Abstract
Background & Aims
Dietary abscisic acid (ABA) has shown efficacy in ameliorating experimental IBD in mice through mechanisms requiring expression of peroxisome proliferator activated-receptor γ (PPAR γ) in immune cells. The goal of this study was to determine whether PPAR γ expression in colonic epithelial cells is required for the anti-inflammatory actions of ABA.
Methods
Conditional knockout mice expressing a transgenic recombinase in intestinal epithelial cells under the control of a villin promoter (PPAR γ flfl; Villin Cre+ or VC+) with defective expression of PPAR γ in intestinal cells (IEC) and PPAR γ-expressing wild type (PPAR γ flfl; Villin Cre− or VC−) mice in a C57BL/6 background were fed diets with and without ABA (0.1 g/kg) for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) in the drinking water for 7 days. Clinical disease severity was assessed daily and colonic lesions on day 7 through macroscopic and histopathological examination. Immune cell phenotypes were examined systemically and at the mesenteric lymph nodes (MLN). Epithelial gene expression was assayed in the colon.
Results
Dietary ABA-supplementation prevented colitis, reduced disease severity, improved colonic histopathology, and upregulated epithelial lanthionine synthetase C-like protein 2 (LANCL2) expression in VC+ mice. Dietary ABA significantly increased the percentages of MLN CD4+IL-10+ T cells, and blood CD4+CD25+FoxP3+ T cells and CD8+IL-10+ T cells.
Conclusion
Expression of PPAR γ in IECs was not required for the anti-inflammatory efficacy of ABA in IBD. LANCL2 in IECs and T cell-derived IL-10 may be implicated in the mechanism underlying ABA’s immune modulatory activity in IBD.
Keywords: Inflammatory bowel disease, ulcerative colitis, Crohn’s disease, abscisic acid, PPARγ, intestinal epithelial cells, lanthionine synthetase C-like protein 2
Introduction
Inflammatory bowel disease (IBD), with its two clinical manifestations Crohn’s Disease (CD) and ulcerative colitis (UC), is characterized by a chronic remitting and relapsing course with varying degrees of mucosal inflammation and immune cell infiltration into the gastrointestinal mucosa [1-3]. IBD arises from the interaction of genetic factors, environmental risks, the gut microbiota, and the immune system. CD and UC are immune-mediated diseases ultimately caused by immune dysregulation [1-5]. Current IBD treatment are modestly effective and associated with significant complications, therefore, there is a critical need for developing more effective prophylactic and therapeutic interventions against IBD [6].
The gut mucosal surface is covered with a thick mucous layer and a one-cell-thick mucosal epithelium, and forms an interface between the external and internal environments. Gastrointestinal epithelial cells provide a unique immunologic homeostasis barrier against microbial pathogens and represent a key component of mucosal innate immunity [7]. Damage to the mucosal immune system, followed by exposure of the lamina propria (LP) to microbial antigens can trigger immuno-inflammatory responses; thus, promoting the pathological characteristics of IBD [1,2,8]. A comprehensive array of immune competent cells reside within the gastrointestinal LP including polymorphonuclear neutrophils, dendritic cells, macrophages, T cells, plasma cells, natural killer cells and eosinophils [1,2]. Epithelial cells regulate the migration, differentiation and activation of these immune cells by releasing immune mediators, including growth factors, cytokines and chemokines [2].
Most epithelial and immune cells in the colonic mucosa express peroxisome proliferator-activated receptor (PPAR γ), a nuclear receptor that plays an important role in the regulation of IBD due to its anti-inflammatory properties [8,9] arising from its ability to antagonize NF-kB, STAT and AP-1 [10]. Human colonic epithelial cells from healthy individuals express high levels of PPAR γ [7,11]. However, expression of PPAR γ is impaired in the IECs from IBD patients [8]. Accordingly, a possible approach to suppress the release of pro-inflammatory stimuli from intestinal epithelial cells (IECs) may be through activation of IEC PPAR γ [9,12], thereby offering a potential therapeutic target for inflammatory enteropathies.
ABA has shown preventive or therapeutic efficacy against chronic diseases such as diabetes [13], obesity [14], atherosclerosis [15] and IBD [16,17]. Previously we reported that dietary ABA ameliorates experimental IBD by down-regulating cellular adhesion molecule expression and suppressing immune cell infiltration [16]. Further, T cell PPAR γ was required for the anti-inflammatory efficacy of ABA against experimental IBD [17], thereby suggesting a high potential for using ABA to treat gut inflammatory diseases. Our previous studies used mice lacking PPAR γ either broadly on both immune and epithelial cells or on T cells. Using T cell-specific PPAR γ deficient mice we demonstrated that ABA’s anti-inflammatory effect required expression of PPAR γ in T cells [17]. Indeed, T cell-specific PPAR γ null mice did not respond to ABA treatment despite expressing PPAR γ in macrophages, dendritic cells, neutrophils, endothelial and IECs; all of which contribute to DSS colitis. This study aimed to determine whether IEC PPAR γ is required for the anti-inflammatory effect of ABA in DSS colitis. Also, since lanthionine synthetase C-like-2 (LANCL2) has been identified as a putative target of ABA [18], we also examined the impact of ABA on colonic LANCL2.
Research Design and Methods
Dietary treatments and development of experimental colitis
Six to eight week-old PPAR γ fl/fl; Villin-Cre+ (VC+; IEC-specific PPAR γ null) and PPAR γ-expressing floxed Villin-Cre− (VC−; PPARγ floxed), littermates corresponding to a wild-type phenotype all in a C57BL/6 background, were genotyped for the PPAR γ gene using previously published genotyping protocols [19,20]. Mice were housed in laboratory animal facilities at the Virginia Tech in a room maintained at 22°C, with a 12-h-light/-dark cycle starting from 0600 hours. Mice were fed purified AIN-93G rodent diets (Table 1) with and without ABA (0.1 g/kg) for 35 days prior to induction of colitis with water containing 2.5% dextran sodium sulfate (DSS), 36,000-44,000 mol Wt (ICN Biomedicals, Aurora, OH).
Table 1.
Composition of Experimental Diets
| Ingredient | Control diet (g/kg) | ABA diet (g/kg) |
|---|---|---|
| Casein | 200 | 200 |
| L-cystine | 3 | 3 |
| Corn Starch | 397.5 | 397.5 |
| Maltodextrin | 132 | 132 |
| Sucrose | 100 | 100 |
| Cellulose | 50 | 50 |
| Mineral mix (AIN-93) 1 | 35 | 35 |
| Vitamin mix (AIN-93) 2 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 |
|
Tert-
butylhydroquinone 3 |
0.014 | 0.014 |
| Soybean Oil | 70 | 70 |
| Abscisic acid 3 | 0 | 0.1 |
Per kg of diet: 3 g nicotinic acid, 1.6 g calcium pantotenate, 0.7 g pyridoxine HCl, 0.6 g thiamin HCl, 0.6 g riboflavin, 0.2 g folic acid, 0.02 g D-biotin, 2.5 g vitamin B12 (0.1% in mannitol), 15 g d,l-α tocopheryl acetate (500 IU/g), 0.8 g vitamin A palmitate (500,000 IU/g), 0.2 g vitamin D3 (cholecalciferol, 500,000 IU/g), 0.075 g vitamin K (phylloquinone), and 974.705 g sucrose.
Per kg of diet: 357 g calcium carbonate, 196 g potassium phosphate monobasic, 70.78 g potassium citrate, 74 g sodium chloride, 46.6 g potassium sulfate, 24.3 g magnesium oxide, 6.06 g ferric citrate, 1.65 g zinc carbonate, 0.63 g manganous carbonate, 0.31 g cupric carbonate, 0.01 g potassium iodate, 0.01025 g sodium selenate, 0.00795 g ammonium paramolybdate, 1.45 g sodium meta-silicate, 0.275 g chromium potassium
Abscisic acid (ABA), or (2-cis,4-trans)-5-(1-Hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexen-1-yl)-3-methyl-2,4-pentadienoic acid, was added as a 50:50 mixture of the (S)-5 and (R)-5 isomers (Sigma).
Assessment of colitis
After the DSS challenge mice were weighed on a daily basis and examined for clinical signs of the disease associated with colitis (i.e., perianal soiling, rectal bleeding, diarrhea, and piloerection). For the DSS challenge, the disease activity indices and rectal bleeding scores were calculated using a modification of a previously published compounded clinical scoring [19]. Briefly, disease activity index consisted of a scoring for diarrhea and lethargy (0-3), whereas rectal bleeding consisted of a visual observation of blood in feces and the perinanal area (0 - 4). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Tech.
Histopathology
Colonic samples were fixed in 10% buffered formalin, embedded in paraffin and sectioned at 5 μm. Sections were stained with hematoxylin and eosin (H&E) and then examined for microscopic lesions and alterations in the mucosal architecture as previously described [21]. Sections were graded for leukocyte infiltration, mucosal thickening, and epithelial cell erosion. For each category, the sections were graded for the severity with a score of 0 – 4 and analyzed as a compounded score.
Real-time quantitative RT-PCR
Epithelial cells were isolated from colonic tissue by incubating the minced tissue in 10 ml of CMF/FBS/EDTA for 15 minutes. The samples were then passed through a 100− μm filter and the supernatant containing the epithelial cells was then centrifuged at 1200 rpm for 10 minutes. The resulting epithelial cell pellet was then lysed with 600 μl of RLT buffer (Qiagen). Total RNA was isolated from epithelial cells using the RNA isolation Minikit (Qiagen) according to the manufacturer’s instructions. Total RNA (1 g) was used to generate complementary DNA (cDNA) template using the iScript cDNA synthesis Kit (Bio-Rad, Hercules, CA). The total reaction volume was 20 l, with reaction incubated as follows in a MJ MiniCycler: 5 min at 25°C, 30 min at 52°C, 5 min at 85°C, and hold at 4°C. PCR was performed on the cDNA using Taq DNA polymerase (Invitrogen, Carlsbad, CA) and using previously described conditions [19]. Each gene amplicon was purified with the MiniElute PCR Purification Kit (Qiagen) and quantitated on an agrose gel by using a DNA mass ladder (Promega). These purified amplicons were used to optimize real-time PCR conditions and to generate standard curves in the real-time PCR assay. Primer concentrations and annealing temperatures were optimized for the iCycler iQ System (Bio-Rad) for each set of primers using the system’s gradient protocol. PCR efficiencies were maintained above 90%, and correlation coefficients were maintained above 0.98 for each primer set during optimization and also during the real-time PCR of sample DNA. Results are presented as the starting quantity of target cDNA pg/μg of total RNA.
Flow Cytometry
MLN and spleen were excised, crushed with frosted slides and resuspended in PBS. The splenocytes were subjected to red blood cells lysis. Cells isolated from spleen and MLN were enumerated with a Coulter Counter (Beckman Coulter, Fullerton, CA). MLN and spleen-derived cells (2 × 105 cells/well) were then seeded onto 96-well plates, centrifuged at 3000 rpm at 4°C for 4 minutes, and w ashed with PBS containing 5% fetal bovine serum and 0.09% sodium azide (FACS buffer). To assess differential monocyte/macrophage subsets, the cells were then incubated in the dark at 4°C for 20 minutes in FcBlock (BD Pharmingen), and then for an additional 20 minutes with fluorochrome-conjugated primary antibodies. For lymphocyte assessment, cells were incubated with anti-CD4-Alexa Fluor 700 (BD Pharmingen), anti-CD8-PerCp-Cy5.5 (eBioscience), anti-CD3 PE-Cy5 (BD Pharmingen), anti-CD25-FITC (BD Pharmingen), anti-CD44-APC (BD Pharmingen), anti-FoxP3-PE (ebioscience), anti-IFN-γ-PE (ebioscience), anti-GATA3-PE (eBioscince) and anti-IL10-FITC (eBioscience) as previously shown [22]. Flow results were computed with a BD LSR II flow cytometer and data analyses was performed by using the FACS Diva software (BD).
Statistics
Analysis of variance (ANOVA) was performed by using the general linear model procedure of Statistical Analysis Software (SAS Institute Inc., Cary, NC) as previously described [23]. ANOVA was used to determine the statistical significance of the model for the main effects (diet, genotype) and interaction (diet × genotype). When the model was significant, the analysis was followed by Fisher’s Least Significant Difference multiple comparisons method. For the disease activity index results over time were analyzed as a 2-way (diet × genotype) and as a 3-way (diet × genotype × time) repeated measures ANOVA. Data were expressed as the mean ± standard error of the mean. Statistical significance was assessed at a probability value of P< 0.05.
Results
Dietary ABA decreases clinical activity in IBD
IEC-specific PPAR γ null (VC+) and PPAR γ-expressing (VC−) mice, challenged with 2.5% DSS from day 0 to 7, were assessed for disease activity index (DAI) scores (Figure 1). Dietary ABA ameliorated DAI both in VC− and VC+ mice when compared to mice fed control diets, although its beneficial effects on disease activity were greater in VC− mice. Dietary ABA-supplementation significantly reduced inflammation, regardless of the PPAR γ genotype, in colon, MLN and spleen (Figure 2).
Figure 1.
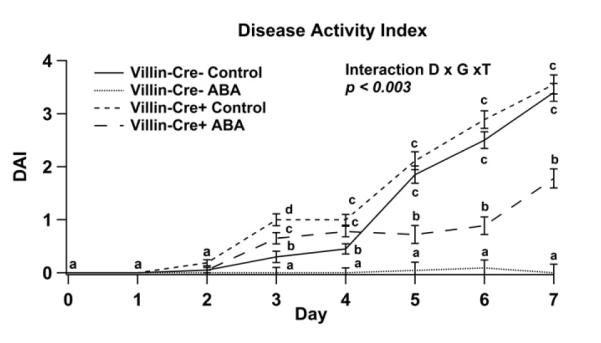
Effect of dietary abscisic acid (ABA)-supplementation on disease severity. C57BL/6J mice were fed ABA supplemented (0.1 g/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) in the drinking water for 7 days. Disease activity index (DAI), a composite score reflecting clinical signs of the disease were assessed daily for intestinal epithelial cell-specific PPAR γ null (VC+) and PPAR γ expressing (VC−) mice undergoing ABA fed and control diet. Significant effects (P<0.05) of the genotype (G) by diet (D) by time (T) interaction are shown for DAI. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Figure 2.
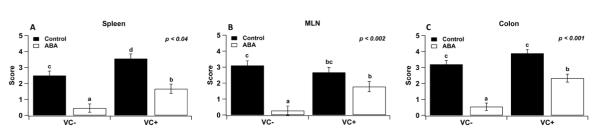
Effect of dietary abscisic acid (ABA)-supplementation on disease severity: a macroscopically evaluation. C57BL/6J mice were fed ABA supplemented (0.1 g/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) in the drinking water for 7 days. On day 7, intestinal epithelial cell-specific PPAR γ null (VC+) and PPAR γ-expressing (VC−) mice fed ABA and control diets were euthanized, and colon, spleen, and mesenteric lymph nodes (MLN) were scored for inflammatory lesions. Significant effects (P<0.05) of the genotype (G) by diet (D) interaction are shown. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Dietary ABA ameliorates colonic immunopathology in mice with IBD
To more closely examine the effect of ABA in experimental IBD, H&E-stained colonic specimens were examined microscopically for the presence of inflammatory lesions. In agreement with reduced DAI scores, ABA-fed mice showed significant histological improvements in the colon. Specifically, ABA significantly decreased leukocytic infiltration, mucosal thickness and epithelial erosion in the colonic mucosa when compared with control groups regardless of PPAR γ expression in IECs (Figure 3).
Figure 3.

Effect of dietary abscisic acids (ABA)-supplementation on colonic histopathology. Intestinal epithelial cell-specific PPAR γ null (VC+) and PPAR γ-expressing (VC−) mice fed ABA (0.1 g/kg) and control diets for 42 days and challenged with 2.5% dextran sodium sulfate (DSS) water for 7 days. All specimens underwent blinded histological examination for epithelial erosion (A), mucosal thickening (B), and leukocyte infiltration (C). The main effects of diet (D) for erosion, mucosal thickening and infiltration are P<0.001, 0.005 and 0.004, respectively. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Effect of dietary ABA on LANCL2 mRNA expression in colonic epithelial cells from mice with IBD
Lanthionine synthetase C-like protein 2 has been identified as a potential target of ABA in mouse models of inflammation [24]. To characterize the effect of ABA on LANCL2 expression in the epithelial compartment we isolated the colonic epithelium and used it for gene expression analyses. Dietary ABA upregulated LANCL2 mRNA expression in colonic epithelia of IEC-specific PPAR γ null mice with experimental IBD (Figure 4).
Figure 4.
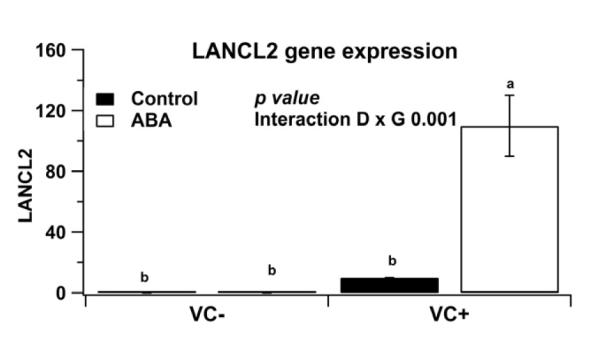
Effect of dietary abscisic acid (ABA)-supplementation on colonic epithelial lanthionine synthetase C-like protein 2 (LANCL2) mRNA expression. C57BL/6J mice were fed with ABA-supplement (0.1 g/kg) or control diets for 35 days and challenged with 2.5% dextran sodium sulfate (DSS) water for 7 days. Expression of LANCL2 mRNA was assessed in colonic epithelial cells by real-time quantitative PCR. Significant effects (P<0.05) of the genotype (G) by diet (D) interaction are shown. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Effect of dietary ABA on immune cell subsets in MLN, blood and spleen
Blood, spleen and MLN-derived mononuclear cells from VC− and VC+ mice were immunophenotyped on day 7 to better understand the immunoregulatory effects of ABA systemically and at the gut mucosa. Dietary ABA significantly increased the percentages of CD4+ T cells, regardless of the genotype (Figure 5) in blood, spleen and MLN. In contrast, dietary ABA resulted in significantly decreased percentages of CD8+ T cells regardless of the genotype (Figure 5) with the exception of spleen CD8+ T cells (Figure 5E). On day 7 of DSS challenge, the percentages of CD4+CD25+FoxP3+T cells in blood were significantly augmented in ABA-fed PPAR γ-expressing mice, although the deficiency of PPAR γ in IECs abrogated this effect (Figure 6A). However, the percentages of CD4+CD25+FoxP3+Treg cells were significantly decreased in spleen and MLN of ABA-fed mice when compared with control–fed mice regardless of the PPAR γ genotype (Figure 6A and 6C), suggesting an effect of ABA on Treg tissue distribution. Surprisingly, the percentages of CD4+CD44+ T cells were significantly higher in blood and spleen of PPAR γ-expressing mice fed ABA when compared with untreated mice, but this effect was abrogated by the deficiency of PPAR γ in IECs (Figure 6D and 6E). Consistent with the anti-inflammatory actions of ABA, the percentages of CD4+IL-10+ T cells in MLN and CD8+IL10+ T cells in blood were significantly increased in ABA-fed mice regardless of the PPAR γ genotype in IECs (Figure 7 A and B).
Figure 5.

Effect of dietary abscisic (ABA)-supplementation on CD4+ and CD8+ T cell subsets in blood, spleen and mesenteric lymph nodes (MLN) of conditional knockout mice with intestinal epithelial cell-specific PPAR γ deficiency (VC+) and PPARγ-expressing (VC−) mice. Significant effects (P<0.05) of the genotype (G) by diet (D) interaction and the main effects of D are shown. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Figure 6.

Effect of dietary abscisic (ABA)-supplementation on lymphocyte subsets in blood, spleen and mesenteric lymph nodes (MLN) of mice with intestinal epithelial cell-specific PPARγ deficiency (VC+) and PPARγ expressing (VC−) mice. Percentages of CD4+CD25+FoxP3+ T cells in blood (A), spleen (B), and MLN (C). Percentages of CD4+CD44+ T cells in blood (D) and spleen (E). Significant effects (P<0.05) of genotype (G) by diet (D) interaction and the main effects of D are shown. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Figure 7.
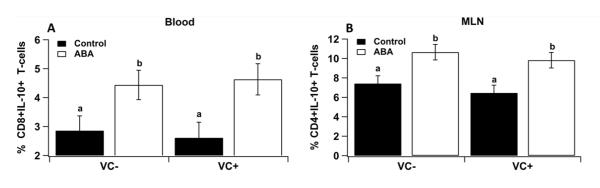
Effect of dietary abscisic (ABA)-supplementation on lymphocyte subsets in blood, spleen and mesenteric lymph nodes (MLN) of mice with intestinal epithelial cell-specific PPAR γ deficiency (VC+) and PPARγ expressing (VC−) mice. CD8+IL10+ T cells in blood (A), and CD4+IL10+ T cells in MLN (B). The main effects of diet (D) for blood CD8+IL10+ T cells and MLN CD4+IL10+ T cells are P<0.04 and 0.005, respectively. Values are means ± SEM, n = 10. Means without a common letter differ, P<0.05.
Discussion
ABA is a naturally occurring isoprenoid phytohormone critical for plant development, including response to abiotic stress [25]. In addition, ABA can be detected in human monocytes and the central nervous system [26]. However, the function of ABA in mammalian cells is incompletely understood. ABA promotes fusion of synthetic phospholipid vesicles in vitro [27] and the intracellular levels of ABA are increased in heat-stressed granulocytes and during the process of phagocytosis [26]. Moreover, monocytes release ABA when exposed to thrombin-activated platelets, which indicates that ABA can act as a human paracrine hormone [26]. Recently, ABA has been identified as a signal molecule in vivo involved in a regulation of inflammation [28].
We provide evidence showing that dietary ABA-supplementation significantly suppressed colonic inflammation and reduced the severity of DSS-induced colitis. ABA’s anti-inflammatory effect was generally independent of PPAR γ activation in colonic epithelial cells, although some effects involving T cells showed a PPAR γ dependency. Previous results from our laboratory demonstrate that the anti-inflammatory effects of ABA on white adipose tissue inflammation are partially dependent on the nuclear receptor PPAR γ [14]. We have also showed that ABA down-regulates gut adhesion molecules and suppresses inflammation in mice with DSS colitis [16]. On the other hand, we demonstrated that T cell PPAR γ activation is required for the anti-inflammatory efficacy of ABA in mice with colitis [17].
This study demonstrates for the first time that PPAR γ expression in the intestinal epithelium is not required to achieve the full spectrum of ABA’s anti-inflammatory effect in the colonic mucosa. However, it is important to note that T cell PPAR γ is critical for mediating ABA’s immune modulatory actions since the loss of PPAR γ in T cells abrogates the anti-inflammatory effect of this compound against DSS colitis [17]. Therefore, there appears to be distinct cell specific (i.e., T cells versus epithelial cells) mechanisms of action orchestrating the anti-inflammatory effects of ABA in the gut mucosa. This observation in nontrivial given that ABA is not a direct ligand of PPAR γ’s ligand-binding domain (LBD) and it is believed to activate the PPAR γ pathway indirectly by binding to upstream proteins such as LANCL2 and triggering a signaling cascade [24].
Flow cytometry analyses demonstrated that the percentages of CD4+ T cells in blood, spleen and MLN were significantly increased in ABA-fed mice regardless of the PPAR γ genotype in IECs. With the increase in CD4+ T cells there was a corresponding decrease in the percentages of CD8+ T cells in MLN following dietary ABA supplementation. Additionally, MLN CD4+IL-10+ T cells and blood CD4+CD25+Foxp3+ Treg cells and CD8+IL-10+ T cells were significantly increased in ABA-fed mice regardless of the PPAR γ genotype in IECs. Moreover, the percentages of CD4+CD44+ T cells were significantly increased in blood and spleen of PPAR γ-expressing mice fed ABA, but this immune modulatory effect was abrogated in IEC-specific PPAR γ null mice. CD44 is a widely expressed adhesion receptor associated with a diverse set of biological processes involving migrating cells, including inflammation, angiogenesis, bone metabolism and wound healing [29]. CD44 expression is indicative of an effector-memory T-cell phenotype [29]. Thus, dietary ABA supplementation favored an increase the percentages of memory CD4+ T cells through a PPARγdependent mechanism.
The increased percentages of MLN CD4+IL-10+ T cells, blood CD8+IL-10+ T cells and blood Treg cells observed in ABA-fed mice were consistent with the predominance of a regulatory phenotype favored by ABA at the gut mucosa and systemically. IL-10 is a potent anti-inflammatory cytokine that can suppress the antigen presentation capacity of antigen presenting cells and thereby helps in maintaining immune homeostasis and tolerance to self-antigens. Indeed, patients with Crohn’s disease demonstrate clinical improvements in disease activity following treatment with bacteria producing recombinant IL-10, delineating the importance of IL-10 for counteracting excessive or uncontrolled immune responses [30]. Therefore, the ability of dietary ABA to increase the percentages of CD4+IL10+ T cells in mucosal inductive sites might contribute to lowering the severity of local inflammation in PPAR γ expressing mice. This finding is consistent with a previous report showing increased percentages of CTLA4-expressing CD4+ T cells in blood of ABA-fed mice [17].
The mammalian peptide-modifying lanthionine synthetase c-like protein (LANCL) family which shares a high amino acid identity with G protein coupled receptor 2 (GCR2), has been proposed as one of the ABA receptors [31]. Recently, LANCL2 has been identified as a critical component of the ABA-sensing protein complex [32]. Moreover, LANCL2 knockout studies demonstrated that ABA-mediated activation of macrophage PPAR γ was dependent on LANCL2 expression [24], suggesting a potential cross-talk between PPAR γ and LANCL2. Furthermore, ABA treatment increased cAMP accumulation in immune cells [24], indicating that additional mechanisms of action are implicated in the anti-inflammatory effects of ABA. Herein we demonstrate that dietary ABA upregulated LANCL2 mRNA expression in colonic epithelial cells isolated from IEC-specific PPAR γ deficient mice, providing in vivo evidence supporting a potential crosstalk between PPAR γ and LANCL2 in intestinal epithelium of potential significance to maintaining barrier function. Surprisingly, the loss of PPAR γ in IECs resulted in upregulation of LANCL2 in epithelial cells, suggesting that PPAR γ may play an inhibitory role in the transcriptional regulation of LANCL2 through a feedback loop. The molecular mechanisms underlying the suppressive effect of ABA in intestinal epithelial inflammation remain incompletely understood, however, both PPAR γ and LANCL2 seem to be implicated in the prevention of experimental IBD by this compound. This study demonstrates for the first time that the PPAR γ activation in colonic epithelial cells is not required for ABA to exert its anti-inflammatory effects during experimental IBD, thereby shedding new light on the mechanism by which ABA regulates mucosal homeostasis and ameliorates IBD. Based on the results of this study showing that IEC PPAR γ is dispensable and previous studies showing the importance of T cells and macrophages [18], we propose that T cells and macrophages might be the likely cellular targets of ABA involved in colitis prevention. This pattern of cellular specificity resembles that of conjugated linoleic acid [33], another natural compound with potent gut anti-inflammatory effects.
Acknowledgements
This research was supported by grant number 5R01AT004308 of National Center for Complementary and Alternative Medicine at the National Institute of Health to J.B-R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement No conflicts
Statement of authorship JBR and RH designed research, conducted research, analyzed data, and wrote paper. All authors have read and approved the final manuscript
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg RS. Inflammation in the intestinal tract: Pathogenesis and treatment. Dig Dis. 2009;27:455–64. doi: 10.1159/000235851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braus NA, Elliott DE. Advances in the pathogenesis and treatment of ibd. Clin Immunol. 2009;132:1–9. doi: 10.1016/j.clim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih DQ, Targan SR. Insights into ibd pathogenesis. Curr Gastroenterol Rep. 2009;11:473–80. doi: 10.1007/s11894-009-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer L. Evolving paradigms in the pathogenesis of ibd. J Gastroenterol. 45:9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 6.Fiocchi C. Future of ibd pathogenesis: How much work is left to do? Inflamm Bowel Dis. 2008;14(Suppl 2):S145–7. doi: 10.1002/ibd.20659. [DOI] [PubMed] [Google Scholar]
- 7.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–53. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, et al. Ppargamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341–9. doi: 10.1136/gut.2006.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 11.Lyles BE, Akinyeke TO, Moss PE, Stewart LV. Thiazolidinediones regulate expression of cell cycle proteins in human prostate cancer cells via ppargamma-dependent and ppargamma-independent pathways. Cell Cycle. 2009;8:268–77. doi: 10.4161/cc.8.2.7584. [DOI] [PubMed] [Google Scholar]
- 12.Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, et al. Immunoregulatory actions of epithelial cell ppar gamma at the colonic mucosa of mice with experimental inflammatory bowel disease. PLoS One. 2010;5:e10215. doi: 10.1371/journal.pone.0010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guri AJ, Hontecillas R, Ferrer G, Casagran O, Wankhade U, Noble AM, et al. Loss of ppar gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19:216–28. doi: 10.1016/j.jnutbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–16. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Guri AJ, Misyak S, Hontecillas R, Hasty A, Liu D, Si H, et al. Abscisic acid ameliorates atherosclerosis by suppressing macrophage and cd4+ t cell recruitment into the aortic wall. Journal of Nutritional Biochemistry. 2011;21:1178–85. doi: 10.1016/j.jnutbio.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guri AJ, Hontecillas R, Bassaganya-Riera J. Abscisic acid ameliorates experimental ibd by downregulating cellular adhesion molecule expression and suppressing immune cell infiltration. Clinical Nutrition. 2010;29:824–31. doi: 10.1016/j.clnu.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guri AJ, Evans NP, Hontecillas R, Bassaganya-Riera J. T cell ppar gamma is required for the anti-inflammatory efficacy of abscisic acid against experimental inflammatory bowel disease. Journal of Nutritional Biochemistry. 2011;22:812–9. doi: 10.1016/j.jnutbio.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassaganya-Riera J, Guri AJ, Lu P, Climent M, Carbo A, Sobral BW, et al. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of ppar gamma. Journal of Biological Chemistry. 2011;286:2504–16. doi: 10.1074/jbc.M110.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, et al. Activation of ppar gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–91. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 20.Guri AJ, Mohapatra SK, Horne WT, 2nd, Hontecillas R, Bassaganya-Riera J. The role of t cell ppar gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterol. 2010;10:60. doi: 10.1186/1471-230X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassaganya-Riera J, Thacker BJ, Yu S, Strait E, Wannemuehler MJ, Thacker EL. Impact of immunizations with porcine reproductive and respiratory syndrome virus on lymphoproliferative recall responses of cd8+ t cells. Viral Immunol. 2004;17:25–37. doi: 10.1089/088282404322875430. [DOI] [PubMed] [Google Scholar]
- 22.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. Ppar gamma is highly expressed in f4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–46. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassaganya-Riera J, Guri AJ, Noble AM, Reynolds KA, King J, Wood CM, et al. Arachidonic acid-and docosahexaenoic acid-enriched formulas modulate antigen-specific t cell responses to influenza virus in neonatal piglets. Am J Clin Nutr. 2007;85:824–36. doi: 10.1093/ajcn/85.3.824. [DOI] [PubMed] [Google Scholar]
- 24.Bassaganya-Riera J, Guri AJ, Lu P, Climent M, Carbo A, Sobral BW, et al. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of ppar gamma. Journal of Biological Chemistry. 2010;286:2504–16. doi: 10.1074/jbc.M110.160077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuteja N. Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2007;2:135–8. doi: 10.4161/psb.2.3.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnone M, Bruzzone S, Guida L, Damonte G, Millo E, Scarfi S, et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009;284:17808–18. doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stillwell W, Brengle B, Wassall SR. Abscisic acid enhances aggregation and fusion of phospholipid vesicles. Biochem Biophys Res Commun. 1988;156:511–6. doi: 10.1016/s0006-291x(88)80871-4. [DOI] [PubMed] [Google Scholar]
- 28.Bassaganya-Riera J, Skoneczka J, Kingston DG, Krishnan A, Misyak SA, Guri AJ, et al. Mechanisms of action and medicinal applications of abscisic acid. Curr Med Chem. 2010;17:467–78. doi: 10.2174/092986710790226110. [DOI] [PubMed] [Google Scholar]
- 29.Baaten BJ, Li CR, Bradley LM. Multifaceted regulation of t cells by cd44. Commun Integr Biol. 2011;3:508–12. doi: 10.4161/cib.3.6.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, et al. A phase i trial with transgenic bacteria expressing interleukin-10 in crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754–9. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Klingler JP, Batelli G, Zhu JK. Aba receptors: The start of a new paradigm in phytohormone signalling. J Exp Bot. 2010;61:3199–210. doi: 10.1093/jxb/erq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturla L, Fresia C, Guida L, Bruzzone S, Scarfi S, Usai C, et al. Lancl2 is necessary for abscisic acid binding and signaling in human granulocytes and in rat insulinoma cells. J Biol Chem. 2009;284:28045–57. doi: 10.1074/jbc.M109.035329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, et al. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage ppar gamma to suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]


