10.49 | --- | 7.22 |
Values from [36], while others were calculated using Advanced Chemistry Development Software (www.acdlabs.com).
Figure 3. Mono β-D-galactopyranosides 6-13.
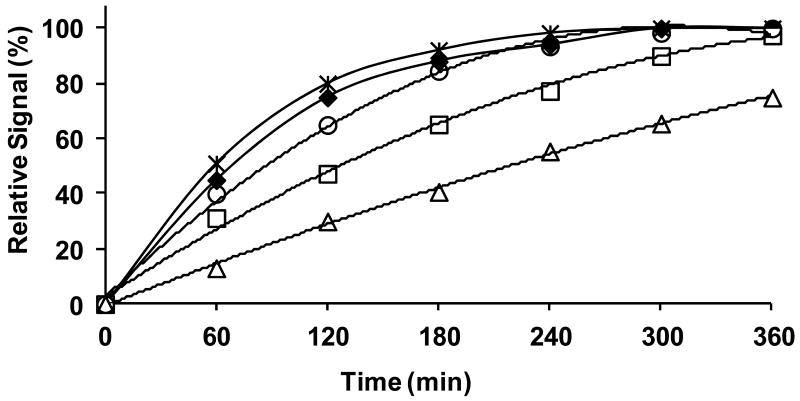
The coumarins 1∼5 are strongly fluorescent (365/440 nm) in PBS (0.1M, pH=7.4), however, their β-D-galactopyranosides, S-Gal™ and 10∼13, are weakly or non-fluorescent. The measurement of fluorescence intensity increased following reaction of the coumarin with β-gal(E801A) in PBS (0.1M, pH=7.4) at 20∼22°C showing that all the β-D-galactopyranosides were effective substrates though with varying hydrolytic rates in the order: v1 > v11 > v13 > v10 > v12 (Figure 4). Given that 11 and 13 were considerably better substrates these were favored for further evaluation. In addition the MRI contrast generated by the aglycones 1∼5 in the presence of Fe3+ (Figure 1) indicated that both 11 and 13 would show considerable T1 contrast upon hydrolysis by β-gal and since 13 showed much less T2 sensitivity it was chosen for further evaluation.
Figure 4. The kinetic hydrolysis time courses of mono β-D-galactopyranosides.
MRI in solution
T1 and T2 maps were measured for vials containing various combinations of mono β-D-galactopyranoside 13 and Fe3+ ions, with or without 5 units of β-gal (E801A) (Figure 5). Ferric ions alone enhanced R1 relaxation, but the presence of 13 made no difference. Addition of β-gal to the mixture of 13 + Fe3+ generated much more rapid relaxation, which depended on the ratio of the two components: specifically ΔR1 5.9 s-1 (3:1), 5.1 s-1 (2:1), and 2.3 s-1 (1:1), respectively (Figure 5a). T2-weighted MR contrast showed a very similar effect though Fe3+ alone caused minimal relaxation, as expected: for the complexes [ΔR2 9.4 s-1 (3:1), 7.0 s-1 (2:1) and 3.2 s-1 (1:1)] (Figure 5b). The relaxation rates R1 and R2 varied linearly as a function of the concentration of 13 at a fixed concentration of Fe3+ (Figure 5c).
Figure 5. T1 and T2 response to β-gal.
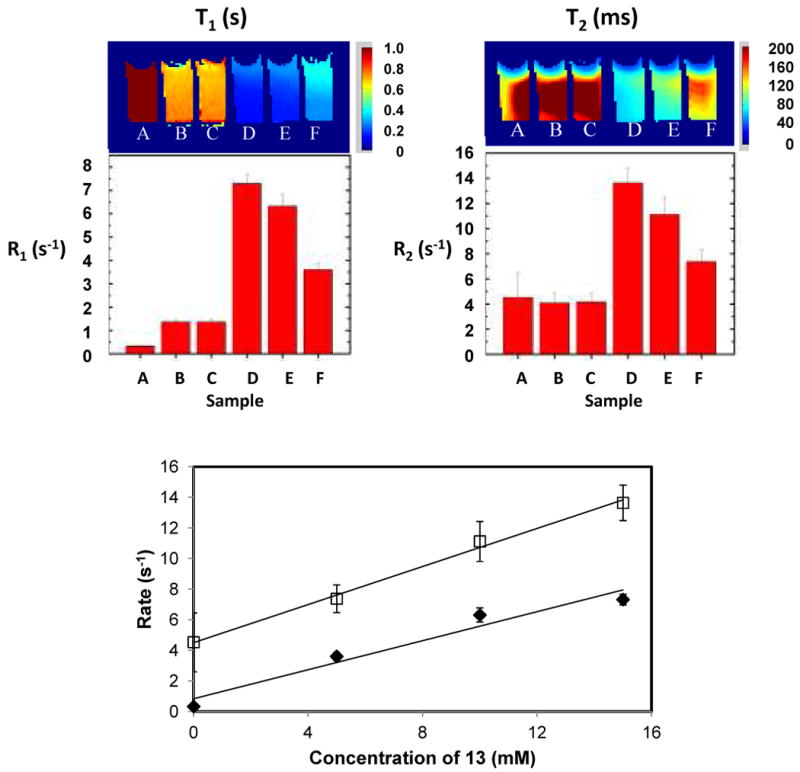
The T1 (a) and T2 (b) maps of solutions of various concentrations of mono β-D-galactopyranoside 13 in PBS (0.1M, pH=7.4) in the presence of ferric ammonium citrate (FAC; 5mM) together with bar charts for corresponding R1 and R2 values. β-gal (E801A, 5 units) was added to D-F. (A) 13 (15mM) alone in PBS; (B) FAC (5mM) alone in H2O; (C) 13 (15mM) plus FAC (5mM) in PBS; (D) 13 (15mM), FAC (5mM) and β-gal in PBS; (E) 13 (10mM), FAC (5mM) and β-gal in PBS; (F) 13 (5mM), FAC (5mM) and β-gal in PBS. 1H MRI at 200 MHz. C) Dependence of relaxation rates R1 (♦) and R2 (□) on concentration of 13 for constant β-gal (E801A, 5 units) and FAC (5mM) in PBS (0.1M, pH=7.4).
MRI in cells
To demonstrate the potential for detecting β-gal activity in vivo, various cells (human MCF7 breast and PC3 prostate cancer), as well as stably transfected clones expressing β-gal (MCF7-lacZ and PC3-lacZ) were incubated with 15 mM 13 and 5 mM Fe3+ in PBS (0.1M, pH=7.4) at 37°C under 5% CO2 in air with 95% humidity for 30 min. A significant difference in T1 and T2 was observed between the lacZ transfected and wild type (WT) cells. In MCF7-WT cells T1 = 1.32 ± 0.12 s and T2 = 45 ± 6 ms, while for MCF7-lacZ T1= 0.70 ± 0.10 and T2 = 32 ± 9 ms (Figure 6). Similarly, in PC3-WT cells T1=1.50 ± 0.07 s, T2= 39 ± 6 ms while for PC3-lacZ T1=1.16 ± 0.04 s, T2= 28 ± 4 ms, respectively).
Figure 6. T1 and T2 effects due to lacZ transfected cells.
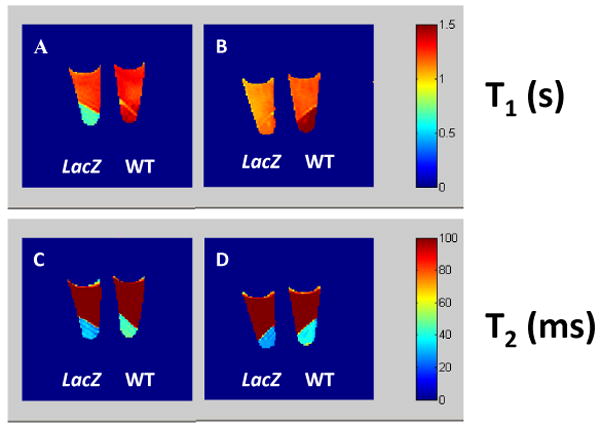
The T1 and T2 maps of mono β-D-galactopyranoside 13 (15mM) and FAC (5mM) in PBS (0.1M, pH=7.4, 200 μ;L) incubated at 37 °C under 5% CO2 in air with 95% humidity for 30 min. T1 maps: (A) MCF7-lacZ and MCF7-WT: 4 × 106 cells each; (B) PC3-lacZ and PC3-WT: 4 × 106 cells each; corresponding T2 maps (C,D). MRI parameters: 200 MHz, matrix=128 × 128, FOV=40 × 40, 2 mm slice
To be useful in vivo, reporter molecules must exhibit sufficient water solubility and it appeared that 10∼13 were less soluble than S-Gal™. Thus, we sought to enhance solubility by conjugating an additional β-D-galactopyranosyl unit to S-Gal™ and 10∼13, as applied successfully to 19F NMR β-gal reporters previously[41].
Di-β-D-galactopyranosides
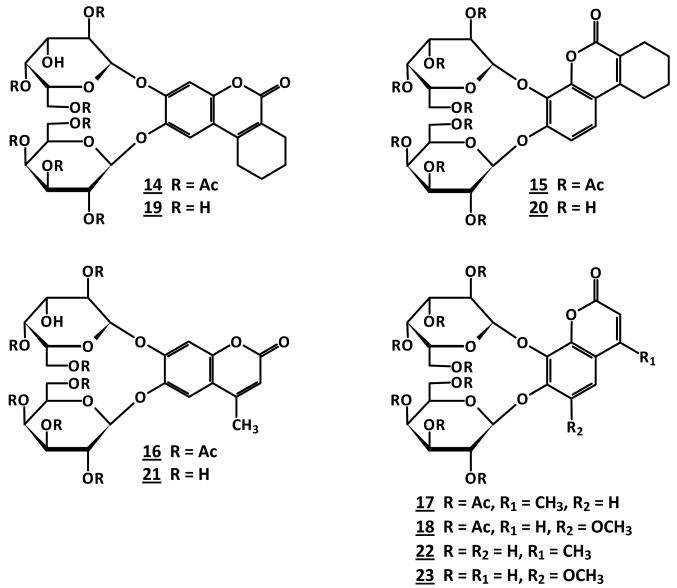
condensation of the coumarins 1∼5 directly with 2.2 equivalents of 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide in anhydrous CH2Cl2/MeCN catalyzed by Hg(CN)2 as a promoter, furnished the fully galactopyranosylated coumarins: 6,7-di-O-(2″, 3″, 4″, 6″-tetra-O-acetyl-β-D-galactopyranosyl)-3, 4-cyclohexenocoumarin 14 (90%), 7,8-di-O-(2″, 3″, 4″, 6″-tetra-O-acetyl-β-D-galactopyranosyl)-3, 4-cyclohexenocoumarin 15 (86%), 6,7-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-4-methylcoumarin 16 (73%), 7,8-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-4-methylcoumarin 17 (77%) and 7,8-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-6-methoxycoumarin 18 (87%), respectively (Figure 7). Deacetylation of 14∼18 in NH3/MeOH from 0 °C to room temperature accomplished the free di-β-D-galactopyranosides 19∼23 in high yields (Figure 7). The ESI-MS of 19∼23 showed the expected molecular ions, corresponding to the fully galactopyranosylated derivatives. Again, the identities of 19∼23 were established from their 1H and 13C NMR spectra. The anomeric protons H-1′, H-1″ or H-1‴ of D-galactoses linked to 7 and 6 or 8 positions of coumarins 1∼5 at 5.26∼4.82 ppm with the well resolved doublets (J1,2 = 8.0 Hz, J2,3 = 10 Hz) confirming both D-galactoses in the β-configuration.
Figure 7. The structures of di β-D-galactopyranosides 14-23.
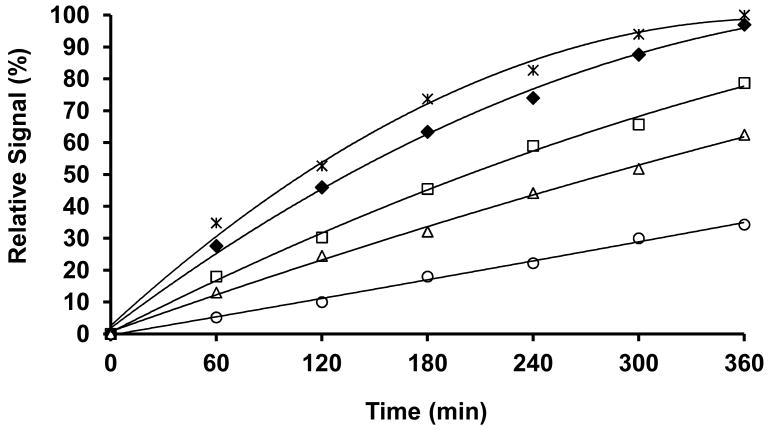
As expected, the synthesized di-β-D-galactopyranosides 19∼23 are soluble in PBS (0.1M, pH= 7.4) in high concentrations, unlike 10∼13, which required the addition of DMSO. The hydrolysis of di-β-D-galactopyranosides 19∼23 by β-gal (E801A) in PBS (0.1M, pH=7.4) at 20∼22°C showed that relative hydrolytic rates were similar though a little slower than for the corresponding mono-galactopyranosides and 23 was considerably slower than expected (Figure 8).
Figure 8. The kinetichydrolysis time courses of di-β-D-galactopyranosides.
Evolution of fluorescence (365/440 nm) following addition of β-gal (10 units, E801A) to solutions of 19-23 (10mM) in PBS (1.0mL, 0.1M, pH=7.4) at 20-22°C showing release of the corresponding aglycones 19→1(*), 20→2(□), 21→3(♦), 22→4(Δ), 23→5(○).
In conclusion we have successfully synthesized 9 novel β-D-galactopyranosides and demonstrated the potential to detect β-gal activity based on MRI contrast in the presence of Fe3+ ions. The di-β-D-galactopyranosides react a little slower, but exhibit much higher water solubility suggesting greater potential for use in vivo. MRI clearly revealed WT versus lacZ expressing cells in culture upon incubation with 13 based on significant differences in both T1 and T2. Signal gain providing contrast in T1-weighted images is potentially preferable to T2-weighted signal loss observed previously with S-Gal™ in vivo. However the combination of both T1 and T2 response may be most promising since the concerted effect will add certainty to observations in vivo, where tissue heterogeneity may otherwise be misinterpreted. These mono and di-β-D-galactopyranosides show promise as 1H MRI lacZ gene reporters and we are currently evaluating them for potential application in human tumor xenografts in vivo.
Experimental
General methods ---
NMR spectra were recorded on a Varian Unity INOVA 400 spectrometer (400 MHz for 1H, 100 MHz for 13C) with CDCl3, or DMSO-d6 as solvents at 25°C, and 1H and 13C chemical shifts are referenced to internal TMS. Microanalyses were performed on a Perkin-Elmer 2400CHN microanalyser. Mass spectra were obtained by positive and negative ESI-MS using a Micromass Q-TOF hybrid quadrupole/time-of-flight instrument (Micromass UK Ltd). Solutions in organic solvents were dried with anhydrous sodium sulfate, and concentrated in vacuo below 45 °C. 3,4-cyclohexenoesculetin-β-D-galactopyranoside (S-Gal™), 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide and 6-methoxy-7, 8-dihydroxycoumarin 5 were purchased from the Sigma Chemical Company. β-Gal (E801A) was purchased from Aldrich Chemical Company and enzyme reactions performed at 20-22 °C in PBS solution (0.1M, pH=7.4). Fluorescence was measured using a Fluorolog 3 spectrometer (Jobin-Yvon Horiba, Edison, NJ) with λex at 365 nm and λem 440 nm. Column chromatography was performed on silica gel (200∼300 mesh) by elution with cyclohexane-ethyl acetate and silica gel GF254 used for analytical TLC (Aldrich Chemical Company). Detection was effected by spraying the plates with 5% ethanolic H2SO4 (followed by heating at 110 °C for 10 min.) or by direct UV illumination of the plate.
Pechmann condensation for synthesis of coumarins 1∼4
General procedure -
To an equimolar mixture of the phenol (pyrogallol or 1,2,4-benzenetriol, 10 mmol) and the β-ketoester (ethyl cyclohexanone-2-carboxylate or ethyl acetoacetate, 10 mmol) in toluene (40mL) was added ZrCl4 (377.3 mg, 1.0 mmol, 10mol%) and the mixture was stirred at 80 °C under N2 until TLC showed complete reaction (<30 minutes). After solvent evaporation under reduced pressure, the mixture was washed with cold water, and recrystallized from hot EtOH/H2O to give the pure coumarins 1∼4.
3,4-cyclohexenoesculetin 1 (2.14 g, 92%), δh ([D6]DMSO, 400 MHz): 6.93 (1 H, s, H-5), 9.25 (1 H, s, OH-6), 9.98 (1 H, s, OH-7), 6.69 (1 H, s, H-8), 2.64 (2 H, t, J = 4.0 Hz, H-1′), 1.69 (4 H, m, H-2′, 3′), 2.34 (2 H, t, J = 4.0 Hz, H-4′) ppm; δC ([D6]DMSO, 100 MHz): 164.02 (-CO), 102.64 (C-3), 148.04 (C-4), 142.74 (C-5), 118.81 (C-6), 146.00 (C-7), 108.40 (C-8), 148.81 (C-9), 111.57 (C-10), 24.78 (CH2-1′), 21.37, 21.05 (CH2-2′, 3′), 23.66 (CH2-4′) ppm.
Anal. Calcd. for C13H12O4 (%): C, 67.23, H, 5.21; Found: C, 67.21, H, 5.19.
7,8-dihydroxy-3,4-cyclohexenocoumarin 2 (2.16 g, 93%), δH ([D6]DMSO, 400 MHz): 7.00 (1 H, d, J = 8.0 Hz, H-5), 6.75 (1 H, d, J = 8.0 Hz, H-6), 9.84 (1 H, s, OH-7), 9.20 (1 H, s, OH-8), 2.68 (2 H, t, J = 4.0 Hz, H-1′), 1.69 (4 H, m, H-2′, 3′), 2.36 (2 H, t, J = 4.0 Hz, H-4′) ppm; δC ([D6]DMSO, 100 MHz): 160.08 (-CO), 148.15 (C-9), 141.61 (C-4), 132.00 (C-7), 118.34 (C-5), 113.90 (C-6), 112.82 (C-10), 112.10 (C-8), 107.23 (C-3), 24.79 (CH2-1′), 21.37, 21.02 (CH2-2′, 3′), 23.61 (CH2-4′) ppm.
Anal. Calcd. for C13H12O4 (%): C, 67.23, H, 5.21; Found: C, 67.21, H, 5.20.
6,7-dihydroxy-4-methylcoumarin 3 (1.83 g, 95%), δH ([D6]DMSO, 400 MHz): 6.07 (1 H, s, H-3), 6.98 (1 H, s, H-5), 9.38 (1 H, brs, OH-6), 10.19 (1 H, brs, OH-7), 6.71 (1 H, s, H-8), 2.29 (3 H, s, CH3-4) ppm; δC ([D6]DMSO, 100 MHz): 160.69 (-CO), 102.74 (C-3), 150.20 (C-4), 142.86 (C-5), 111.58 (C-6), 147.80 (C-7), 110.45 (C-8), 153.31 (C-9), 110.49 (C-10), 18.29 (CH3-4) ppm.
Anal. Calcd. for C10H8O4 (%): C, 62.50, H, 4.20; Found: C, 62.47, H, 4.18.
7,8-dihydroxy-4-methylcoumarin 4 (1.81 g, 94%), δH ([D6]DMSO, 400 MHz): 6.10 (1 H, s, H-3), 7.06 (1 H, d, J = 8.2 Hz, H-5), 6.80 (1 H, d, J = 8.2 Hz, H-6), 10.03 (1 H, s, OH-7), 9.27 (1 H, s, OH-8), 2.33 (3 H, s, CH3-4) ppm; δC ([D6]DMSO, 100 MHz): 160.29 (-CO), 110.27 (C-3), 149.47 (C-4), 132.24 (C-5), 115.59 (C-6), 143.36 (C-7), 112.19 (C-8), 154.01 (C-9), 112.84 (C-10), 18.33 (CH3-4) ppm.
Anal. Calcd. for C10H8O4 (%): C, 62.50, H, 4.20; Found: C, 62.48, H, 4.19.
Regioselective mono β-D-galactopyranosylation of coumarins 2∼5 for preparation of acetylated mono β-D-galactopyranosides 6∼9
General procedure ---
A solution of 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide (1.04 g, 2.52 mmol) in CH2Cl2 (30 mL) was added dropwise to a vigorously stirred biphasic mixture (pH 8∼9) of coumarins 2∼5 (2.52 mmol) and tetrabutylammonium bromide (TBAB) (160 mg, 0.5 mmol) in CH2Cl2-H2O (50 mL, 1:1 V/V′) over a period of 1 hr at room temperature under N2, and the stirring continued until TLC showed that the reaction complete (∼3 hr). Extraction with CH2Cl2 (4 × 30 mL), wash, dry (Na2SO4), and evaporation under reduced pressure gave a syrup, which was purified by column chromatography on silica gel yielding acetylated mono β-D-galactopyranosides 6∼9.
7-O-(2″, 3″, 4″, 6″-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-3, 4-cyclohexeno-coumarin 6 (1.25 g, 88%), Rf 0.40 (1:1 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 7.23 (1 H, d, J = 8.0 Hz, H-5), 6.83 (1 H, d, J = 8.0 Hz, H-6), 4.84 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 5.53 (1 H, dd, J2″,3″ = 12.0 Hz, H-2″), 5.07 (1 H, dd, J3″,4″ = 4.0 Hz, H-3″), 5.40 (1 H, d, J 4″,5″ = 3.2 Hz, H-4″), 3.95 (1 H, m, H-5″), 4.19 (1 H, dd, J5″,6a″ = 7.2 Hz, J6a″,6b″ = 11.8 Hz, H-6a″), 4.16 (1 H, dd, J5″,6b″ = 5.6 Hz, H-6b″), 2.68 (2 H, t, J = 4.0 Hz, H-1′), 1.76 (4 H, m, H-2′, 3′), 2.47 (2 H, t, J = 4.0 Hz, H-4′), 2.00, 1.99, 1.98, 1.96 (12 H, 4 s, 4 × CH3CO), ppm; δC (CDCl3, 100 MHz): 170.92, 170.56, 170.30, 170.14 (4 × CH3CO), 161.24 (-CO), 112.83 (C-3), 147.64 (C-4), 131.06 (C-5), 120.88 (C-6), 145.87 (C-7), 114.35 (C-8), 151.96 (C-9), 120.69 (C-10), 104.52 (C-1″), 68.11 (C-2″), 70.68 (C-3″), 66.83 (C-4″), 71.87 (C-5″), 61.12 (C-6″), 25.49 (CH2-1′), 21.80, 21.49 (CH2-2′, 3′), 24.00 (CH2-4′), 20.91, 20.82, 20.75, 20.70 (4 × CH3CO) ppm.
Anal. Calcd. for C27H30O13 (%): C, 57.65, H, 5.38; Found: C, 57.63, H, 5.35.
7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-6-hydroxy-4-methylcoumarin 7 (0.95 g, 72%), Rf 0.33 (2:3 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.16 (1 H, s, H-3), 7.07 (1 H, s, H-5), 6.95 (1 H, s, H-8), 5.03 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.45 (1 H, dd, J2′,3′ = 114 Hz, H-2′), 5.15 (1 H, dd, J3′,4′ = 3.4 Hz, H-3′), 5.42 (1 H, d, J4′,5′ = 7.2 Hz, H-4′), 4.10 (1 H, m, H-5′), 4.16 (1 H, dd, J5′,6a′ = 4.0 Hz, J6a′,6b′ = 7.2 Hz, H-6a′), 4.13 (1 H, dd, J5′,6b′ = 5.0 Hz, H-6b′), 2.32 (3 H, s, CH3-4), 2.15, 2.08, 2.07, 1.99 (12 H, 4 s, 4 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.95, 170.64, 170.24, 170.04 (4 × CH3CO), 161.15 (-CO), 104.15 (C-3), 147.88 (C-4), 143.45 (C-5), 116.17 (C-6), 146.77 (C-7), 110.03 (C-8), 152.30 (C-9), 114.03 (C-10), 100.85 (C-1′), 69.18 (C-2′), 70.23 (C-3′), 66.82 (C-4′), 71.91 (C-5′), 61.46 (C-6′), 21.06, 20.84, 20.77, 20.69 (4 × CH3CO), 18.98 (CH3-4) ppm.
Anal. Calcd. for C24H26O13 (%): C, 55.17, H, 5.02; Found: C, 55.15, H, 5.00.
7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-4-methylcoumarin 8 (1.07 g, 81%), Rf 0.47 (1:1 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.17 (1 H, s, H-3), 7.01 (1 H, d, J = 8.0 Hz, H-5), 6.93 (1 H, d, J = 8.0 Hz, H-6), 4.96 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.44 (1 H, dd, J2′,3′ = 10.7 Hz, H-2′), 5.09 (1 H, dd, J3′,4′ = 4.0 Hz, H-3′), 5.41 (1 H, d, J4′,5′ = 3.0 Hz, H-4′), 4.01 (1 H, m, H-5′), 4.18 (1 H, dd, J5′,6a′ = 5.8 Hz, J6a′,6b′ = 11.6 Hz, H-6a′), 4.16 (1 H, dd, J5′,6b′ = 7.8 Hz, H-6b′), 2.34 (3 H, s, CH3-4), 2.14, 2.06, 2.01, 1.96 (12 H, 4 s, 4 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.58, 170.46, 170.33, 170.24 (4 × CH3CO), 160.06 (-CO), 113.77 (C-3), 146.35 (C-4), 135.34 (C-5), 117.28 (C-6), 143.65 (C-7), 113.93 (C-8), 152.63 (C-9), 115.25 (C-10), 101.64 (C-1′), 69.01 (C-2′), 70.42 (C-3′), 66.83 (C-4′), 71.54 (C-5′), 61.37 (C-6′), 20.91, 20.83, 20.81, 20.75 (4 × CH3CO), 18.99 (CH3-4) ppm.
Anal. Calcd. for C24H26O13 (%): C, 55.17, H, 5.02; Found: C, 55.16, H, 5.01.
7-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-8-hydroxy-6-methoxycoumarin 9 (1.06 g, 78%), Rf 0.52 (1:4 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.29 (1 H, d, J = 9.8 Hz, H-3), 7.51 (1 H, d, J = 9.8 Hz, H-4), 6.90 (1 H, s, H-5), 4.80 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.44 (1 H, dd, J2′,3′ = 10.2 Hz, H-2′), 5.04 (1 H, dd, J3′,4′ = 3.5 Hz, H-3′), 5.34 (1 H, d, J4′,5′ = 6.8 Hz, H-4′), 3.93 (1 H, m, H-5′), 4.12 (1 H, dd, J5′,6a′ = 4.4 Hz, J6a′,6b′ = 7.8 Hz, H-6a′), 4.04 (1 H, dd, J5′,6b′ = 5.2 Hz, H-6b′), 3.79 (3 H, s, CH3O-6), 2.14, 2.08, 1.97, 1.94 (12 H, 4 s, 4 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.64, 170.34, 170.20, 169.75 (4 × CH3CO), 160.24 (-CO), 115.49 (C-3), 143.36 (C-4), 138.34 (C-5), 136.07 (C-6), 139.23 (C-7), 116.27 (C-8), 149.31 (C-9), 116.77 (C-10), 103.57 (C-1′), 68.60 (C-2′), 70.51 (C-3′), 66.74 (C-4′), 71.76 (C-5′), 61.03 (C-6′), 56.41 (CH3O-6), 20.06, 20.88, 20.79, 20.76 (4 × CH3CO) ppm.
Anal. Calcd. for C24H26O14 (%): C, 53.53, H, 4.87; Found: C, 53.52, H, 4.85.
Mono β-D-galactopyranosides 10∼13
General procedure ---
A solution of 7-O-(acetylated β-D-galactopyranosyl) coumarins 6∼9 (900 mg) in anhydrous ammoniacal MeOH (0.5M 100 mL) was vigorously stirred from 0 °C to room temperature overnight until TLC showed that the reaction was complete, evaporated to dryness in vacuo. Chromatography of the crude syrup on silica gel with EtOAc/MeOH afforded the corresponding free mono β-D-galactopyranosides 10∼13 in nearly quantitative yields.
7-O-(β-D-galactopyranosyl)-8-hydroxy-3, 4-cyclohexenocoumarin 10 (599.43 mg, 95%), Rf 0.41 (1:3 MeOH-EtOAc), δh ([D6]DMSO, 400 MHz): 7.30 (1 H, d, J = 8.4 Hz, H-5), 6.82 (1 H, d, J = 8.4 Hz, H-6), 4.75 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 3.67 (1 H, dd, J2″,3″ = 10.2 Hz, H-2″), 3.56 (1 H, dd, J3″,4″ = 4.0 Hz, H-3″), 3.45 (1 H, d, J4″,5″ = 6.3 Hz, H-4″), 3.40 (1 H, m, H-5″), 3.38 (2 H, m, H-6″), 2.74 (2 H, t, J = 4.0 Hz, H-1′), 1.72 (4 H, m, H-2′, 3′), 2.51 (2 H, t, J = 4.0 Hz, H-4′) ppm; δC ([D6]DMSO, 100 MHz): 160.81 (-CO), 112.12 (C-3), 147.99 (C-4), 131.60 (C-5), 119.62 (C-6), 145.89 (C-7), 113.45 (C-8), 153.46 (C-9), 118.15 (C-10), 105.53 (C-1″), 71.40 (C-2″), 73.27 (C-3″), 67.86 (C-4″), 75.77 (C-5″), 59.95 (C-6″), 24.83 (CH2-1′), 21.36, 21.02 (CH2-2′, 3′), 23.60 (CH2-4′) ppm.
Anal. Calcd. for C19H22O9 (%): C, 57.87, H, 5.62; Found: C, 57.84, H, 5.58.
7-O-(β-D-galactopyranosyl)-6-hydroxy-4-methylcoumarin 11 (567.62 mg, 93%), Rf 0.40 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.16 (1 H, s, H-3), 7.07 (1 H, s, H-5), 6.95 (1 H, s, H-8), 5.03 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.45 (1 H, dd, J2′,3′ = 11.4 Hz, H-2′), 5.15 (1 H, dd, J3′,4′ = 3.4 Hz, H-3′), 5.42 (1 H, d, J4′,5′ = 7.2 Hz, H-4′), 4.10 (1 H, m, H-5′), 4.16 (1 H, dd, J5′,6a′ = 4.0 Hz, J6a′,6b′ = 7.2 Hz, H-6a′), 4.13 (1 H, dd, J5′,6b′ = 5.0 Hz, H-6b′), 2.32 (3 H, s, CH3-4) ppm; δ C ([D6]DMSO, 100 MHz): 161.15 (-CO), 104.15 (C-3), 147.88 (C-4), 143.45 (C-5), 116.17 (C-6), 146.77 (C-7), 110.03 (C-8), 152.30 (C-9), 114.03 (C-10), 100.85 (C-1′), 69.18 (C-2′), 70.23 (C-3′), 66.82 (C-4′), 71.91 (C-5′), 61.46 (C-6′), 18.98 (CH3-4) ppm.
Anal. Calcd. for C16H18O9 (%): C, 54.24, H, 5.12; Found: C, 54.20, H, 5.09.
7-O-(β-D-galactopyranosyl)-8-hydroxy-4-methylcoumarin 12 (585.93 mg, 96%), Rf 0.38 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.17 (1 H, s, H-3), 7.01 (1 H, d, J = 8.0 Hz, H-5), 6.93 (1 H, d, J = 8.0 Hz, H-6), 4.96 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.44 (1 H, dd, J2′,3′ = 10.7 Hz, H-2′), 5.09 (1 H, dd, J3′,4′ = 4.0 Hz, H-3′), 5.41 (1 H, d, J4′,5′ = 3.0 Hz, H-4′), 4.01 (1 H, m, H-5′), 4.18 (1 H, dd, J5′,6a′ = 5.8 Hz, J6a′,6b′ = 11.6 Hz, H-6a′), 4.16 (1 H, dd, J5′,6b′ = 7.8 Hz, H-6b′), 2.34 (3 H, s, CH3-4) ppm; δC ([D6]DMSO, 100 MHz): 160.06 (-CO), 113.77 (C-3), 146.35 (C-4), 135.34 (C-5), 117.28 (C-6), 143.65 (C-7), 113.93 (C-8), 152.63 (C-9), 115.25 (C-10), 101.64 (C-1′), 69.01 (C-2′), 70.42 (C-3′), 66.83 (C-4′), 71.54 (C-5′), 61.37 (C-6′), 18.99 (CH3-4) ppm.
Anal. Calcd. for C16H18O9 (%): C, 54.24, H, 5.12; Found: C, 54.19, H, 5.08.
7-O-(β-D-galactopyranosyl)-8-hydroxy-6-methoxycoumarin 13 (575.63 mg, 93%), Rf 0.45 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.29 (1 H, d, J = 9.8 Hz, H-3), 7.51 (1 H, d, J = 9.8 Hz, H-4), 6.90 (1 H, s, H-5), 4.80 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.44 (1 H, dd, J2′,3′ = 10.2 Hz, H-2′), 5.04 (1 H, dd, J3′,4′ = 3.5 Hz, H-3′), 5.34 (1 H, d, J4′,5′ = 6.8 Hz, H-4′), 3.93 (1 H, m, H-5′), 4.12 (1 H, dd, J5′,6a′ = 4.4 Hz, J6a′,6b′ = 7.8 Hz, H-6a′), 4.04 (1 H, dd, J5′,6b′ = 5.2 Hz, H-6b′), 3.79 (3 H, s, CH3O-6) ppm; δC ([D6]DMSO, 100 MHz): 160.24 (-CO), 115.49 (C-3), 143.36 (C-4), 138.34 (C-5), 136.07 (C-6), 139.23 (C-7), 116.27 (C-8), 149.31 (C-9), 116.77 (C-10), 103.57 (C-1′), 68.60 (C-2′), 70.51 (C-3′), 66.74 (C-4′), 71.76 (C-5′), 61.03 (C-6′), 56.41 (CH3O-6) ppm.
Anal. Calcd. for C16H18O10 (%): C, 51.90, H, 4.90; Found: C, 51.85, H, 4.86.
Full β-D-galactopyranosylation of coumarins 1∼5 for preparation of acetylated di-β-D-galactopyranosides 14∼18
General procedure ---
To a vigorously stirred solution of coumarins 1∼5 (2.52 mmol) and Hg(CN)2 (2.10 g, 8.31 mmol) in anhydrous MeCN (100 mL) containing freshly activated 4Å molecular sieves (5.00 g) was added dropwise 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide (2.29 g, 5.54 mmol, 2.2 equiv.) in CH2Cl2 (50 mL). The mixture was stirred in the dark at room temperature under N2 atmosphere until TLC indicated complete reaction, then diluted with CH2Cl2 (150 mL), filtered through Celite, washed, dried (Na2SO4), evaporated under reduced pressure to give a syrup, which was purified by column chromatography on silica gel to give the acetylated di-β-D-galactopyranosides 14∼18.
6,7-di-O-(2″, 3″, 4″, 6″-tetra-O-acetyl-β-D-galactopyranosyl)-3, 4-cyclohexenoesculetin 14 (2.03 g, 90%), Rf 0.41 (2:3 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 7.32 (1 H, s, H-5), 7.08 (1 H, s, H-8), 5.14 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 5.20 (1 H, d, J1‴,2‴ = 8.0 Hz, H-1‴), 5.48 (2 H, dd, J2″,3″ = J2‴,3‴ = 10.0 Hz, H-2″, 2‴), 5.16 (2 H, dd, J 3″,4″ = J3‴,4‴ = 4.0 Hz, H-3″, 3‴), 5.43 (2 H, d, J4″,5″ = J4‴,5‴ = 3.2 Hz, H-4″, 4‴), 4.06 (2 H, m, H-5″, 5‴), 4.20 (2 H, dd, J5″,6a″ = J5‴,6a‴ = 5.0 Hz, J6a″,6b″ = J6a‴,6b‴ = 11.2 Hz, H-6a″, 6a‴), 4.13 (2 H, dd, J5″,6b″ = J5‴,6b‴ = 4.0 Hz, H-6b″, 6b‴), 2.72 (2 H, t, J = 4.0 Hz, H-1′), 1.81 (4 H, m, H-2′, 3′), 2.57 (2 H, t, J = 4.0 Hz, H-4′), 2.21, 2.14, 2.10, 2.09, 2.03, 2.02, 2.01, 2.00 (24 H, 8 s, 8 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.63, 170.43, 170.35, 170.27, 170.24, 170.22, 169.36, 169.20 (8 × CH3CO), 161.71 (-CO), 105.34 (C-3), 148.84 (C-4), 143.17 (C-5), 122.95 (C-6), 146.53 (C-7), 114.10 (C-8), 149.30 (C-9), 115.68 (C-10), 100.88 (C-1″), 101.02 (C-1‴), 68.66 (C-2″), 69.00 (C-2‴), 70.87 (C-3″), 70.96 (C-3‴), 67.17 (C-4″), 67.22 (C-4‴), 71.48 (C-5″), 71.82 (C-5‴), 61.57 (C-6″), 61.64 (C-6‴), 25.38 (CH2-1′), 21.72, 21.46 (CH2-2′, 3′), 24.13 (CH2-4′), 20.95, 20.90, 20.86, 20.83, 20.80, 20.78, 20.76, 20.74 (8 × CH3CO) ppm; ESIMS: m/z 893 [M+] (27%), 894 [M+1] (11%).
Anal. Calcd. for C41H48O22 (%): C, 55.16, H, 5.42; Found: C, 55.14, H, 5.40.
7,8-di-O-(2″, 3″, 4″, 6″-tetra-O-acetyl-β-D-galactopyranosyl)-3, 4-cyclohexenocoumarin 15 (1.94 g, 86%), Rf 0.36 (2:3 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 7.27 (1 H, d, J = 8.0 Hz, H-5), 7.06 (1 H, d, J = 8.0 Hz, H-6), 5.37 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 5.25 (1 H, d, J1‴,2‴ = 8.0 Hz, H-1‴), 5.48 (1 H, dd, J2″,3″ = 10.4 Hz, H-2″), 5.45 (1 H, dd, J2‴,3‴ = 10.2 Hz, H-2‴), 5.13 (1 H, dd, J3″,4″ = 4.0 Hz, H-3″), 5.10 (1 H, dd, J3‴,4‴ = 4.0 Hz, H-3‴), 5.44 (1 H, d, J4″,5″ = 3.6 Hz, H-4″), 5.42 (1 H, d, J4‴,5‴ = 3.8 Hz, H-4‴), 3.99 (1 H, m, H-5″), 3.93 (1 H, m, H-5‴), 4.21 (2 H, dd, J5″,6a″ = J5‴,6a‴ = 7.6 Hz, J6a″,6b″ = J6a‴,6b‴ = 6.4 Hz, H-6a″, 6a‴), 4.16 (2 H, dd, J5″,6b″ = J5‴,6b″″ = 5.8 Hz, H-6b″, 6b‴), 2.73 (2 H, t, J = 4.0 Hz, H-1′), 1.84 (4 H, m, H-2′, 3′), 2.54 (2 H, t, J = 4.0 Hz, H-4′), 2.19, 2.18, 2.17, 2.16, 2.12, 2.00, 1.99, 1.94 (24 H, 8 s, 8 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.36, 170.33, 170.31, 170.27, 170.13, 170.10, 169.87, 169.65 (8 × CH3CO), 160.44 (-CO), 116.79 (C-3), 148.91 (C-4), 134.35 (C-5), 122.81 (C-6), 145.51 (C-7), 117.87 (C-8), 150.07 (C-9), 118.62 (C-10), 101.45 (C-1″), 100.72 (C-1‴), 69.29 (C-2″), 68.80 (C-2‴), 71.07 (C-3″), 70.62 (C-3‴), 67.01 (C-4″, 4‴), 71.31 (C-5″), 71.24 (C-5‴), 61.25 (C-6″), 61.20 (C-6‴), 25.47 (CH2-1′), 21.58, 21.36 (CH2-2′, 3′), 24.06 (CH2-4′), 20.91, 20.90, 20.76, 20.74, 20.71, 20.69, 20.67, 20.66 (8 × CH3CO) ppm; ESIMS: m/z 893 [M+] (29%), 894 [M+1] (15%).
Anal. Calcd. for C41H48O22 (%): C, 55.16, H, 5.42; Found: C, 55.13, H, 5.38.
6,7-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-4-methylcoumarin 16 (1.56 g, 73%), Rf 0.35 (1:2 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.17 (1 H, s, H-3), 7.30 (1 H, s, H-5), 7.04 (1 H, s, H-8), 5.15 (2 H, d, J1′,2′ = J1″,2″ = 8.0 Hz, H-1′,1″), 5.41 (2 H, dd, J2′,3′ = J2″,3″ = 10.4 Hz, H-2′, 2″), 5.10 (2 H, dd, J3′,4′ = J3″,4″ = 4.0 Hz, H-3′, 3″), 5.37 (2 H, d, J4′,5′ = J4′,5′ = 3.2 Hz, H-4′, 4″), 4.07 (1 H, m, H-5′), 4.00 (1 H, m, H-5″), 4.15 (2 H, dd, J5′,6a′ = J5″,6a″ = 5.4 Hz, J6a′,6b′ = J6a″,6b″ = 110 Hz, H-6a′, 6a″), 4.13 (2 H, dd, J5′,6b′ = J5″,6b″ = 4.2 Hz, H-6b′, 6b″), 2.34 (3 H, s, CH3-4), 2.14, 2.12, 2.07, 2.04, 2.03, 1.96, 1.95, 1.94 (24 H, 8 s, 8 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.52, 170.33, 170.25, 170.16, 170.13, 170.10, 169.27, 169.11 (8 × CH3CO), 160.69 (-CO), 105.43 (C-3), 150.41 (C-4), 132.52 (C-5), 115.27 (C-6), 143.05 (C-7), 114.00 (C-8), 151.74 (C-9), 114.38 (C-10), 100.68 (C-1′), 100.74 (C-1″), 68.53 (C-2′), 68.90 (C-2″), 70.79 (C-3′), 70.85 (C-3″), 67.05 (C-4′, 4″), 71.49 (C-5′), 71.87 (C-5″), 61.41 (C-6′), 61.51 (C-6″), 21.12, 20.86, 20.75, 20.73, 20.70, 20.68, 20.66, 20.64 (8 × CH3CO), 18.69 (CH3-4) ppm; ESIMS: m/z 853 [M+] (25%), 854 [M+1] (9%).
Anal. Calcd. for C38H44O22 (%): C, 53.52, H, 5.20; Found: C, 53.50, H, 5.18.
7,8-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-4-methylcoumarin 17 (1.66 g, 77%), Rf 0.30 (1:2 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.16 (1 H, s, H-3), 7.25 (1 H, d, J = 8.0 Hz, H-5), 7.04 (1 H, d, J = 8.0 Hz, H-6), 5.33 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 5.21 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 5.42 (1 H, dd, J2′,3′ = 10.8 Hz, H-2′), 5.38 (1 H, dd, J2″,3″ = 10.4 Hz, H-2″), 5.08 (1 H, dd, J3′,4′ = 4.0 Hz, H-3′), 5.05 (1 H, dd, J3″,4″ = 4.0 Hz, H-3″), 5.34 (1 H, d, J4′,5′ = 2.8 Hz, H-4′), 5.32 (1 H, d, J4″,5″ = 3.0 Hz, H-4″), 3.95 (1 H, m, H-5′), 3.90 (1 H, m, H-5″), 4.16 (2 H, dd, J5′,6a′ = J5″,6a″ = 5.6 Hz, J6a′,6b′ = J6a″,6b″ = 11.2 Hz, H-6a′, 6a″), 4.09 (2 H, dd, J5′,6b′ = J5″,6b″ = 4.8 Hz, H-6b′, 6b″), 2.35 (3 H, s, CH3-4), 2.15, 2.14, 2.12, 2.06, 1.97, 1.96, 1.95, 1.94 (24 H, 8 s, 8 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.35, 170.31, 170.29, 170.27, 170.26, 170.10, 169.78, 169.56 (8 × CH3CO), 159.39 (-CO), 113.93 (C-3), 151.33 (C-4), 134.49 (C-5), 120.02 (C-6), 147.17 (C-7), 116.53 (C-8), 152.25 (C-9), 117.53 (C-10), 101.37 (C-1′), 100.55 (C-1″), 69.32 (C-2′), 68.75 (C-2″), 71.04 (C-3′), 70.58 (C-3″), 67.01 (C-4′), 66.92 (C-4″), 71.39 (C-5′), 71.30 (C-5″), 61.23 (C-6′), 61.16 (C-6″), 21.13, 20.88, 20.77, 20.75, 20.71, 20.69, 20.67, 20.65 (8 × CH3CO), 18.97 (CH3-4) ppm; ESIMS: m/z 853 [M+] (29%), 854 [M+1] (17%).
Anal. Calcd. for C38H44O22 (%): C, 53.52, H, 5.20; Found: C, 53.49, H, 5.17.
7,8-di-O-(2′, 3′, 4′, 6′-tetra-O-acetyl-β-D-galactopyranosyl)-6-methoxycoumarin 18 (1.91 g, 87%), Rf 0.38 (1:3 cyclohexane-EtOAc), δh (CDCl3, 400 MHz): 6.27 (1 H, d, J = 8.2 Hz, H-3), 7.54 (1 H, d, J = 8.2 Hz, H-4), 6.69 (1 H, s, H-5), 4.08 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 4.01 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 5.42 (1 H, dd, J2′,3′ = 10.1 Hz, H-2′), 5.40 (1 H, dd, J2″,3″ = 10.0 Hz, H-2″), 5.04 (1 H, dd, J3′,4′ = 3.0 Hz, H-3′), 5.01 (1 H, dd, J3″,4″ = 3.2 Hz, H-3″), 5.36 (1 H, d, J4′,5′ = 6.4 Hz, H-4′), 5.32 (1 H, d, J4″,5″ = 6.2 Hz, H-4″), 3.89 (1 H, m, H-5′), 3.83 (1 H, m, H-5″), 4.04 (4 H, m, H-6′, 6″), 3.78 (3 H, s, CH3O-6), 2.11, 2.10, 2.05, 1.96, 1.91, 1.90, 1.88, 1.85 (24 H, 8 s, 8 × CH3CO) ppm; δC (CDCl3, 100 MHz): 170.44, 170.36, 170.26, 170.20, 170.15, 170.08, 169.83, 169.42 (8 × CH3CO), 159.58 (-CO), 105.61 (C-3), 143.15 (C-4), 141.77 (C-5), 136.64 (C-6), 142.44 (C-7), 115.30 (C-8), 149.75 (C-9), 116.11 (C-10), 105.58 (C-1′), 101.45 (C-1″), 69.48 (C-2′), 69.17 (C-2″), 70.96 (C-3′, 3″), 66.93 (C-4′), 66.88 (C-4″), 71.20 (C-5′), 71.10 (C-5″), 60.93 (C-6′), 60.88 (C-6″), 56.74 (CH3O-6), 21.07, 20.86, 20.84, 20.80, 20.65, 20.62, 20.60, 20.58 (8 × CH3CO) ppm; ESIMS: m/z 869 [M+] (27%), 870 [M+1] (18%).
Anal. Calcd. for C38H44O23 (%): C, 52.54, H, 5.11; Found: C, 52.50, H, 5.08.
Free di-β-D-galactopyranosides 19∼23
Di-O-(2′, 3′, 4′, 6-tetra-O-acetyl-β-D-galactopyranosyl) coumarins 14∼18 (1.00 g) were deacetylated as described above for free mono β-D-galactopyranosides 10∼13 to afford the corresponding free di-β-D-galactopyranosides 19∼23 in high yields.
6,7-di-O-(β-D-galactopyranosyl)-3, 4-cyclohexenoesculetin 19 (585.93 mg, 94%), Rf 0.29 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 7.40 (1 H, s, H-5), 7.14 (1 H, s, H-8), 5.29 (1 H, br, HO-2″, 2‴, exchangeable with D2O), 4.67 (2 H, br, HO-3″, 3‴, exchangeable with D2O), 5.00 (2 H, br, HO-4″, 4‴, exchangeable with D2O), 4.70 (2 H, br, HO-6″, 6‴, exchangeable with D2O), 4.82 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 4.91 (1 H, d, J1‴,2‴ = 8.0 Hz, H-1‴), 3.68 (2 H, dd, J2″,3″ = J2‴,3‴ = 10.0 Hz, H-2″, 2‴), 3.65 (2 H, dd, J3″,4″ = J3‴,4‴ = 4.0 Hz, H-3″, 3‴), 3.47 (2 H, d, J4″,5″ = J4‴,5‴ = 3.4 Hz, H-4″, 4‴), 3.52 (2 H, m, H-5″, 5‴), 3.72 (2 H, m, H-6″, 6‴), 2.74 (2 H, t, J = 4.0 Hz, H-1′), 1.75 (4 H, m, H-2′, 3′), 2.39 (2 H, t, J = 4.0 Hz, H-4′) ppm; δC ([D6]DMSO, 100 MHz): 161.30 (-CO), 103.82 (C-3), 147.74 (C-4), 143.49 (C-5), 120.55 (C-6), 147.23 (C-7), 103.92 (C-8), 149.28 (C-9), 113.66 (C-10), 101.23 (C-1″), 102.05 (C-1‴), 70.05 (C-2″), 70.26 (C-2‴), 72.76 (C-3″), 72.94 (C-3‴), 67.99 (C-4″), 68.17 (C-4‴), 75.61 (C-5″), 75.70 (C-5‴), 60.34 (C-6″), 60.54 (C-6‴), 24.60 (CH2-1′), 21.14, 20.84 (CH2-2′, 3′), 23.58 (CH2-4′) ppm; ESIMS: m/z 557 [M+] (7%), 558 [M+1] (10%).
Anal. Calcd. for C25H32O14 (%): C, 53.96, H, 5.80; Found: C, 53.94, H, 5.76.
7,8-di-O-(β-D-galactopyranosyl)-3, 4-cyclohexenocoumarin 20 (592.16 mg, 95%), Rf 0.31 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 7.43 (1 H, d, J = 8.0 Hz, H-5), 7.26 (1 H, d, J = 8.0 Hz, H-6), 5.43 (2 H, br, HO-2″, 2‴, exchangeable with D2O), 4.47 (2 H, br, HO-3″, 3‴, exchangeable with D2O), 4.95 (2 H, br, HO-4″, 4‴, exchangeable with D2O), 4.63 (2 H, br, HO-6″, 6‴, exchangeable with D2O), 5.08 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 4.82 (1 H, d, J1‴,2‴ = 8.0 Hz, H-1‴), 3.72 (2 H, dd, J2″,3″ = J2‴,3‴ = 10.2 Hz, H-2″, 2‴), 3.45 (2 H, dd, J3″,4″ = J3‴,4‴ = 4.0 Hz, H-3″, 3‴), 3.39 (2 H, d, J4″,5″ = J4‴,5‴ = 3.6 Hz, H-4″, 4‴), 3.35 (2 H, m, H-5″, 5‴), 3.53 (4 H, m, H-6″, 6‴), 2.77 (2 H, t, J = 4.0 Hz, H-1′), 1.78 (4 H, m, H-2′, 3′), 2.43 (2 H, t, J = 4.0 Hz, H-4′) ppm; δC ([D6]DMSO, 100 MHz): 160.51 (-CO), 112.98 (C-3), 147.40 (C-4), 132.95 (C-5), 120.66 (C-6), 145.51 (C-7), 115.42 (C-8), 151.56 (C-9), 118.94 (C-10), 103.85 (C-1″), 102.95 (C-1‴), 71.37 (C-2″), 70.66 (C-2‴), 73.28 (C-3″), 72.60 (C-3‴), 68.15 (C-4″), 68.05 (C-4‴), 75.94 (C-5″), 75.77 (C-5‴), 60.46 (C-6″), 60.20 (C-6‴), 24.76 (CH2-1′), 21.13, 20.83 (CH2-2′, 3′), 23.61 (CH2-4′) ppm; ESIMS: m/z 557 [M+] (9%), 558 [M+1] (13%).
Anal. Calcd. for C25H32O14 (%): C, 53.96, H, 5.80; Found: C, 53.92, H, 5.77.
6,7-di-O-(β-D-galactopyranosyl)-4-methylcoumarin 21 (545.07 mg, 90%), Rf 0.31 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.24 (1 H, s, H-3), 7.45 (1 H, s, H-5), 7.16 (1 H, s, H-8), 5.10 (1 H, d, JH-2′,OH-2′ = 4.0 Hz, HO-2′, exchangeable with D2O), 5.12 (1 H, d, JH-2″,OH-2″ = 4.0 Hz, HO-2″, exchangeable with D2O), 4.54 (1 H, d, JH-3′,OH-3′ = 4.0 Hz, HO-3′, exchangeable with D2O), 4.58 (1 H, d, JH-3″,OH-3″ = 4.0 Hz, HO-3″, exchangeable with D2O), 4.88 (1 H, d, JH-4′,OH-4′ = 4.0 Hz, HO-4′, exchangeable with D2O), 4.90 (1 H, d, JH-4″,OH-4″ = 4.0 Hz, HO-4″, exchangeable with D2O), 4.69 (1 H, d, JH-6a′,OH-6′ = 4.0 Hz, JH-6b′,OH-6′ = 6.1 Hz, HO-6′, exchangeable with D2O), 4.71 (1 H, d, JH-6a″,OH-6″ = 4.0 Hz, JH-6b″,OH-6″ = 6.0 Hz, HO-6″, exchangeable with D2O), 4.85 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 4.97 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 3.68 (2 H, dd, J2′,3′ = J2″,3″ = 10.1 Hz, H-2′, 2″), 3.71 (2 H, dd, J3′,4′ = J3″,4″ = 4.0 Hz, H-3′, 3″), 3.53 (2 H, d, J4′,5′ = J4″,5″ = 3.6 Hz, H-4′, 4″), 3.46 (2 H, m, H-5′, 5″), 3.58 (4 H, m, H-6′, 6″), 2.37 (3 H, s, CH3-4) ppm; δC ([D6]DMSO, 100 MHz): 160.24 (-CO), 104.25 (C-3), 150.70 (C-4), 143.65 (C-5), 113.51 (C-6), 149.09 (C-7), 112.04 (C-8), 153.25 (C-9), 113.04 (C-10), 101.30 (C-1′), 102.24 (C-1″), 70.24 (C-2′), 70.40 (C-2″), 73.11 (C-3′), 73.33 (C-3″), 68.18 (C-4′), 68.35 (C-4″), 75.78 (C-5′), 75.92 (C-5″), 60.49 (C-6′), 60.65 (C-6″), 18.13 (CH3-4) ppm; ESIMS: m/z 517 [M+] (5%), 518 [M+1] (9%).
Anal. Calcd. for C22H28O14 (%): C, 51.16, H, 5.47; Found: C, 51.12, H, 5.44.
7,8-di-O-(β-D-galactopyranosyl)-4-methylcoumarin 22 (563.24 mg, 93%), Rf 0.26 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.27 (1 H, s, H-3), 7.48 (1 H, d, J = 8.0 Hz, H-5), 7.26 (1 H, d, J = 8.0 Hz, H-6), 5.36 (1 H, d, JH-2′,OH-2′ = 4.0 Hz, HO-2′, exchangeable with D2O), 4.94 (1 H, d, JH-2″,OH-2″ = 4.0 Hz, HO-2″, exchangeable with D2O), 4.60 (1 H, d, JH-3′,OH-3′ = 4.0 Hz, HO-3′, exchangeable with D2O), 4.58 (1 H, d, JH-3″,OH-3″ = 4.0 Hz, HO-3″, exchangeable with D2O), 4.91 (1 H, d, JH-4′,OH-4′ = 4.0 Hz, HO-4′, exchangeable with D2O), 4.89 (1 H, d, JH-4″,OH-4″ = 4.0 Hz, HO-4″, exchangeable with D2O), 4.73 (1 H, d, JH-6a′,OH-6′ = 4.0 Hz, JH-6b′,OH-6′ = 6.2 Hz, HO-6′, exchangeable with D2O), 4.43 (1 H, d, JH-6a″,OH-6″ = 4.0 Hz, JH-6b″,OH-6″ = 6.6 Hz, HO-6″, exchangeable with D2O), 5.07 (1 H, d, J1′,2′ = 8.0 Hz, H-1′), 4.83 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 3.70 (2 H, dd, J2′,3′ = J2″,3″ = 10.0 Hz, H-2′, 2″), 3.44 (2 H, dd, J3′,4′ = J3″,4″ = 4.2 Hz, H-3′, 3″), 3.76 (2 H, d, J4′,5′ = J4″,5″ = 3.8 Hz, H-4′, 4″), 3.36 (2 H, m, H-5′, 5″), 3.51 (4 H, m, H-6′, 6″), 2.40 (3 H, s, CH3-4) ppm; δC ([D6]DMSO, 100 MHz): 159.72 (-CO), 112.29 (C-3), 152.63 (C-4), 133.08 (C-5), 120.48 (C-6), 147.12 (C-7), 112.98 (C-8), 153.32 (C-9), 115.39 (C-10), 103.82 (C-1′), 102.72 (C-1″), 71.38 (C-2′), 70.64 (C-2″), 73.26 (C-3′), 72.61 (C-3″), 68.16 (C-4′), 68.02 (C-4″), 75.97 (C-5′), 75.76 (C-5″), 60.45 (C-6′), 60.19 (C-6″), 18.23 (CH3-4) ppm; ESIMS: m/z 517 [M+] (7%), 518 [M+1] (15%).
Anal. Calcd. for C22H28O14 (%): C, 51.16, H, 5.47; Found: C, 51.13, H, 5.43.
7,8-di-O-(β-D-galactopyranosyl)-6-methoxycoumarin 23 (706.17 mg, 97%), Rf 0.35 (1:3 MeOH-EtOAc), δH ([D6]DMSO, 400 MHz): 6.41 (1 H, d, J = 8.0 Hz, H-3), 7.96 (1 H, d, J = 8.0 Hz, H-4), 7.15 (1 H, s, H-5), 5.36 (1 H, br, HO-2′, exchangeable with D2O), 5.32 (1 H, br, HO-2″, exchangeable with D2O), 4.46 (2 H, br, HO-3′, 3″, exchangeable with D2O), 4.88 (2 H, br, HO-4′, 4″, exchangeable with D2O), 4.57 (2 H, br, HO-6′, 6″, exchangeable with D2O), 5.22 (1H, d, J1′,2 = 8.0 Hz, H-1′), 5.26 (1 H, d, J1″,2″ = 8.0 Hz, H-1″), 3.72 (2 H, dd, J2′,3′ = J2″,3″ = 10.0 Hz, H-2′, 2″), 3.57 (2 H, dd, J3′,4′ = J3″,4″ = 4.0 Hz, H-3′, 3″), 3.40 (2 H, d, J4′,5′ = J4″,5″ = 3.8 Hz, H-4′, 4″), 3.46 (2 H, m, H-5′, 5″), 3.75 (4 H, m, H-6′, 6″), 3.83 (3 H, s, CH3O-6) ppm; δC ([D6]DMSO, 100 MHz): 159.94 (-CO), 105.99 (C-3), 144.23 (C-4), 141.58 (C-5), 136.52 (C-6), 142.56 (C-7), 114.38 (C-8), 149.89 (C-9), 114.83 (C-10), 103.33 (C-1′), 103.23 (C-1″), 71.31 (C-2′), 71.26 (C-2″), 73.26 (C-3′), 73.18 (C-3″), 68.06 (C-4′, 4″), 75.88 (C-5′), 75.82 (C-5″), 60.09 (C-6′, 6″), 56.65 (CH3O-6) ppm; ESIMS: m/z 533 [M+] (8%), 534 [M+1] (16%).
Anal. Calcd. for C22H28O15 (%): C, 49.63, H, 5.30; Found: C, 49.59, H, 5.26.
Preparation of stable lacZ transfected MCF7 and PC3 cell lines
E.coli lacZ
gene (from pSV-β-gal vector, Promega, Madison, WI) was inserted into high expression human cytomegalovirus (CMV) immediate-early enhancer/promoter vector phCMV (Gene Therapy Systems, San Diego, CA) giving a recombinant vector phCMV/lacZ. This was used to transfect wild type MCF7 (human breast cancer) and PC3 (human prostate cancer) cells (ATCC, Manassas, VA) using GenePORTER2 (Gene Therapy Systems, Genlantis, Inc., San Diego, CA), as described in detail previously [24, 25]. The highest β-gal expressing colony was selected using the antibiotic G418 disulfate (Research Products International Corp, Mt Prospect, IL, USA); 800 μg/ml) and G418 (200 μg/ml) was also included for routine culture. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Mediatech Inc., Herndon, VA, USA) containing 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA) with 100 units/ml of penicillin, 100 units/ml streptomycin, and cultured in a humidified 5% CO2 incubator at 37°C. The β-gal activity of tumor cells was measured using a β-gal assay kit with o-nitrophenyl-β-D-galactopyranoside (Promega, Madison, WI).
MRI
MRI studies were performed using a 4.7 T horizontal bore magnet equipped with a Varian INOVA Unity system (Palo Alto, CA, USA). T1 and T2 maps were acquired using a spin-echo sequence with varying repetition times (TR) and echo times (TE), respectively.
Acknowledgments
This research was supported in part by the NIH National Cancer Institute (R21 CA120774) and the Southwestern Small Animal Imaging Research Program (SW-SAIRP), which is supported in part by U24 CA126608 and P30 CA142543. NMR experiments were performed at the Advanced Imaging Research Center, an NIH BTRP facility (P41RR02584). We are also grateful to Dr. Jian Zhou and Mindy Zhou for technical assistance.
Contributor Information
Jian-Xin Yu, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-9058, USA.
Praveen K. Gulaka, Joint Program in Biomedical Engineering, The University of Texas at Arlington and The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-9058, USA
Li Liu, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-9058, USA.
Vikram D. Kodibagkar, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-9058, USA.
Ralph P. Mason, Department of Radiology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, Texas 75390-9058, USA.
References
- 1.Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, Phelps ME, Larson SM, Balatoni J, Finn R, Sadelain M, Tjuvajev J, Blasberg R. Neoplasia (New York) 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilad AA, Winnard PT, van Zijl PCM, Bulte JWM. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 3.Kruger A, Schirrmacher V, Khokha R. Cancer Metastasis Rev. 1999;17:285–294. doi: 10.1023/a:1006066706040. [DOI] [PubMed] [Google Scholar]
- 4.Serebriiskii IG, Golemis EA. Anal Biochem. 2000;285:1–15. doi: 10.1006/abio.2000.4672. [DOI] [PubMed] [Google Scholar]
- 5.James AL, Perry JD, Chilvers K, Robson IS, Armstrong L, Orr KE. Letters Appl Microbiol. 2000;30:336–340. doi: 10.1046/j.1472-765x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- 6.Browne NK, Huang Z, Dockrell M, Hashmi P, Price RG. J Appl Microbiol. 2009;108:1828–1838. doi: 10.1111/j.1365-2672.2009.04588.x. [DOI] [PubMed] [Google Scholar]
- 7.Nolan GP, Fiering S, Nicolas JF, Herzenberg LA. Proc Natl Acad Sci (USA) 1988;85:2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. Cancer Res. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- 9.Kamiya M, Kobayashi H, Hama Y, Koyama Y, Bernardo M, Nagano T, Choyke PL, Urano Y. J Am Chem Soc. 2007;129:3918–3929. doi: 10.1021/ja067710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehrman TS, von Degenfeld G, Krutzik P, Nolan GP, Blau HM. Nature Methods. 2006;3:295–301. doi: 10.1038/nmeth868. [DOI] [PubMed] [Google Scholar]
- 11.Takayasu S, Maeda M, Tsuji A. J Immunolog Methods. 1985;83:317–325. doi: 10.1016/0022-1759(85)90253-4. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Kirn TJ, Artis D, Waldman SA, Kricka LJ. Luminescence. 2009;25:463–465. doi: 10.1002/bio.1173. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Mason RP. Plos One. 2010;5:e12024. doi: 10.1371/journal.pone.0012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celen S, Deroose C, de Groot T, Chitneni SK, Gijsbers R, Debyser Z, Mortelmans L, Verbruggen A, Bormans G. Bioconj Chem. 2008;19:441–449. doi: 10.1021/bc700216d. [DOI] [PubMed] [Google Scholar]
- 15.Van Dort ME, Lee KC, Hamilton CA, Rehemtulla A, Ross BD. Molec Imaging. 2008;7:187–197. [PMC free article] [PubMed] [Google Scholar]
- 16.Moats RA, Fraser SE, Meade TJ. Angew Chem Int Ed. 1997;36:726–728. [Google Scholar]
- 17.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. Nature Biotechnol. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 18.Chang YT, Cheng CM, Su YZ, Lee WT, Hsu JS, Liu GC, Cheng TL, Wang YM. Bioconj Chem. 2007;18:1716–1727. doi: 10.1021/bc070019s. [DOI] [PubMed] [Google Scholar]
- 19.Chauvin T, Durand P, Bernier M, Meudal H, Doan BT, Noury F, Badet B, Beloeil JC, Toth E. Angew Chem Int Ed. 2008;47:4370–4372. doi: 10.1002/anie.200800809. [DOI] [PubMed] [Google Scholar]
- 20.Cui W, Liu L, Kodibagkar VD, Mason RP. Magn Reson Med. 2010;64:65–71. doi: 10.1002/mrm.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui W, Otten P, Li Y, Koeneman K, Yu J, Mason RP. Magn Reson Med. 2004;51:616–620. doi: 10.1002/mrm.10719. [DOI] [PubMed] [Google Scholar]
- 22.Yu JX, Otten P, Ma Z, Cui W, Liu L, Mason RP. Bioconj Chem. 2004;15:1334–1341. doi: 10.1021/bc049936d. [DOI] [PubMed] [Google Scholar]
- 23.Kodibagkar VD, Yu J, Liu L, Hetherington HP, Mason RP. Magn Reson Imaging. 2006;24:959–962. doi: 10.1016/j.mri.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Kodibagkar VD, Yu JX, Mason RP. FASEB J. 2007;21:2014–2019. doi: 10.1096/fj.06-7366lsf. [DOI] [PubMed] [Google Scholar]
- 25.Yu JX, Kodibagkar VD, Liu L, Mason RP. NMR Biomed. 2008;21:704–712. doi: 10.1002/nbm.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizukami S, Matsushita H, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Chemical Sci. 2011;2:1151–1155. [Google Scholar]
- 27.Li L, Zemp RJ, Lungu G, Stoica G, Wang LHV. J Biomed Optics. 2007;12:020504. doi: 10.1117/1.2717531. [DOI] [PubMed] [Google Scholar]
- 28.Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, Gewirtz DA, Holt SE. J Biol Chem. 2002;277:35509–35515. doi: 10.1074/jbc.M205477200. [DOI] [PubMed] [Google Scholar]
- 29.Bassaneze V, Miyakawa AA, Krieger JE. Anal Biochem. 2008;372:198–203. doi: 10.1016/j.ab.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson NE, Brown G, Scott EW, Walter GA. Magn Reson Med. 2010;63:745–753. doi: 10.1002/mrm.22235. [DOI] [PubMed] [Google Scholar]
- 31.Heuermann K, Cosgrove J. Biotechniques. 2001;30:1142–1147. doi: 10.2144/01305pf01. [DOI] [PubMed] [Google Scholar]
- 32.Schwert DD, Richardson N, Ji GJ, Raduchel B, Ebert W, Heffner PE, Keck R, Davies JA. J Med Chem. 2005;48:7482–7485. doi: 10.1021/jm0501984. [DOI] [PubMed] [Google Scholar]
- 33.Davies JA, Dutremez SG, Hockensmith CM, Keck R, Richardson N, Selman S, Smith DA, Ulmer CW, Wheatley LS, Zeiss J. Acad Radiol. 1996;3:936–945. doi: 10.1016/s1076-6332(96)80305-9. [DOI] [PubMed] [Google Scholar]
- 34.Raymond KN, Muller G, Matzanke BF. Topics Curr Chem. 1984;123:49–102. [Google Scholar]
- 35.Wu L, Wang X, Xu W, Farzaneh F, Xu R. Curr Med Chem. 2009;16:4236–4260. doi: 10.2174/092986709789578187. [DOI] [PubMed] [Google Scholar]
- 36.Sharma GVM, Reddy JJ, Lakshmi PS, Krishna PR. Tetrahedron Letters. 2005;46:6119–6121. [Google Scholar]
- 37.James AL, Perry JD, Ford M, Armstrong L, Gould FK. J Appl Microbiol. 1997;82:532–536. doi: 10.1046/j.1365-2672.1997.00370.x. [DOI] [PubMed] [Google Scholar]
- 38.Shamis M, Barbas CF, Iii, Shabat D. Bioorg Med Chem Letters. 2007;17:1172–1175. doi: 10.1016/j.bmcl.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 39.d'Antuono P, Botek E, Champagne B, Maton L, Taziaux D, Habib-Jiwan JL. Theoret Chem Acc. 2010;125:461–470. doi: 10.1039/c003514a. [DOI] [PubMed] [Google Scholar]
- 40.Yu JX, Ma Z, Li Y, Koeneman KS, Liu L, Mason RP. Med Chem. 2005;1:255–262. doi: 10.2174/1573406053765495. [DOI] [PubMed] [Google Scholar]
- 41.Yu JX, Mason RP. J Med Chem. 2006;49:1991–1999. doi: 10.1021/jm051049o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu JX, Liu L, Kodibagkar VD, Cui W, Mason RP. Bioorg Med Chem. 2006;14:326–333. doi: 10.1016/j.bmc.2005.08.021. [DOI] [PubMed] [Google Scholar]