Abstract
Significance: Calorie restriction (CR) is a known intervention that delays most aging processes. Most of the beneficial effects of CR are mediated by improved maintenance of mitochondrial performance in aged individuals. The control of mitochondrial biogenesis, apoptosis, and protein turnover is required for healthy aging. CR is able to induce molecular mechanisms that preserve oxidative capacity and decrease oxidative damage. Recent Advances and Critical Issues: Published data indicate that peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) is activated in old animals under CR conditions compared to ad libitum counterparts, enhancing mitochondrial biogenesis. Molecular regulation of PGC-1α has recently attracted significant research interest. We discuss the master regulators of energy metabolism such as AMP-activated protein kinase and sirtuin 1 among others that have been demonstrated to activate mitochondrial biogenesis through increased PGC-1α activity at transcriptional and post-translational levels. Additionally, we describe the latest findings that explain how CR promotes mitochondrial efficiency and decreases mitochondrial-derived oxidative damage. Future Directions: Understanding the beneficial mitochondrial changes conferred by CR will aid design of therapies for age-related diseases and help slow the aging process. Given the difficulty for humans to adhere to CR, we also explore new molecules that have been proposed during the last years to mimic the CR phenotype and their potential as future therapeutics. Antioxid. Redox Signal. 19, 310–320.
Introduction
Calorie restriction (CR) is a well-known intervention that ameliorates most of the alterations that occur with aging and extends lifespan. During the last decades a large body of research has focused on understanding the molecular mechanisms that confer these benefits. CR has been shown to improve the health and lifespan in organisms ranging from yeast to humans. Recently, long-term studies have examined these benefits in nonhuman primates. Monkeys under CR have shown a delayed onset of age-associated pathologies compared with their ad libitum (AL) counterparts (38). There are also documented data that suggest CR has beneficial effects in humans. In the 15th century, the Italian Luigi Cornaro started a nutritional regimen comparable to CR at the age of 40 years. His diet was based on 400 g of food daily plus wine and daily exercise; he died at the age of 91, almost three times the average lifespan in developed countries at that time (47). Another known case is the population of Okinawa, who consumed very few calories during World War II. During this time this population showed delayed aging and the lowest incidence of age-related diseases in the world. Interestingly, the subsequent diet normalization after the war raised the incidence of these diseases and aging up to regular rates (68). Further, there are several human studies that suggest CR may be responsible for health benefits such as decreased cardiovascular complications (17, 44).
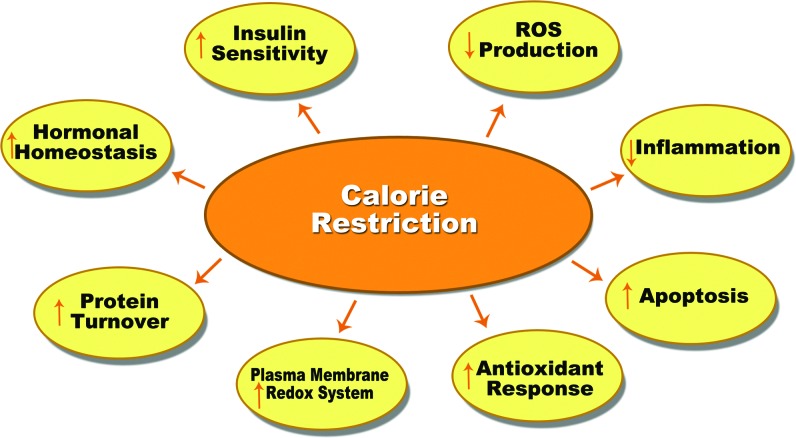
It is well known that CR decreases reactive oxygen species (ROS) production, enhances the plasma membrane redox system, decreases inflammation, and improves insulin signaling pathways (Fig. 1). Most of these benefits are thought to be associated with better mitochondrial function. In this venue, CR has been shown to produce very efficient mitochondrial performance, which is equivalent to a maintained ATP production while reducing ROS generation in the complexes of the electron transport chain (ETC) of the mitochondria. Thereby, this improvement on mitochondrial efficiency under CR is able to maintain cellular metabolism with a lower oxidative stress damage accumulation, resulting in a decreased aging rate at cellular and organismic levels.
FIG. 1.
Calorie restriction (CR) benefits. CR affects many processes in organisms that promote benefits to age-related diseases and delays aging. ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Since adherence to a CR diet is quite difficult for humans, investigators have recently pursued compounds that could mimic CR, providing benefits to health and lifespan. In this review, we focus on the changes in molecular mechanisms produced by CR and CR-mimetics in the mitochondria that promote health benefits.
CR Prevents the Decline of Oxidative Capacity with Aging
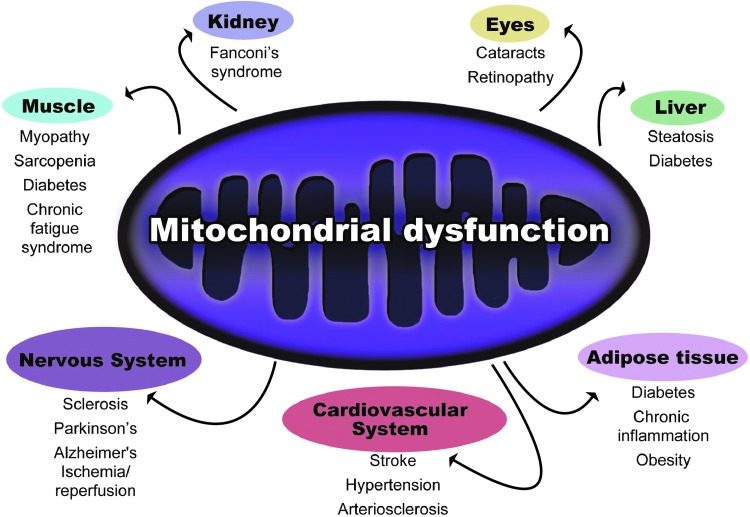
Mitochondria are responsible for production of ∼90% of intracellular ATP. Mitochondrial respiration plays a major role in preserving longevity, as it has been demonstrated that increments in respiratory activity enhance cell and organism longevity (6). It is known that impaired mitochondrial functioning occurs with aging and leads to deterioration in the structure and function of tissues, implicating this process in the development of several age-related diseases (Fig. 2) (76). The causes of declining oxidative capacity could be based on decreased mitochondrial content and/or function. There are several contradictory reports concerning changes of mitochondrial content with aging, but the impairment of mitochondrial function is a well-established fact (76).
FIG. 2.
Mitochondrial dysfunction promotes diseases. Impaired mitochondrial metabolism has been correlated to many diseases. The organs with higher energetic requirements have been found to be most altered by mitochondrial dysfunction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The proposed causes of mitochondrial dysfunction involve the synthesis of nonfunctional complexes of the ETC, damaged mitochondrial DNA (mtDNA), and/or the accumulation of nonfunctional proteins by ROS-induced modifications (76, 88). For instance, it has been demonstrated that aged tissues exhibit dysfunctional complex IV of the ETC. This alteration mainly affects the muscle because it requires more energy than most other tissues and is also one of the tissues most affected by aging (25). It has been recently demonstrated that the decline in oxidative capacity is the primary contributing factor to age-related muscle atrophy (88). Moreover, sarcopenia, the decline of muscle mass associated with aging, makes individuals more sedentary and is often accompanied by an increase in fat mass that compromises the quality of life and survival of the patients (63). Aged muscle tissue is characterized by a decline in the number and size of fibers, predominately type II fibers (54). CR has been shown to completely prevent the decline in mass-specific skeletal muscle aerobic performance between young and old rats (42). The protection of aerobic function was due to maintenance of skeletal muscle oxidative capacity with aging and enhanced mitochondrial function in CR animals. Accordingly, the metabolic rate when normalized to body weight does not decline with age in CR animals but does decrease in AL counterparts. It has been demonstrated that even a short-term CR period improves mitochondrial function in as little as two weeks (9). Mitochondrial oxidative capacity is a strong predictor of insulin sensitivity in skeletal muscle (12). The maintenance of skeletal muscle oxidative capacity by CR has been demonstrated even after accounting for the extension of life span. Baker et al. elegantly determined that CR completely prevented the 40% decline in plantaris and gastrocnemius muscle oxidative capacity observed in AL counterparts. Surprisingly, no differences were found in citrate synthase protein levels (5). In agreement with that, human studies in healthy overweight but nonobese individuals have shown that key mitochondrial enzymes of the tricarboxylic acid cycle, beta-oxidation, and ETC are unchanged. These data imply that CR improves the quality of individual mitochondria and ultimately results in the maintenance of healthy mitochondria with high bioenergenetic capacity and reduced propensity toward oxidant production. Improved mitochondrial function does not solely occur in the muscle tissue. For example, within the context of the liver and the white adipose tissue (WAT), increased mitochondrial biogenesis contributes to an improved metabolic profile, increasing the fatty acid oxidation and decreasing circulating triglycerides to maintain insulin sensitivity in peripheral tissues (8, 71). These events result in reduced fat accumulation, which is known to promote lifespan extension.
CR Induces Mitochondrial Biosynthesis Through PGC-1α Activation
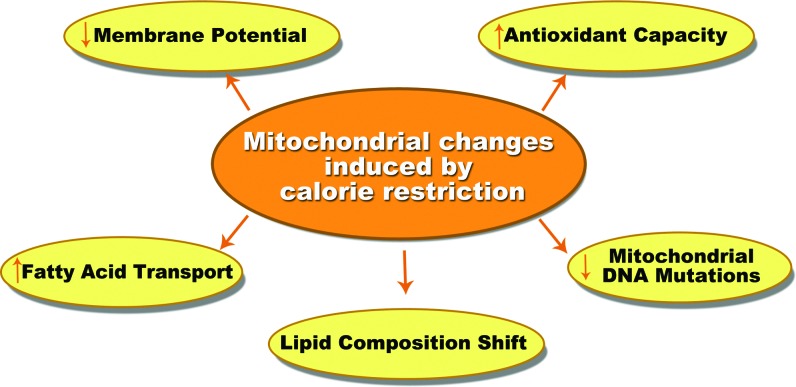
One of the hallmarks of CR is the increase of functional respiratory units (mitochondrial biogenesis) and the promotion of changes in dynamics and composition of this organelle (Fig. 3) (17, 27, 58). Mitochondrial respiration and content actually increases in yeast and nematodes under CR (46, 57). There are some reports that show increased amounts of mtDNA, ATP concentration, and oxygen consumption in WAT and many other tissues of CR mice when compared with AL mice. Additionally, it has been reported that CR may alter mitochondrial membrane fatty acid composition, allowing for increased mitochondrial function (27).
FIG. 3.
Mitochondrial changes induced by CR. Besides the increase in mitochondrial content observed in CR conditions, mitochondrial components and dynamics are also modulated by CR. These changes have been proposed to play a role in the benefits of CR to mitochondrial function. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
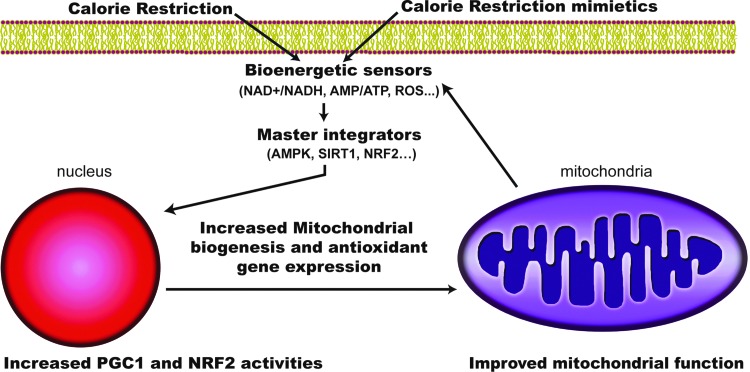
In mammals, the regulation of mitochondrial biogenesis is complex, and it is not known whether the effects of CR on mitochondrial biogenesis are tissue-specific. Mitochondrial biogenesis is a highly regulated process that coordinates the activity of the ∼1000 genes involved in mitochondrial function (Fig. 4). This process requires coordination of the nuclear and mitochondrial genomes. Under CR, transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) is expressed and activated, leading to an increase of mitochondrial mass. PGC-1α gene expression has been shown to be maintained with aging in CR models (5). In addition to PGC-1α there are other master regulators that are expressed under CR conditions like peroxisome proliferator activated receptor (PPAR) family and liver X receptor, which control fatty acid metabolism. PGC-1α physiological importance has been demonstrated since repression of this protein by a mutant form of the huntingtin protein leads to mitochondrial dysfunction, whereas its overexpression rescues cells from the deleterious effect of huntingtin (20). PGC-1α specifically modulates the activity of several transcription factors and coactivators involved in mitochondrial respiration and biogenesis such as nuclear respiratory factor 1 (NRF-1), NRF-2, PPARγ, steroid receptor coactivator-1, and mitochondrial transcriptor factor A. NRF-1 and NRF-2 coordinate the expression of nuclear and mitochondrial genes that encode most of the subunits of mitochondrial complexes (91). In the same sense, PGC-1α activates the shift of substrate utilization from carbohydrates to fatty acids through coregulation of PPARγ (75). Although it has been reported that CR enhances mitochondrial activities, the mechanism remains controversial as reports conflict about the extent to which CR changes expression of genes involved in nutrient sensing, mitochondrial biogenesis, and other key mitochondrial enzymes involved in the Krebs cycle, β-oxidation, and ETC activities in humans (17). The differences found in the literature might be based on the species used in the studies, the different ages of the animals, the tissues analyzed, and/or the way that the data are presented (e.g., activity/g of wet tissue weight versus activity/g of protein extracted). In any case, we consider the quantification of mitochondrial number by electron microscopy the best option to determine changes in mitochondrial number (58).
FIG. 4.
Diagram of enhanced mitochondrial function under CR and mimetics. CR and mimetics modulate stress and bioenergetic sensors, leading to the activation of master integrators, such as AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) among others. These proteins activate mitochondrial biogenesis and antioxidant response through the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and nuclear respiratory factor 2 (NRF2), leading to an improvement in mitochondrial function. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Regulation of PGC-1α Activity
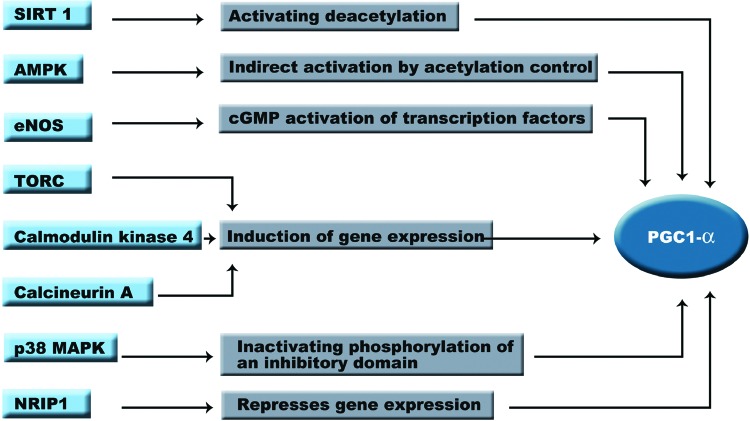
CR activates several regulatory pathways that lead to the activation of PGC-1α and increases mitochondrial biogenesis. During the years a new field has developed to understand the regulation of PGC-1α and its downstream target activities. In this section, we will describe the present knowledge about molecular pathways that regulate PGC-1α (Fig. 5).
FIG. 5.
Regulation of PGC-1α activity. Mitochondrial biogenesis is regulated through PGC-1α to control the amount of mitochondria depending on the environmental conditions. Under CR, mitochondrial biogenesis is activated by deacetylation of PGC-1α, while inactivation of this protein is achieved by negative regulatory domains present in its amino acid sequence. eNOS, endothelial nitric oxide synthase; TORC, transducer of regulated CREB; MAPK, mitogen-activated protein kinase; NRIP1, nuclear receptor-interacting protein 1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Sirtuin 1
Sirtuin 1 (SIRT1) is a NAD+-dependent histone deacetylase that is highly expressed under CR in most of the animal models. For instance, it has been reported that SIRT1 expression is induced under CR and exercise in humans (17). SIRT1 belongs to the sirtuin family. This family consists of seven enzymes that share a conserved deacetylation catalytic domain but differ in cellular localization, tissue distribution, and substrate preference (23). Increasing evidence implicates mammalian sirtuins as regulators of CR-induced physiological responses. Although there are controversial reports, additional copies of SIRT1 orthologues have been shown to extend lifespan in yeast, worms, and flies (13). In mammals, SIRT1 performs an important role in epigenetic silencing and genomic stability, regulating energy metabolism and endocrine signaling. This protein is also expressed under stress conditions and low-nutritional status. SIRT1 overexpression or activation confers a metabolic state that resembles CR; however, it does not extend lifespan in mammals (10). The lack of lifespan extension on Sirt1 transgenic mice could be due to the mild overexpression obtained in Sir1 transgenic mice, between two- to four-fold increased mRNA expression, which could not be high enough to produce an increase in longevity. Another possibility could be that Sirt1 might not protect against all the age-related diseases and could be beneficial only in protecting against a subset of these diseases, such as some types of cancer and diabetes (43).
It has been proposed that SIRT1 acts as a master regulator of the cellular metabolism via the redox status. Under CR there is a molecular adaptation that shifts the NAD+/NADH ratio toward higher NAD+ levels inducing SIRT1 activity (30). SIRT1-mediated effects result from deacetylation of lysine residues on substrate proteins. To date, several proteins such as nuclear factor-κB, forkhead box class O3, p53, PGC-1α, and endothelial nitric oxide synthase (eNOS) have been identified as targets of SIRT1 (28). Increased SIRT1 activity triggered by elevated NAD+ levels has been shown to increase the transcriptional activity of PGC-1α (75). Accordingly, decreased acetylation status of PGC-1α has also been demonstrated to increase mitochondrial PGC-1α-dependent expression. These data suggest a direct link between SIRT1 and mitochondrial bioenergetics. Studies with mice lacking SIRT1 have shown that PGC-1α mRNA is reduced while PGC-1α is acetylated in skeletal muscle, suggesting that SIRT1 regulates PGC-1α by both transcriptional and post-translational mechanisms (34). Further, the addition of a SIRT1 inhibitor, nicotinamide, decreases the expression of downstream targets of PGC-1α in muscle cells (34).
SIRT1 appears to be involved in the regulation of the antioxidant defenses within the mitochondria since SIRT1 overexpression attenuates the high fat-induced hepatic steatosis by induction of antioxidant proteins, such as mitochondrial superoxide dismutase and NRF-1, while SIRT1 deficiency enhances liver steatosis. Moreover, the regulatory role of SIRT1 on cellular respiration has been demonstrated by studies with SIRT1-silenced cells treated with serum from CR mice.
AMP-activated protein kinase
Another master integrator of external signals involved in PGC-1α regulation is the AMP-activated protein kinase (AMPK). This kinase coordinates the vast majority of the responses to the energy status of the cell and is activated by an increase in AMP/ATP ratio. Impaired regulation of AMPK activity is associated with several aging-associated processes, such as obesity and insulin resistance. AMPK expression is also induced under CR conditions (17, 74). However, it is not clear if CR directly affects AMPK function, but short term CR increases phosphorylation and thereby the activity of AMPK in young and old animals (35). Interestingly, chronic AMPK inactivation is associated with age-related complications (74) and constitutively, activated AMPK leads to increased lifespan in nematodes (21). In mammalian studies, it has been shown that AMPK phosphorylates PGC-1α and thereby controls glucose uptake, fatty acid oxidation, and mitochondrial biogenesis in primary muscle cells. Starvation conditions activate AMPK that initiates a signaling process that increases PGC-1α and PPARδ activities, leading to an increase of the mitochondrial content.
Endothelial nitric oxide synthase
Nitric oxide (NO) is a free radical involved in several physiological processes. NO production is essential for mitochondrial biogenesis and is increased under CR conditions. This mechanism also works through the activation of PGC-1α (71). NO is synthesized by eNOS, which is sensitive to nutritional status. AKT modulates eNOS activity through phosphorylation, leading to an increase of the NO levels (31). The consequence of eNOS expression and 3,5-cyclic guanosine monophosphate (cGMP) production is the activation of PGC-1α via cGMP-mediated upregulation of transcriptional factors. Increases in cGMP levels have also been related to the expression of the deacetylase SIRT1 (71). eNOS gene expression is increased in humans under CR and CR plus exercise. Interestingly, NO can induce mitochondrial biogenesis in human skeletal muscle without increasing SIRT1 protein expression in humans (17). These data suggest that the effect of NO in mitochondrial biosynthesis might not be related to SIRT1 activity. Decreased levels of NO in eNOS null-mutant mice attenuated the caloric restriction-induced expression of PGC-1α and TFAM mRNA and the increase of mitochondrial content induced by CR.
Other activators
There are several reports that include other families of coactivators in PGC-1α activation. One of them is the transducer of regulated CREB (TORC) family (90). TORCs proteins induce PGC-1α gene transcription in response to an increase of cAMP through cAMP response element-binding (CREB). Increased expression of TORC1, TORC2, and TORC3 in primary muscle cells induced PGC-1α expression and its downstream targets leading to increased cellular respiration (90). This is a PGC-1α dependent mechanism, since the lack of this gene abolished the increment of mitochondrial content. A different regulation process depends on an increase in muscle cytosolic calcium levels induced by exercise. Calcium stimulates calmodulin kinase IV, which promotes PGC-1α expression through CREBP. Calcineurin A also enhances PGC-1α expression in cardiac muscle through activation of myocyte enhancer factor-2 (22).
The inhibition of PGC-1α
PGC-1α also contains a negative regulatory domain. The p160 myb binding protein (p160MBP) represses PGC-1α by binding it to this regulatory region. This interaction is further regulated by p38 mitogen-activated protein kinase (MAPK), which phosphorylates the inhibitory domain of PGC-1α, thereby disrupting p160MBP-binding and inducing mitochondrial biogenesis (26). Another protein that inactivates PGC-1α is the nuclear receptor-interacting protein 1 (NRIP1). This protein seems to function primarily as a scaffold protein that links nuclear receptors to chromatin remodeling enzymes repressing PGC-1α activity. NRIP1 is a coregulator that shares a number of features with the PGC-1 proteins and has been shown to repress PGC-1α activity (89).
CR Promotes Mitochondrial Efficiency
Preservation of mitochondrial structural and functional integrity by CR is believed to result from the attenuation of oxidative damage, which is considered one of the major factors contributing to slowing the aging process and preventing tumor formation. ROS are produced by mitochondrial oxidative phosphorylation and extracellular oxidants. When ROS levels are elevated, they can modify cellular molecules resulting in lipid peroxidation, DNA strand break, and protein carbonylation. All these alterations impair cellular functions and increase the chance of cell death (Fig. 1). The accumulation of oxidative damage eventually impairs the function of the organs, which has been identified in many age-related diseases. We and others have shown that serum from CR animals decreases membrane potential and oxygen consumption in human cells lines and primary culture cells, thereby decreasing the electron flow (58). Therefore, CR prevents the electron leakage from mitochondrial complexes to form superoxide, decreasing the accumulation of oxidative damage. Interestingly, the ATP levels and the ATP/ADP ratio do not change in CR. Thus, these results agree with the hypothesis that CR produces very efficient mitochondrial electron transport based on low-potential mitochondria that sustain reduced oxygen consumption while maintaining cellular ATP levels and reducing ROS production.
Results from different laboratories support that, besides the decreased ROS production, CR also upregulates the expression of genes involved in ROS scavenging (51, 59, 60, 69). One of the most important transcription factors involved in the upregulation of the antioxidant defenses is the nuclear factor erythoid-derived 2-like 2 (NFE2L2). For instance, under CR, SKN-1 (NFE2L2 orthologue) is upregulated in the ASIs neurons in Caenorhabditis elegans. Studies in our lab have focused on its antioxidant activity and its implications in cancer and aging. CR was not effective against chemically induced tumorigenesis in the NFE2L2 knockout (KO) mice. AL NFE2L2 KO and CR NFE2L2 KO mice reached total tumor incidence at the age of 30 weeks, while 40% CR wt mice did not show any papillomas until 42 weeks of treatment (73). These data suggest that the anticarcinogenic effect of CR solely depends on the activity of NFE2L2 due to the lack of activation of the antioxidant defenses. Moreover, several reports have shown that rodents overexpressing antioxidant enzymes, such as mitochondrial catalase and copper-zinc superoxide dismutase in fruit fly, increase lifespan through increased antioxidant capacity.
There is extensive evidence that mitochondrial dysfunction and loss of mitochondria are due in large part to mutations in mtDNA since the vast majority of the ROS production is localized within the mitochondria. Interestingly, there is a body of literature showing that uncoupled mitochondria could give some health and lifespan benefits since several organisms have been shown to live longer (81). Supporting this hypothesis, studies with mice lacking uncoupling protein 2 (UCP2) presented shortened lifespan and increased oxidative damage (2). On the other hand, mice overexpressing UCP1 displayed increased lifespan, consumed more oxygen, increased body temperature, lowered body weight, and reduced oxidative damage accumulation (32, 49). The explanation of the increased longevity found in these animals could be that uncoupled mitochondria decreases mitochondrial potential and thereby ROS production. These events might produce the induction of mitochondrial biogenesis, as suggested by the increased Sirt1 activity and decreased acetylation of PGC-1α, which in turn, would be able to maintain ATP production (32).
The mitochondrial theory of aging proposes that mutations of mtDNA induced by ROS are the primary cause of cellular energy decline with aging. Accordingly, complex I is particularly affected since seven of its subunits are encoded by mtDNA (33). Moreover, the lack of protective histones and the low abundance of introns increase the probability to mutate coding sequences. Mutations in mtDNA have been found to accumulate and colocalize with impaired oxidative phosphorylation in muscle fibers (88). Mitochondria presenting mutated mtDNA have been shown to produce lower activities in the ETC complexes that are codified by mtDNA in several research models and humans. On the other hand, several studies have refuted the involvement of mtDNA mutations as a major contributing factor in the decline of the skeletal muscle oxidative capacity (82). These reports suggest that the different amount of mtDNA mutations in muscles under AL and CR are not associated with a decreased oxidative capacity. Further, different oxidative capacities in several muscles from very old CR and AL rats do not correlate with increased mtDNA mutations (5).
CR Promotes Apoptosis
Another cellular consequence of reduced energy intake in CR models is the induction of pro-apoptotic gene expression. It is known that aside from their role as energy suppliers, mitochondria are also involved in the regulation of the apoptotic program through caspase-dependent and caspase-independent mechanisms (62, 87). Apoptosis activation depends on the release of mitochondrial enzymes such as apoptosis inducing factor (AIF) and endonuclease G (EndoG), which induce DNA-fragmentations (87). When apoptosis is induced, cytochrome c is released into the cytoplasm and promotes the oligomerization of the apoptosis protease activating factor-1, which finally activates the caspase pathway. In addition, some reports have shown that AIF and EndoG can also translocate to the nucleus and start a caspase-independent apoptosis mechanism.
CR Enhances Protein Turnover
CR promotes autophagy in many species, leading to the selective elimination of damaged proteins and whole organelles. The autophagic response persists in old CR individuals while becoming dysfunctional in AL counterparts (61). Autophagy is the main cellular mechanism attributed to the degradation of dysfunctional and damaged mitochondria. This process is critical for cellular survival since the progressive accumulation of damaged proteins in cells lacking autophagy results in cell death and loss of tissue function (29). Although the autophagic activity in liver declines with age, there must be a tissue-specific regulation of this process, since macroautophagy is maintained in heart and skeletal muscle in old rodents. Macroautophagy plays an important role promoting the health benefits of CR because the inhibition of this process suppressed the lifespan extension promoted by CR in worms. Under CR, autophagy is regulated by at least two signaling pathways. The first one involves the activation of the phosphoinositide 3-kinase class III, which is bound to Beclin 1. The second one acts through the mitochondrial target of rapamycin (19, 83). Experimental evidence indicates that protective effects of CR on mitochondrial function rely on the ability to reduce the incidence of mitochondrial abnormalities through maintenance of proper autophagic responses. Additionally, CR may induce a shift toward an enhanced turnover of mitochondrial proteins and increased mitochondrial biogenesis by PGC-1α. This hypothesis would explain the lack of differences in citrate synthase protein content between AL and CR old animals (17).
CR Mimetics
Due to the difficulty for humans to adhere to CR similar to that performed under laboratory conditions, many investigators have sought compounds that could mimic CR and produce physiological and anti-aging benefits similar to CR. Epidemiological studies have clearly documented that the action of some phytochemicals resembles, at least partially, the CR phenotype (Table 1) (15, 16).
Table 1.
Calorie Restriction Mimetics
| Compound | Health/lifespan benefits |
|---|---|
| Green tea polyphenols | Longevity (11, 50) |
| SRT1720 and SRT501 | Diabetes, longevity (65, 66) |
| Curcumin | Longevity, cancer (55, 56, 60) |
| Resveratrol | Longevity, diabetes, apoptosis, steatosis, sarcopenia, cancer (7, 73, 93) |
| 2-deoxy-D-glucose | Diabetes, cancer (36, 52, 67, 79) |
| Rimonabant | Energetics, obesity, diabetes (18) |
| Rapamycin | Longevity, cancer (39, 41, 48, 64, 85) |
| Metformin | Longevity, diabetes, cancer (4, 72, 77, 94) |
| AICAR | Energetics (70) |
| Glaucarubinone | Longevity (92) |
| Lonidamine | Longevity (78) |
One of the most known CR mimetics proposed is resveratrol. This is a naturally occurring polyphenol that has been shown to increase the activity of PGC-1α, leading to an increased mitochondrial biogenesis in liver, muscle, and brain (7, 53). SRT1720 is an analog of resveratrol that acts through the same enzymatic mechanism, binding to an allosteric site in the enzyme-substrate complex and lowers the Km of SIRT1 (80). It has been described as 1000 times more potent than resveratrol. We and others have shown that resveratrol and other polyphenols such as SRT1720 and SRT501 activate SIRT1 directly or indirectly in a variety of models leading to increased deacetylation of PGC-1α when administered in the diet (86). In fact, resveratrol is completely unable to modulate PGC-1α function in SIRT1−/− MEFs (53). SRT501 and SRT1720 generate a gene expression profile similar to CR (80). An analysis of the gene expression shift induced by CR compared to SRT1720 and SRT501 depicts that the gene expression profile is significantly shifted in the same direction, including the mitochondrial biogenesis network among others. Further, this analysis also showed that the PPAR family and estrogen-related receptor are induced by SRT501. These genes are known to be activated when mitochondrial biogenesis is required. Correspondingly, the data show that NRIP1, an inhibitor of PGC-1α, is reduced. These data suggest that both act via the same mechanism in vivo.
Another CR mimetic candidate is Metformin. This CR mimetic has been used since 1960 in the treatment of diabetes due its ability to reduce blood glucose levels thereby reducing the risk of glucotoxic effects such as glycosylation and oxidative damage. This compound induces a gene expression profile that closely aligns to long-term CR (24). Metformin supplementation in diet has also been shown to extend lifespan in several laboratory models, including mammals (3, 72). Metformin also induces the expression of glycolytic genes, which is known to occur in CR as well. It has been proposed that metformin compromises ATP production in the mitochondria. The mechanism of action would be based in a mild inhibition of the mitochondrial complex I. Interestingly, although the increase of oxidative damage due to the inhibition of mitochondrial complexes is a well-established fact, metformin seems to reduce ROS generation and oxidative damage accumulation (1, 37, 40, 84). Supporting these findings, an increase of the expression of antioxidant proteins has been reported in cells and animals treated with metformin (45, 72). A slightly inhibited complex I activity under metformin treatment may lead to lower ATP production without affecting, or even decreasing, oxidative damage. In this context, the decrease of ATP production renders the increase of the AMP/ATP ratio, which is known to activate AMPK. Although AMPK activation is not responsible for all the benefits achieved by CR, it is likely that AMPK activation due to metformin treatment can mimic certain aspects of CR (14).
The regulation of AMPK, SIRT1, and PGC-1α pathways using CR mimetics would induce mitochondrial biogenesis and antioxidant gene expression. The post-translational regulation on PGC-1α by CR mimetics may confer the gene expression changes that induce a switch in the source of energy utilized from glucose to lipids in the mitochondria leading to the improvement of the mitochondrial function and thereby, protecting against metabolic stresses.
Concluding Remarks
During the last decades, researchers have been trying to determine the mechanisms whereby CR delays aging and prevents age-related diseases. Understanding the metabolic reprogramming in the mitochondria induced by CR would offer potential to develop mechanism-based interventions to promote longevity and healthy aging. The next step in future investigations would be developing a mechanism to stimulate the regulatory components of mitochondrial biogenesis, mainly PGC-1α, SIRT1, AMPK, and their downstream targets to shift the metabolic balance. To develop a preventive strategy to treat the age-related complications in humans, we propose the use of nontoxic CR mimetics as a potential therapeutic target. A plausible aging prevention strategy could be based on an approach that includes moderate CR, physical activity, and CR mimetic supplementation, which seem to be the best way to maintain mitochondrial activity.
Abbreviations Used
- AIF
apoptosis inducing factor
- AL
ad libitum
- AMPK
AMP-activated protein kinase
- cGMP
3,5-cyclic guanosine monophosphate
- CR
calorie restriction
- CREB
cAMP response element-binding
- EndoG
endonuclease G
- eNOS
endothelial nitric oxide synthase
- ETC
electron transport chain
- mtDNA
mitochondrial DNA
- NFE2L2
nuclear factor erythoid-derived 2-like 2
- NO
nitric oxide
- NRF
nuclear respiratory factor
- NRIP1
nuclear receptor-interacting protein 1
- p160MBP
p160 myb binding protein
- PGC-1α
peroxisome proliferator activated receptor-activated receptor gamma coactivator 1 alpha
- PPAR
peroxisome proliferator activated receptor
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- TORC
transducer of regulated CREB
- UCP
uncoupling protein
- WAT
white adipose tissue
Acknowledgment
This work has been supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. We thank Jessica Curtis for editing the article.
References
- 1.Algire C. Moiseeva O. Deschenes-Simard X. Amrein L. Petruccelli L. Birman E. Viollet B. Ferbeyre G. Pollak MN. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 2.Andrews ZB. Horvath TL. Uncoupling protein-2 regulates lifespan in mice. Am J Physiol. 2009;296:E621–E627. doi: 10.1152/ajpendo.90903.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov VN. Berstein LM. Egormin PA. Piskunova TS. Popovich IG. Zabezhinski MA. Tyndyk ML. Yurova MV. Kovalenko IG. Poroshina TE. Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle (Georgetown, TX) 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 4.Anisimov VN. Berstein LM. Popovich IG. Zabezhinski MA. Egormin PA. Piskunova TS. Semenchenko AV. Tyndyk ML. Yurova MN. Kovalenko IG. Poroshina TE. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging. 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker DJ. Betik AC. Krause DJ. Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J Gerontol. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- 6.Barros MH. Bandy B. Tahara EB. Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- 7.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G. Lewis K. Pistell PJ. Poosala S. Becker KG. Boss O. Gwinn D. Wang M. Ramaswamy S. Fishbein KW. Spencer RG. Lakatta EG. Le Couteur D. Shaw RJ. Navas P. Puigserver P. Ingram DK. de Cabo R. Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berge RK. Tronstad KJ. Berge K. Rost TH. Wergedahl H. Gudbrandsen OA. Skorve J. The metabolic syndrome and the hepatic fatty acid drainage hypothesis. Biochimie. 2005;87:15–20. doi: 10.1016/j.biochi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Bevilacqua L. Ramsey JJ. Hagopian K. Weindruch R. Harper ME. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol. 2004;286:E852–E861. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bordone L. Cohen D. Robinson A. Motta MC. van Veen E. Czopik A. Steele AD. Crowe H. Marmor S. Luo J. Gu W. Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown MK. Evans JL. Luo Y. Beneficial effects of natural antioxidants EGCG and alpha-lipoic acid on life span and age-dependent behavioral declines in Caenorhabditis elegans. Pharmacol, Biochem, Behav. 2006;85:620–628. doi: 10.1016/j.pbb.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Bruce CR. Anderson MJ. Carey AL. Newman DG. Bonen A. Kriketos AD. Cooney GJ. Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88:5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 13.Burnett C. Valentini S. Cabreiro F. Goss M. Somogyvari M. Piper MD. Hoddinott M. Sutphin GL. Leko V. McElwee JJ. Vazquez-Manrique RP. Orfila AM. Ackerman D. Au C. Vinti G. Riesen M. Howard K. Neri C. Bedalov A. Kaeberlein M. Soti C. Partridge L. Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canto C. Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda, MD) 26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JM. Giovannucci EL. Vegetables, fruits, associated micronutrients, and risk of prostate cancer. Epidemiol Rev. 2001;23:82–86. doi: 10.1093/oxfordjournals.epirev.a000799. [DOI] [PubMed] [Google Scholar]
- 16.Chen C. Yu R. Owuor ED. Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23:605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 17.Civitarese AE. Carling S. Heilbronn LK. Hulver MH. Ukropcova B. Deutsch WA. Smith SR. Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colagiuri S. Diabesity: therapeutic options. Diabetes Obes Metab. 2010;12:463–473. doi: 10.1111/j.1463-1326.2009.01182.x. [DOI] [PubMed] [Google Scholar]
- 19.Corradetti MN. Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 20.Cui L. Jeong H. Borovecki F. Parkhurst CN. Tanese N. Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Curtis R. O'Connor G. DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 22.Czubryt MP. McAnally J. Fishman GI. Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dali-Youcef N. Lagouge M. Froelich S. Koehl C. Schoonjans K. Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 24.Dhahbi JM. Mote PL. Fahy GM. Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 25.Essen-Gustavsson B. Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126:107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 26.Fan M. Rhee J. St-Pierre J. Handschin C. Puigserver P. Lin J. Jaeger S. Erdjument-Bromage H. Tempst P. Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18:278–289. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faulks SC. Turner N. Else PL. Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 28.Finkel T. Deng CX. Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 30.Fulco M. Cen Y. Zhao P. Hoffman EP. McBurney MW. Sauve AA. Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulton D. Gratton JP. McCabe TJ. Fontana J. Fujio Y. Walsh K. Franke TF. Papapetropoulos A. Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gates AC. Bernal-Mizrachi C. Chinault SL. Feng C. Schneider JG. Coleman T. Malone JP. Townsend RR. Chakravarthy MV. Semenkovich CF. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Genova ML. Pich MM. Bernacchia A. Bianchi C. Biondi A. Bovina C. Falasca AI. Formiggini G. Castelli GP. Lenaz G. The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann N Y Acad Sci. 2004;1011:86–100. doi: 10.1007/978-3-662-41088-2_10. [DOI] [PubMed] [Google Scholar]
- 34.Gerhart-Hines Z. Rodgers JT. Bare O. Lerin C. Kim SH. Mostoslavsky R. Alt FW. Wu Z. Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez AA. Kumar R. Mulligan JD. Davis AJ. Weindruch R. Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S. Mathur R. Dwarakanath BS. The glycolytic inhibitor 2-deoxy-D-glucose enhances the efficacy of etoposide in ehrlich ascites tumor-bearing mice. Cancer Biol Ther. 2005;4:87–94. doi: 10.4161/cbt.4.1.1381. [DOI] [PubMed] [Google Scholar]
- 37.Halicka HD. Zhao H. Li J. Traganos F. Zhang S. Lee M. Darzynkiewicz Z. Genome protective effect of metformin as revealed by reduced level of constitutive DNA damage signaling. Aging (Albany, NY) 3:1028–1038. doi: 10.18632/aging.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen BC. Ortmeyer HK. Bodkin NL. Prevention of obesity in middle-aged monkeys: food intake during body weight clamp. Obes Res. 1995;3(Suppl 2):199s–204s. doi: 10.1002/j.1550-8528.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 39.Hansen M. Taubert S. Crawford D. Libina N. Lee SJ. Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 40.Hariharakrishnan J. Satpute RM. Prasad GB. Bhattacharya R. Oxidative stress mediated cytotoxicity of cyanide in LLC-MK2 cells and its attenuation by alpha-ketoglutarate and N-acetyl cysteine. Toxicol Lett. 2009;185:132–141. doi: 10.1016/j.toxlet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Harrison DE. Strong R. Sharp ZD. Nelson JF. Astle CM. Flurkey K. Nadon NL. Wilkinson JE. Frenkel K. Carter CS. Pahor M. Javors MA. Fernandez E. Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hepple RT. Baker DJ. Kaczor JJ. Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J. 2005;19:1320–1322. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- 43.Herranz D. Munoz-Martin M. Canamero M. Mulero F. Martinez-Pastor B. Fernandez-Capetillo O. Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holloszy JO. Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou X. Song J. Li XN. Zhang L. Wang X. Chen L. Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 396:199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Houthoofd K. Braeckman BP. Lenaerts I. Brys K. De Vreese A. Van Eygen S. Vanfleteren JR. No reduction of metabolic rate in food restricted Caenorhabditis elegans. Exp Gerontol. 2002;37:1359–1369. doi: 10.1016/s0531-5565(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 47.Howell TH. The art of living long by Luigi Cornaro. Age Ageing. 1987;16:194–195. doi: 10.1093/ageing/16.3.194. [DOI] [PubMed] [Google Scholar]
- 48.Kapahi P. Zid BM. Harper T. Koslover D. Sapin V. Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keipert S. Klaus S. Heldmaier G. Jastroch M. UCP1 ectopically expressed in murine muscle displays native function and mitigates mitochondrial superoxide production. Biochim Biophys Acta. 2010;1797:324–330. doi: 10.1016/j.bbabio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Kitani K. Osawa T. Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8:567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- 51.Klaassen CD. Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–328. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- 52.Klenow H. Inhibition by cordycepin and 2-deoxyglucose of the incorporation of (32p)orthophosphate into the nucleic acids of ehrlich ascites-tumor cells in vitro. Biochim Biophys Acta. 1963;76:354–365. [PubMed] [Google Scholar]
- 53.Lagouge M. Argmann C. Gerhart-Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P. Geny B. Laakso M. Puigserver P. Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Larsson L. Sjodin B. Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22—65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee KS. Lee BS. Semnani S. Avanesian A. Um CY. Jeon HJ. Seong KM. Yu K. Min KJ. Jafari M. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 13:561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- 56.Liao VH. Yu CW. Chu YJ. Li WH. Hsieh YC. Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 57.Lin SJ. Kaeberlein M. Andalis AA. Sturtz LA. Defossez PA. Culotta VC. Fink GR. Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Lluch G. Hunt N. Jones B. Zhu M. Jamieson H. Hilmer S. Cascajo MV. Allard J. Ingram DK. Navas P. de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandlekar S. Hong JL. Kong AN. Modulation of metabolic enzymes by dietary phytochemicals: a review of mechanisms underlying beneficial versus unfavorable effects. Cur Drug Metab. 2006;7:661–675. doi: 10.2174/138920006778017795. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Montalvo A. Villalba JM. Navas P. de Cabo R. NRF2, cancer and calorie restriction. Oncogene. 30:505–520. doi: 10.1038/onc.2010.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marzetti E. Lees HA. Wohlgemuth SE. Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. BioFactors (Oxford, England) 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marzetti E. Wohlgemuth SE. Lees HA. Chung HY. Giovannini S. Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metter EJ. Talbot LA. Schrager M. Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 64.Miller RA. Harrison DE. Astle CM. Baur JA. Boyd AR. de Cabo R. Fernandez E. Flurkey K. Javors MA. Nelson JF. Orihuela CJ. Pletcher S. Sharp ZD. Sinclair D. Starnes JW. Wilkinson JE. Nadon NL. Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A: Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Milne JC. Lambert PD. Schenk S. Carney DP. Smith JJ. Gagne DJ. Jin L. Boss O. Perni RB. Vu CB. Bemis JE. Xie R. Disch JS. Ng PY. Nunes JJ. Lynch AV. Yang H. Galonek H. Israelian K. Choy W. Iffland A. Lavu S. Medvedik O. Sinclair DA. Olefsky JM. Jirousek MR. Elliott PJ. Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minor RK. Baur JA. Gomes AP. Ward TM. Csiszar A. Mercken EM. Abdelmohsen K. Shin Y-K. Canto C. Scheibye-Knudsen M. Krawczyk M. Irusta PM. Martin-Montalvo A. Hubbard BP. Zhang Y. Lehrmann E. White AA. Price NL. Swindell WR. Pearson KJ. Becker KG. Bohr VA. Gorospe M. Egan JM. Talan MI. Auwerx J. Westphal CH. Ellis JL. Ungvari Z. Vlasuk GP. Elliott PJ. Sinclair DA. de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Minor RK. Smith DL., Jr. Sossong AM. Kaushik S. Poosala S. Spangler EL. Roth GS. Lane M. Allison DB. de Cabo R. Ingram DK. Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–339. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miyagi S. Iwama N. Kawabata T. Hasegawa K. Longevity and diet in Okinawa, Japan: the past, present and future. Asia Pac J Public Health. 2003;15(Suppl):S3–S9. doi: 10.1177/101053950301500S03. [DOI] [PubMed] [Google Scholar]
- 69.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Narkar VA. Downes M. Yu RT. Embler E. Wang YX. Banayo E. Mihaylova MM. Nelson MC. Zou Y. Juguilon H. Kang H. Shaw RJ. Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nisoli E. Tonello C. Cardile A. Cozzi V. Bracale R. Tedesco L. Falcone S. Valerio A. Cantoni O. Clementi E. Moncada S. Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science (New York, NY) 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 72.Onken B. Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pearson KJ. Lewis KN. Price NL. Chang JW. Perez E. Cascajo MV. Tamashiro KL. Poosala S. Csiszar A. Ungvari Z. Kensler TW. Yamamoto M. Egan JM. Longo DL. Ingram DK. Navas P. de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reznick RM. Zong H. Li J. Morino K. Moore IK. Yu HJ. Liu ZX. Dong J. Mustard KJ. Hawley SA. Befroy D. Pypaert M. Hardie DG. Young LH. Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodgers JT. Lerin C. Haas W. Gygi SP. Spiegelman BM. Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 76.Rooyackers OE. Adey DB. Ades PA. Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scarpello JH. Improving survival with metformin: the evidence base today. Diabetes Metab. 2003;29:6S36–6S43. doi: 10.1016/s1262-3636(03)72786-4. [DOI] [PubMed] [Google Scholar]
- 78.Schmeisser S. Zarse K. Ristow M. Lonidamine extends lifespan of adult Caenorhabditis elegans by increasing the formation of mitochondrial reactive oxygen species. Horm Metab Res. 43:687–692. doi: 10.1055/s-0031-1286308. [DOI] [PubMed] [Google Scholar]
- 79.Schulz TJ. Zarse K. Voigt A. Urban N. Birringer M. Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Smith JJ. Kenney RD. Gagne DJ. Frushour BP. Ladd W. Galonek HL. Israelian K. Song J. Razvadauskaite G. Lynch AV. Carney DP. Johnson RJ. Lavu S. Iffland A. Elliott PJ. Lambert PD. Elliston KO. Jirousek MR. Milne JC. Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Speakman JR. Talbot DA. Selman C. Snart S. McLaren JS. Redman P. Krol E. Jackson DM. Johnson MS. Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 82.Stuart JA. Bourque BM. de Souza-Pinto NC. Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737–745. doi: 10.1016/j.freeradbiomed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Tassa A. Roux MP. Attaix D. Bechet DM. Class III phosphoinositide 3-kinase—Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–586. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Testa CM. Sherer TB. Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 85.Tuveson D. Hanahan D. Translational medicine: Cancer lessons from mice to humans. Nature. 2011;471:316–317. doi: 10.1038/471316a. [DOI] [PubMed] [Google Scholar]
- 86.Ungvari Z. Labinskyy N. Mukhopadhyay P. Pinto JT. Bagi Z. Ballabh P. Zhang C. Pacher P. Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Gurp M. Festjens N. van Loo G. Saelens X. Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 88.Wanagat J. Cao Z. Pathare P. Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- 89.White R. Morganstein D. Christian M. Seth A. Herzog B. Parker MG. Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett. 2008;582:39–45. doi: 10.1016/j.febslet.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 90.Wu Z. Huang X. Feng Y. Handschin C. Feng Y. Gullicksen PS. Bare O. Labow M. Spiegelman B. Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Z. Puigserver P. Andersson U. Zhang C. Adelmant G. Mootha V. Troy A. Cinti S. Lowell B. Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 92.Zarse K. Bossecker A. Muller-Kuhrt L. Siems K. Hernandez MA. Berendsohn WG. Birringer M. Ristow M. The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans. Horm Metab Res. 43:241–243. doi: 10.1055/s-0030-1270524. [DOI] [PubMed] [Google Scholar]
- 93.Zarse K. Schmeisser S. Birringer M. Falk E. Schmoll D. Ristow M. Differential effects of resveratrol and SRT1720 on lifespan of adult Caenorhabditis elegans. Horm Metab Res. 2010;42:837–839. doi: 10.1055/s-0030-1265225. [DOI] [PubMed] [Google Scholar]
- 94.Zhu Z. Jiang W. Thompson MD. McGinley JN. Thompson HJ. Metformin as an energy restriction mimetic agent for breast cancer prevention. J Carcinog. 2011;10:17. doi: 10.4103/1477-3163.83043. [DOI] [PMC free article] [PubMed] [Google Scholar]