Abstract
Background
In the Women’s Health Initiative (WHI) randomized trial, estrogen plus progestin increased both breast cancer incidence and mortality. In contrast, most observational studies associate estrogen plus progestin with favorable prognosis breast cancers. To address differences, a cohort of WHI observational study participants with characteristics similar to the WHI clinical trial was studied.
Methods
We identified 41 449 postmenopausal women with no prior hysterectomy and mammogram negative within 2 years who were either not hormone users (n = 25 328) or estrogen and progestin users (n = 16 121). Multivariable-adjusted Cox proportional hazard regression was used to calculate hazard ratios (HRs) with 95% confidence intervals (CI). All statistical tests were two-sided.
Results
After a mean of 11.3 (SD = 3.1) years, with 2236 breast cancers, incidence was higher in estrogen plus progestin users than in nonusers (0.60% vs 0.42%, annualized rate, respectively; HR = 1.55, 95% CI = 1.41 to 1.70, P < .001). Women initiating hormone therapy closer to menopause had higher breast cancer risk with linear diminishing influence as time from menopause increased (P < .001). Survival after breast cancer, measured from diagnosis, was similar in combined hormone therapy users and nonusers (HR = 1.03, 95% CI = 0.79 to 1.35). On a population basis, there were somewhat more deaths from breast cancer, measured from cohort entry (HR = 1.32, 95% CI = 0.90 to 1.93, P = .15), and more all-cause deaths after breast cancer (HR = 1.65, 95% CI = 1.29 to 2.12, P < .001) in estrogen plus progestin users than in nonusers.
Conclusions
Consistent with WHI randomized trial findings, estrogen plus progestin use is associated with increased breast cancer incidence. Because prognosis after diagnosis on combined hormone therapy is similar to that of nonusers, increased breast cancer mortality can be expected.
In the Women’s Health Initiative (WHI) randomized, placebo-controlled clinical trial, estrogen plus progestin increased breast cancers not limited to tumors with favorable prognosis (1,2) and increased breast cancer mortality (3). These findings were similar to observational study results regarding incidence (4) but differ from most reports regarding tumor characteristics and clinical outcome, in which a decrease (5–8) in deaths after breast cancer is commonly, but not universally (9), seen.
To explain these patterns, variation in study populations, including difference in time from menopause to first hormone therapy use (gap time) and differential mammography, have been invoked (10,11). Pursuant to this question, Prentice and colleagues (10) combined WHI randomized trial results with WHI observational study results after 5.6 years of follow-up and suggested that estrogen plus progestin initiation with shorter gap time was associated with greater breast cancer risk. We now extend those findings in the WHI observational study by examining associations between estrogen plus progestin and breast cancer incidence, now with 11.3 years of mean follow-up, and, for the first time, with breast cancer mortality, in analyses adjusting for gap time, ongoing mammography, estrogen plus progestin use, and prior hormone therapy use before cohort entry.
Methods
Study Design and Population
The WHI entered postmenopausal women into four clinical trials (n = 68 132) and an observational study (n = 93 176) at 40 US clinical centers. Study details have been published (1,12). Eligible women were postmenopausal, were aged between 50 and 79 years, and had anticipated 3-year survival.
Women ineligible for or not interested in the clinical trials could enroll in the observational study. For these analyses, observational study participants were identified who met entry criteria similar to the WHI clinical trial evaluating estrogen plus progestin (1). Exclusions included prior hysterectomy or breast cancer. A mammogram not suspicious for breast cancer less than 2 years before entry was required, and women had to either be taking estrogen plus progestin or not using any hormone therapy. Applying these criteria, 41 449 postmenopausal women were eligible, with 25 328 non–hormone users and 16 121 estrogen plus progestin users.
Information on demographics, medical history, lifestyle and breast cancer risk factors were collected using self-report instruments. History of medication use was obtained by interviewer-administered questionnaire (12). Hormone therapy information was collected at baseline and at years 3, 6, 7, and 8. Physical activity information was used to calculate metabolic equivalent values (13). Height and weight were measured to generate body mass index (BMI) determinations. Mammography and breast exam frequency were not protocol determined, but information on usage was collected annually by participant self-report.
Age at menopause was defined as the earliest age of last menstrual bleeding, bilateral oophorectomy, or initiation of hormone therapy. Gap time from menopause to first hormone therapy use was the difference between age at menopause and first use of hormone therapy. Women with menopause defined by hormone therapy initiation had a zero gap time.
Information on breast cancer was collected at annual contacts. Breast cancers were initially verified by medical record review by centrally trained physician adjudicators at each clinical center with final adjudication and coding performed centrally by WHI cancer coders (14).
Statistical Analysis
Baseline characteristics of estrogen plus progestin users were compared with those of nonusers with tests of association adjusted for age and race/ethnicity. Invasive breast cancer incidence rates per 10 000 persons per year were calculated by estrogen plus progestin use.
Cox regression models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for breast cancer incidence among estrogen plus progestin users vs nonusers at baseline. The proportionality assumption was assessed and confirmed by statistical tests and graphical methods for the analysis of deaths from breast cancer, all-cause deaths after breast cancer, and survival after breast cancer. For breast cancer incidence analyses, there was no evidence of nonproportionality after adherence was accounted for. Analyses were adjusted for age, race/ethnicity, BMI, education, smoking status, alcohol use, health status, physical activity, family history, Gail model risk (incorporating age, race, age at first menses,age at first live birth, first-degree relatives with breast cancer, number of prior breast biopsies and atypical hyperplasia [yes/no]) (15), and bilateral oophorectomy and were stratified by age. Event times were defined relative to enrollment date, with censoring defined by end of follow-up, loss to follow-up, or death from causes other than breast cancer. Kaplan–Meier curves describe cumulative breast cancer hazard ratios over time. Multivariable Cox regression models that included the aforementioned covariables and age at diagnosis were also used to investigate survival beginning with cohort entry and with breast cancer diagnosis date. Statistical significance of the three subgroups defined by age, BMI, and Gail risk score were based on a 1 degree of freedom test of trend. Statistical significance of gap time was based on a spline fit that allowed the effect of estrogen plus progestin to vary nonparametrically by gap time, with the smoothness parameter chosen objectively by generalized cross-validation.
In the WHI hormone therapy trials, mammograms not suspicious for breast cancer were required before annual dispensing of study medication.1 To mirror this clinical trial component and adjust for cancer screening, additional analyses censored women for breast cancer incidence when a greater than 2-year interval without a mammogram occurred. Additional analyses addressed the influence of ongoing estrogen plus progestin use by censoring breast cancer incidence events 6 months after participants discontinued use when originally hormone therapy users or began hormone therapy if originally nonusers. Both sensitivity analyses included time-varying weights, which were inversely proportional to the estimated probability of continued mammogram use or adherence in the proportional hazards models to adjust for changes in the distribution of sample characteristics during follow-up.
Tumor characteristics were compared in estrogen plus progestin users and nonusers with χ2 statistics. Women who entered the cohort already using estrogen plus progestin could not have experienced an early, fast-growing breast cancer. Therefore, in exploratory analyses, tumor characteristics in women using estrogen plus progestin at entry with ongoing use through follow-up were compared with those of women who began estrogen plus progestin only after cohort entry, women using estrogen plus progestin at entry who discontinued use, and women who never used hormones. Additional analyses examined interactions between estrogen plus progestin, gap time, and combined hormone therapy duration (5-year increments) on breast cancer incidence.
Analyses were conducted using SAS software, version 9.2 (SAS Institute, Cary, NC) and R version 2.11 (R Development Core Team; http://www.R-project.org.). All statistical tests were two sided. The results reflect findings through September 30, 2010, as of March 31, 2011.
Results
Compared with nonusers, estrogen plus progestin users at entry were younger and more likely to be white, use alcohol, have BMI less than 25, and be at somewhat lower 5-year Gail breast cancer risk (Supplementary Table 1, available online).
After a mean follow-up of 11.3 years (SD = 3.1), 2236 invasive breast cancers were diagnosed. Women who were estrogen plus progestin users entered with a mean 5.3 years of prior hormone therapy use. Breast cancer incidence was higher in estrogen plus progestin users than nonusers (0.60% vs 0.42%, annualized rate, respectively; HR = 1.55, 95% CI = 1.41 to 1.70, P < .001) (Figure 1). Analyses censoring women when a 2-year interval without a mammogram occurred had similar breast cancer association (0.63% vs 0.39%, annualized rate, respectively; HR = 1.61, 95% CI = 1.46 to 1.77, P < .001). Sensitivity analyses adjusted for ongoing combined hormone therapy use had stronger breast cancer association than seen in the analyses including the entire study population (0.75% vs 0.41%; HR = 1.99, 95% CI = 1.68 to 2.37, P < .001) (Table 1). In the subgroup analyses (age, BMI, Gail risk score, and gap time), gap time and BMI showed statistically significant interaction with combined hormone therapy and breast cancer risk. The risk of invasive breast cancer associated with estrogen plus progestin use decreased with BMI (P for interaction = .03); for women with BMI less than 25, 25 to less than 30, and 30 or greater, the hazard ratios were 1.70 (95% CI = 1.48 to 1.95), 1.50 (95% CI = 1.29 to 1.75), and 1.34 (95% CI = 1.11 to 1.62), respectively.
Figure 1.
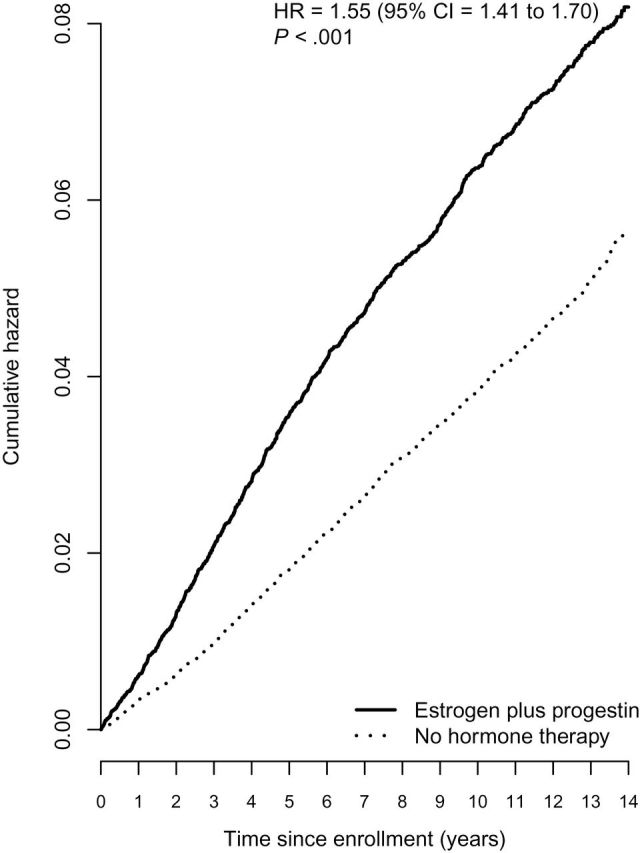
Invasive breast cancer incidence by estrogen plus progestin use at baseline. Analyses were adjusted for age, race or ethnic group, body mass index, education, smoking status, alcohol use, self-reported health, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model, and bilateral oophorectomy and stratified by baseline age group. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
Table 1.
Invasive breast cancer incidence and mortality by estrogen plus progestin use at baseline
| Clinical event Cox regression model | Estrogen plus progestin | No hormone therapy | HR (95% CI) | P |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Invasive breast cancer incidence | ||||
| M1*: Multivariable adjusted | 1097 (0.60%) | 1139 (0.42%) | 1.55 (1.41 to 1.70) | <.001 |
| M2†: M1 censoring for a >2-year interval without a mammogram | 840 (0.63%) | 702 (0.39%) | 1.61 (1.46 to 1.77) | <.001 |
| M3‡: M1 censoring for adherence | 586 (0.75%) | 919 (0.41%) | 1.99 (1.68 to 2.37) | <.001 |
| Deaths from breast cancer | ||||
| M1*: Multivariable adjusted | 54 (0.03%) | 85 (0.03%) | 1.32 (0.90 to 1.93) | .15 |
| M2†: M1 censoring for a >2-year interval without a mammogram | 45 (0.03%) | 55 (0.03%) | 1.31 (0.81 to 2.11) | .27 |
| M3‡: M1 censoring for adherence | 30 (0.04%) | 74 (0.03%) | 1.41 (0.89 to 2.23) | .15 |
| Deaths after breast cancer | ||||
| M1*: Multivariable adjusted | 141 (0.07%) | 192 (0.06%) | 1.65 (1.29 to 2.12) | <.001 |
| M2†: M1 censoring for a >2-year interval without a mammogram | 123 (0.08%) | 120 (0.06%) | 1.87 (1.37 to 2.54) | <.001 |
| M3‡: M1 censoring for adherence | 82 (0.10%) | 169 (0.07%) | 1.62 (1.09 to 2.40) | .02 |
* Analyses were adjusted for age, race or ethnic group, body mass index, education, smoking status, alcohol use, self-reported health, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model, and bilateral oophorectomy and stratified by baseline age group. All statistical tests were two-sided.
† In addition to M1 adjustments, Cox regression model included time-varying weights, which were inversely proportional to the estimated probability of continued mammogram use to adjust for changes in the distribution of sample characteristics during follow-up. All statistical tests were two-sided.
‡ In addition to MI adjustments, Cox regression model included time-varying weights, which were inversely proportional to the estimated probability of adherence (conformation to baseline study group through ongoing use of estrogen plus progestin or no hormone therapy during follow-up) to adjust for changes in the distribution of sample characteristics during follow-up. All statistical tests were two-sided.
Gap time from menopause to initial estrogen plus progestin use was related to breast cancer risk. Highest risk was seen for initiation of combined hormone therapy at menopause (zero gap time) (HR = 1.68, 95% CI = 1.52 to 1.86) and decreased by a factor of 0.87 (95% CI = 0.80 to 0.94) with each 5-year increment in gap time, with subsequent linear decrease in risk through gap times of 15 years (P < .001). Even with long gap times, combined hormone therapy use was associated with increased breast cancer risk (Figure 2). Closely comparable findings were seen in gap time analyses adjusted for hormone therapy duration where a decrease in hazard ratio of 0.88 (95% CI = 0.81 to 0.96) was associated with each 5-year increment in gap time (P = .005).The influence of duration of estrogen plus progestin use on breast cancer risk by gap times less than 5years and 5 or more years are depicted in Supplementary Table 2 (available online). For women with gap time less than 5 years, the hazard ratio for estrogen plus progestin duration of use of less than 5 years was 1.45 (95% CI = 1.13 to 1.88), and for women with gap time of 5 or more years, the hazard ratio for duration of use of less than 5 years was 1.19 (95% CI = 0.92 to 1.55).
Figure 2.
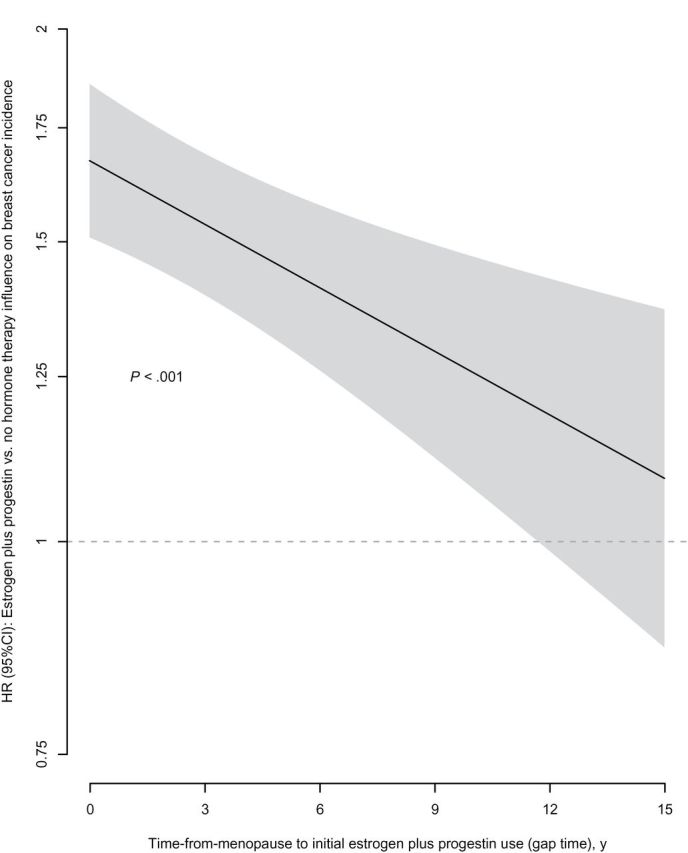
Invasive breast cancer incidence by estrogen plus progestin use at baseline modified by time-from-menopause to hormone therapy initiation (gap time). Nonparametric spline fit for the hazard ratio (HR) (95% confidence interval [CI] in shaded region) of the effect of estrogen plus progestin by gap time. The smoothness of the fit was chosen objectively by generalized cross-validation. P values corresponds to a test of whether the effect of estrogen plus progestin changes with gap time. Analyses were adjusted for age, race or ethnic group, body mass index, education, smoking status, alcohol use, self-reported health, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model, and bilateral oophorectomy and stratified by baseline age group. All statistical tests were two-sided.
Considering all participants classified by hormone therapy use at baseline, tumors in estrogen plus progestin users were more likely to be well differentiated and positive for hormone receptors and less likely to be triple negative (estrogen receptor negative, progestin receptor negative, human epidermal growth factor receptor 2 [HER2] status negative) (Table 2). In exploratory analyses, tumor characteristics in women using estrogen plus progestin at entry with ongoing use through follow-up (n = 586) were compared with those who began estrogen plus progestin only after cohort entry (n = 220), combined hormone therapy users who discontinued use (n = 511), and those who never used hormone (n = 919). The characteristics of breast cancers in women who began combined hormone use only after entry were more likely to be estrogen receptor negative (16.8% vs 9.6%), progesterone receptor negative (28.6% vs 16.6%), triple negative (11.8% vs 2.6%), and poorly differentiated (23.9% vs 19.3%) than those using combined hormone therapy at cohort entry with continuous use (Table 3).
Table 2.
Breast cancer characteristics by estrogen plus progestin use at baseline*
| Estrogen plus progestin | No hormone therapy | ||
|---|---|---|---|
| Characteristic | No. (%) (n = 1097) | No. (%) (n = 1139) | P † |
| Tumor size | .83 | ||
| ≤0.5 cm | 137 (12.9) | 130 (11.9) | |
| >0.5–1 cm | 290 (27.4) | 302 (27.7) | |
| >1–2 cm | 428 (40.5) | 437 (40.1) | |
| >2 cm | 203 (19.2) | 222 (20.3) | |
| No. of positive lymph nodes | .25 | ||
| None | 745 (75.3) | 778 (78.4) | |
| 1–3 | 173 (17.5) | 155 (15.6) | |
| >3 | 71 (7.2) | 59 (5.9) | |
| Positive lymph nodes | 245 (24.7) | 218 (21.9) | .13 |
| Summary stage (SEER) | .04 | ||
| Localized | 827 (76.0) | 869 (77.1) | |
| Regional | 250 (23.0) | 229 (20.3) | |
| Distant | 5 (0.5) | 15 (1.3) | |
| Unknown | 6 (0.6) | 14 (1.2) | |
| SEER stage (regional/distant) | 255 (23.6) | 244 (21.9) | .36 |
| Histology | .07 | ||
| Ductal | 668 (61.4) | 717 (63.6) | |
| Lobular | 121 (11.1) | 124 (11.0) | |
| Ductal and lobular | 177 (16.3) | 158 (14.0) | |
| Tubular | 55 (5.1) | 35 (3.1) | |
| Other | 67 (6.2) | 93 (8.3) | |
| Grade | .008 | ||
| Unknown | 85 (7.8) | 112 (9.9) | |
| Well differentiated | 317 (29.1) | 279 (24.8) | |
| Moderately differentiated | 470 (43.2) | 460 (40.8) | |
| Poorly differentiated/anaplastic | 216 (19.9) | 276 (24.5) | |
| Estrogen receptor assay | <.001 | ||
| Positive | 923 (84.1) | 867 (76.1) | |
| Negative | 104 (9.5) | 175 (15.4) | |
| Borderline | 3 (0.3) | 0 (0.0) | |
| Unknown/not done/missing | 67 (6.1) | 97 (8.5) | |
| Progesterone receptor assay | <.001 | ||
| Positive | 795 (72.5) | 720 (63.2) | |
| Negative | 215 (19.6) | 299 (26.3) | |
| Borderline | 5 (0.5) | 3 (0.3) | |
| Unknown/not done/missing | 82 (7.5) | 117 (10.3) | |
| Erb2 (HER2) status | .74 | ||
| Positive | 118 (10.8) | 122 (10.7) | |
| Negative | 612 (55.8) | 663 (58.2) | |
| Borderline | 9 (0.8) | 8 (0.7) | |
| Unknown/not done/missing | 358 (32.6) | 346 (30.4) | |
| Triple-negative tumor status | <.001 | ||
| Triple negative (ER−/PR−/HER2−) | 42 (3.8) | 92 (8.1) | |
| Other (includes borderline) | 689 (62.8) | 690 (60.6) | |
| Unknown/missing ER/PR/HER2 all/some | 366 (33.4) | 357 (31.3) |
* Some categories do not add up to the total number of cases due to missing data. ER = estrogen receptor; Erb2 and HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor; SEER = Surveillance Epidemiolgy and End Results.
† Based on χ2 test of association where brackets indicate which rows were included in tests. All statistical tests were two-sided.
Table 3.
Breast cancer characteristics by ongoing estrogen plus progestin use§
| Characteristic | Estrogen plus progestin use at baseline and ongoing through follow-up | Estrogen plus progestin use at baseline and stopped | No hormone therapy at baseline and started estrogen plus progestin during follow-up | No hormone therapy at baseline and through follow-up |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Tumor size | ||||
| 0.5 cm | 49 (8.6) | 49 (10.0) | 17 (8.1) | 84 (9.5) |
| >0.5–1 cm | 157 (27.7) | 133 (27.1) | 63 (30.0) | 239 (27.1) |
| >1–2 cm | 233 (41.1) | 195 (39.7) | 80 (38.1) | 357 (40.5) |
| >2 cm | 104 (18.3) | 99 (20.2) | 47 (22.4) | 175 (19.9) |
| Positive lymph node | ||||
| No | 391 (73.8) | 354 (77.0) | 144 (72.0) | 634 (79.6) |
| Yes | 139 (26.2) | 106 (23.0) | 56 (28.0) | 162 (20.4) |
| Summary stage (SEER) | ||||
| Localized | 434 (74.3) | 393 (78.0) | 158 (72.5) | 711 (78.2) |
| Regional | 143 (24.5) | 107 (21.2) | 58 (26.6) | 171 (18.8) |
| Distant | 3 (0.5) | 2 (0.4) | 1 (0.5) | 14 (1.5) |
| Unknown | 4 (0.7) | 2 (0.4) | 1 (0.5) | 13 (1.4) |
| Histology | ||||
| Ductal | 344 (58.9) | 324 (64.3) | 137 (62.8) | 580 (63.8) |
| Lobular | 67 (11.5) | 54 (10.7) | 28 (12.8) | 96 (10.6) |
| Ductal and lobular | 98 (16.8) | 79 (15.7) | 33 (15.1) | 125 (13.8) |
| Tubular/other | 75 (12.8) | 47 (9.3) | 20 (9.2) | 108 (11.9) |
| Grade | ||||
| Unknown | 64 (11.0) | 21 (4.2) | 18 (8.3) | 94 (10.3) |
| Well differentiated | 163 (27.9) | 154 (30.6) | 65 (29.8) | 214 (23.5) |
| Moderately differentiated | 244 (41.8) | 226 (44.8) | 83 (38.1) | 377 (41.5) |
| Poorly differentiated/anaplastic | 113 (19.3) | 103 (20.4) | 52 (23.9) | 224 (24.6) |
| ER | ||||
| Positive | 484 (82.6) | 439 (85.9) | 171 (77.7) | 696 (75.7) |
| Negative | 56 (9.6) | 48 (9.4) | 37 (16.8) | 138 (15.0) |
| Borderline/unknown/missing | 46 (7.8) | 24 (4.7) | 12 (5.5) | 85 (9.2) |
| PR | ||||
| Positive | 433 (73.9) | 362 (70.8) | 141 (64.1) | 579 (63.0) |
| Negative | 97 (16.6) | 118 (23.1) | 63 (28.6) | 236 (25.7) |
| Borderline/unknown/missing | 56 (9.5) | 31 (6.1) | 16 (7.3) | 1.4 (11.3) |
| ERBB2 (HER2) | ||||
| Positive | 55 (9.4) | 63 (12.3) | 27 (12.3) | 95 (10.3) |
| Negative | 252 (43.0) | 360 (70.5) | 154 (70.0) | 509 (55.4) |
| Borderline/unknown/missing | 279 (47.7) | 88 (17.2) | 37 (17.7) | 315 (34.3) |
| Triple-negative tumor | ||||
| Triple negative (ER−/PR−/HER2−) | 15 (2.6) | 27 (5.3) | 26 (11.8) | 66 (7.2) |
| Other (includes borderline) | 293 (50.0) | 396 (77.5) | 155 (70.5) | 535 (58.2) |
| Unknown/missing ER/PR/HER2 all/some | 278 (47.4) | 88 (17.2) | 39 (17.7) | 318 (34.6) |
* The four groups were generated by censoring the follow-up period 6 months after a woman stopped taking the hormone therapy use at baseline, if an estrogen plus progestin user, or 6 months after initiating any hormone therapy, if a nonuser. Tumor characteristics are grouped by participant estrogen plus progestin status as 1) estrogen plus progestin user at baseline and continued through follow-up, 2) estrogen plus progestin user at baseline and stopped, (3) nonuser at baseline and started during follow-up, or (4) nonuser at baseline and through follow-up, and provided in a descriptive table. Groups 1 and 4 represent tumors from adherent estrogen plus progestin users and nonusers, respectively whereas the remaining two groups represent tumors from participants with partial estrogen plus progestin exposure during follow-up. ER = estrogen receptor; Erb2 and HER2 = human epidermal growth factor receptor 2; PR = progesterone receptor; SEER = Surveillance Epidemiology and End Results.
Survival after a breast cancer diagnosis, measured from diagnosis, was similar in estrogen plus progestin users and nonusers (HR = 1.03, 95% CI = 0.79 to 1.35) (Figure 3).
Figure 3.
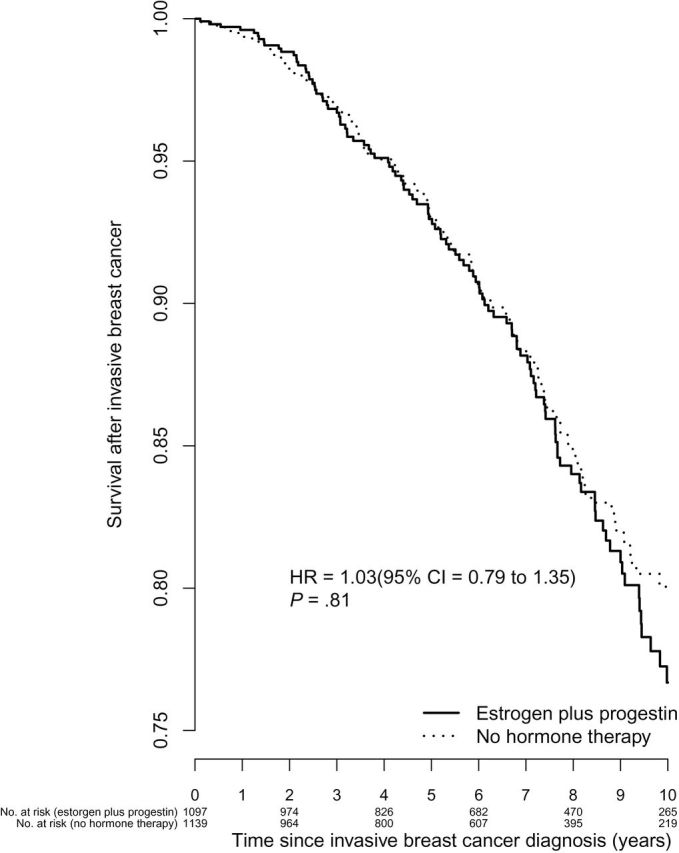
Survival after breast cancer diagnosis by estrogen plus progestin use at baseline. Analyses were adjusted for age, race or ethnic group, body mass index, education, smoking status, alcohol use, self-reported health, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model, bilateral oophorectomy, and age at diagnosis and stratified by baseline age group. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
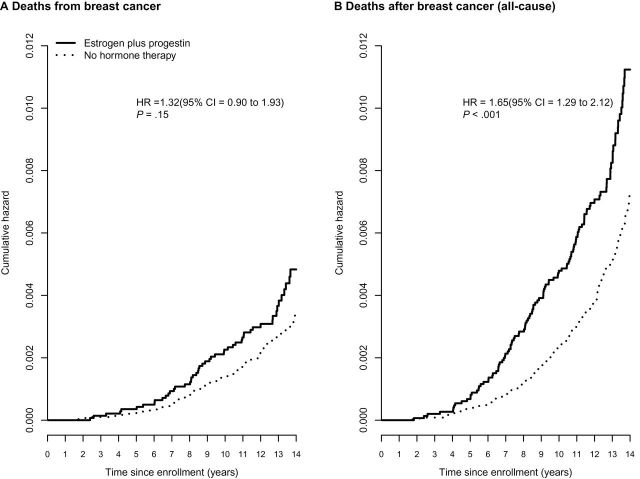
Kaplan–Meier curves of breast cancer mortality, analyzed from cohort entry, are depicted in Figure 4A. The risk of death directly attributed to breast cancer was somewhat greater in estrogen plus progestin users than nonusers (HR = 1.32, 95% CI = 0.90 to 1.93, P = .15), although the difference was not statistically significant. The risk of breast cancer followed by death from any cause was statistically significantly greater in estrogen plus progestin users than nonusers (HR = 1.65, 95% CI = 1.29 to 2.12, P < .001) (Table 1, Figure 4B).
Figure 4.
Breast cancer mortality by estrogen plus progestin use at baseline. Panels depict (A) deaths from breast cancer (deaths directly attributed to the cancer) and (B) deaths after breast cancer (deaths from all causes after the cancer). Analyses were adjusted for age, race or ethnic group, body mass index, education, smoking status, alcohol use, self-reported health, level of physical activity, presence or absence of a family history of breast cancer, estimated breast-cancer risk based on the Gail model, and bilateral oophorectomy. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
In analyses adjusted for ongoing mammography screening, risk of death from breast cancer was greater in estrogen plus progestin users than nonusers (HR = 1.31, 95% CI = 0.81 to 2.11, P = .27), a non-statistically significant difference. However, the risk of breast cancer followed by death from any cause in estrogen plus progestin users was substantially greater than in nonusers (HR = 1.87, 95% CI = 1.37 to 2.54, P < .001). A similar pattern was seen in sensitivity analyses adjusted for ongoing estrogen plus progestin use (Table 1).
Discussion
In a WHI observational study cohort with eligibility corresponding to the WHI randomized clinical trial population, estrogen plus progestin use was associated with increased breast cancer incidence, especially when hormone use was begun closer to menopause. Because survival after breast cancer diagnosis did not differ between estrogen plus progestin users and nonusers, the higher breast cancer incidence of those using estrogen plus progestin may lead to increased breast cancer mortality on a population basis.
These findings for estrogen plus progestin are similar to those from observational studies with respect to breast cancer incidence but differ from most reports with respect to breast cancer characteristics and influence on clinical outcome (5–8). These differences may be related to methodological issues. In the WHI randomized clinical trial, a negative mammogram was an entry requirement, and mammography was protocol defined and closely comparable in the two randomization groups (1,16). Similarly, in the current WHI observational study analyses, the cohort was restricted to women with a recent negative mammogram, and sensitivity analyses were adjusted for ongoing mammography. In other settings, however, hormone therapy users are substantially more likely to have serial mammography than nonusers (17). Because populations screened with mammography have substantially more cancers detected than nonscreened populations (17,18) and screen-detected cancers are commonly early stage with favorable characteristics (19–21), confounding can arise without precise control for mammography in observational studies. This is an issue because mammography frequency is not reliably determined retrospectively (22) and analyses to control for this variable are complex (23).
In contrast with our current findings, a favorable effect on breast cancer survival after diagnosis in estrogen plus progestin users compared with nonusers has been reported in studies unadjusted for mammography (4–7,24), as well as in several recent reports that have adjusted for mammography (8,9,25,26). The adjustments in the later reports were made for “recent mammography” (25), “history of regular mammography” (8), and “mammogram less than 5 years before diagnosis” (26). However, because these analyses did not use an antecedent negative mammogram for cohort eligibility or adjust for ongoing mammography, residual confounding related to differential mammography could persist. A study that adjusted for “a mammogram less than 2 years before the reference date” (9), similar to the eligibility requirement of our cohort, found no difference in survival after breast cancer diagnosis in combined hormone therapy users compared with nonusers.
The current analyses now place findings in the WHI Observational Study in parallel to the WHI randomized trial, where the hazard ratio was 1.25 (95% CI = 1.07 to 1.46) for the estrogen plus progestin influence on invasive breast cancer incidence, with a strong positive dependence on duration of use, and was 1.96 (95% CI = 1.00 to 4.04) for deaths from breast cancer (3).
The findings also support earlier combined analyses in the WHI observational study and clinical trial that demonstrated a somewhat higher breast cancer risk from estrogen plus progestin use when initiated closer to menopause (10). Similar heterogeneity of combined hormone therapy effect modulation by time from menopause was seen in the French E3N cohort (P for interaction = .04) (27), in the Million Women Study (P for interaction < .001) (28), and in updated WHI clinical trial results (P for interaction = .08) (3,11). Thus, the modest breast cancer risk seen with estrogen plus progestin in the WHI clinical trial in women without prior hormone therapy may be related to their longer gap time and may underestimate the actual risk for women beginning hormones close to menopause.
In the WHI randomized trial, breast cancers in estrogen plus progestin were not limited to well-differentiated, hormone receptor–positive cancers (2,3). Although breast cancers seen in all estrogen plus progestin users in the WHI observational study were more likely to be well differentiated and hormone receptor positive, breast cancers in women who began estrogen plus progestin only after cohort entry had breast cancer characteristics approaching those seen in the randomized trial of combined hormone therapy. These findings suggest that, because most cohort reports include current hormone therapy users at entry, a selection bias may have occurred. Becasue hormone receptor–negative and triple-negative cancers “missing” in most observational studies commonly are fast-growing malignancies, women initiating hormone therapy with such preclinical cancers may well develop clinical disease close to therapy initiation and be “selected out” from cohort participation. This concept that reproductive hormones broadly influence breast cancer subtypes has received support from European Prospective Investigation into Cancer and Nutrition (EPIC) cohort studies, in which baseline sex steroid hormones were statistically significantly associated with risk of both hormone receptor–positive (P trend = .002) and –negative (P trend = .05) breast cancers (29) and increased risk of receptor-negative cancers were seen in current hormone therapy users (30).
There are questions regarding the optimal way to translate these research study findings regarding breast cancer into clinical practice. The 96% relative increase in breast cancer deaths in the WHI randomized trial (3), with support by current study findings, suggests a substantial breast cancer mortality risk for combined hormone therapy use. Alternatively, women given absolute risk information of “two additional deaths per 10 000 women per year” (32) may conclude relative breast cancer safety for estrogen plus progestin use. However, from a public health prospective, there should be reasonable caution in recommending a therapy for less than limiting climacteric symptoms, which, broadly applied, will likely increase breast cancer deaths. Although individuation of therapy is commonly recommended (32,33), most women who will be diagnosed with breast cancer have no breast cancer risk factors, and eight of nine cancers are diagnosed in women without a first-degree relative with breast cancer (34). The substantial differences in hormone therapy use across developed Western countries, where about 20% of postmenopausal US women are users (35) compared with only 4% in Spain (36), question what constitutes limiting climacteric symptoms and what factors influence therapy decisions in each culture.
Study strengths include the large, ethnically diverse study population with comprehensive breast cancer risk assessment, eligibility criteria similar to the WHI randomized hormone therapy trial, central adjudication of breast cancers, serial assessment of mammography, and long follow-up. Limitations include the retrospective assessment of prior hormone therapy use and age at menopause, the lack of breast cancer therapy information, and the borderline nature of some breast cancer mortality analyses.
The WHI findings in the randomized clinical trial and the current observational study analyses evaluating estrogen plus progestin in women with no prior hysterectomy differ substantially from those seen in the WHI randomized trial evaluating estrogen alone in women with prior hysterectomy (2,37). Estrogen plus progestin significantly interfered with breast cancer detection and statistically significantly increased breast cancer incidence along with breast cancer mortality (1,3). With estrogen alone, breast cancer incidence was statistically significantly decreased (37), as was breast cancer mortality (38).
In summary, estrogen plus progestin use is associated with increased breast cancer incidence, especially when its use is initiated close to menopause. Because prognosis after a breast cancer diagnosis is similar for combined hormone therapy users and non-users, increased breast cancer mortality on a population basis can be expected. Analyses adjusting for gap time, prior mammography, and prior hormone therapy reconcile much of the difference between observational studies and randomized clinical trial results regarding estrogen plus progestin breast cancer influence on breast cancer characteristics and clinical outcome.
These findings were presented at the 2012 ASCO Annual Meeting.
References
- 1. Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003; 289(24):3243–3253 [DOI] [PubMed] [Google Scholar]
- 2. Chlebowski RT, Anderson GL. Changing concepts: Menopausal hormone therapy and breast cancer. J Natl Cancer Inst. 2012; 104(7):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010; 304(15):1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995; 332(24):1589–1593 [DOI] [PubMed] [Google Scholar]
- 5. Bergkvist L, Adami HO, Persson J, et al. Prognosis after breast cancer diagnosis in women exposed to estrogen and estrogen-progestogen replacement therapy. Am J Epidemiol. 1989; 130(2):221–228 [DOI] [PubMed] [Google Scholar]
- 6. Antoine C, Liebens F, Carly B, et al. Influence of HRT on prognostic factors for breast cancer: a systematic review after the Women’s Health Initiative trial. Human Reprod. 2004; 19(3):741–756 [DOI] [PubMed] [Google Scholar]
- 7. Chen W, Petitti DB, Geiger AM. Mortality following development of breast cancer while using oestrogen or oestrogen plus progestin: a computer record-linkage study. Br J Cancer. 2005; 93(4):392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newcomb PA, Egan KM, Trentham Dietz A, et al. Prediagnostic use of hormone therapy and mortality after breast cancer. Cancer Epidemiol Biomarkers Prev. 2008; 17(4):864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norman SA, Weber AL, Localio AR, et al. Hormone therapy and fatal breast cancer. Pharmacoepidemiol Drug Saf. 2010; 19(5):440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prentice RL, Chlebowski RT, Stefanick ML, et al. Estrogen plus progestin therapy and breast cancer in recently postmenopausal women. Am J Epidemiol. 2008; 167(10):1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chlebowski RT, Anderson GL. The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment. J Natl Cancer Inst. 2011; 103(4):284–285 [DOI] [PubMed] [Google Scholar]
- 12. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Am Epidemiol. 2003; 13(9):S5–S17 [DOI] [PubMed] [Google Scholar]
- 13. McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women. JAMA. 2003; 290(10):1331–1336 [DOI] [PubMed] [Google Scholar]
- 14. National Cancer Institute Surveillance, Epidemiology, and End Results Program. http://seer.cancer.gov/ Accessed May 22, 2012 [Google Scholar]
- 15. Gail MH. Personalized estimates of breast cancer risk in clinical practice and public health. Stat Med. 2011; 30(10):1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chlebowski RT, Anderson GL, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008; 168(4):370–377 [DOI] [PubMed] [Google Scholar]
- 17. Joffe MM, Byrne C, Colditz GA. Postmenopausal hormone use, screening and breast cancer characterization and control of a bias. Epidemiology. 2001; 12(4):429–438 [DOI] [PubMed] [Google Scholar]
- 18. Hofvind S, Sorum R, Haldorsen T, Langmark F. Incidence of breast cancer before and after implementation of mammography screening. Tidsskr Nor Laegeforen. 2006; 126(22):2935–2938 [PubMed] [Google Scholar]
- 19. Kerlikowske K, Miglioretti DL, Ballard-Barbash R, et al. Prognostic characteristics of breast cancer among postmenopausal hormone users in a screened population. J Clin Oncol. 2003; 21(23):4314–4321 [DOI] [PubMed] [Google Scholar]
- 20. Sihto H, Lundin J, Lehtimaki T, et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clin Cancer Res. 2008; 14(13):4103–4110 [DOI] [PubMed] [Google Scholar]
- 21. Dong W, Berry DA, Bevers TB, et al. Prognostic role of detection method and its relationship with tumor biomarkers in breast cancer: the University of Texas MD Anderson Cancer Center experience. Cancer Epidemiol Biomarkers Prev. 2008; 17(5):1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gordon NP, Hiatt RA, Lampert DI. Correspondence of self-reported data and medical record audit for six cancer screening procedures. J Natl Cancer Inst. 1993; 85(7):566–570 [DOI] [PubMed] [Google Scholar]
- 23. Weiss NS. Adjusting for screening history in epidemiologic studies of cancer: why, when, and how to do it. Am J Epidemiol. 2003; 157(11):957–961 [DOI] [PubMed] [Google Scholar]
- 24. Christante D, Pommier S, Garreau J, et al. Improved breast cancer survival among hormone replacement therapy user is durable after 5 years of additional follow-up. Am J Surg. 2008; 196(4):505–511 [DOI] [PubMed] [Google Scholar]
- 25. Rosenberg LU, Granath F, Dickman PW, et al. Menopausal hormone therapy in relation to breast cancer characteristics and prognosis: a cohort study. Breast Cancer Res. 2008; 10(5):R78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reding KW, Doody DR, McTiernan A, et al. Age-related variation in the relationship between menopausal hormone therapy and the risk of dying from breast cancer. Breast Cancer Res Treat. 2001; 126(3):749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fournier A, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Estrogen-progestagen menopausal hormone therapy and breast cancer: does delay from menopause onset to treatment initiation influence risks? J Clin Oncol. 2009; 27(31):5138–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011; 103(4):296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James RE, Lukanova A, Dossus L, et al. Postmenopausal serum sex steroids and risk of hormone receptor-positive and negative breast cancer: a nested case-control study. Cancer Prev Res (Phila). 2011; 4(10):1626–1635 [DOI] [PubMed] [Google Scholar]
- 30. Ritte RE, Lukanova A, Berrino F, et al. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012; 14: R76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malhotra GK, Zhao X, Band H, Band V. Histological, molecular and functional subtypes of breast cancer. Cell Biology & Therapy. 2010; 10 (10):955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. North American Menopause Society The 2012 hormone position statement of the North American Menopause Society. Menopause. 2012; 19(3):257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gompel A, Rozenberg S, Barlow DH. The EMAS 2008 update on clinical recommendations on postmenopausal hormone replacement therapy. Maturitas. 2008; 61(3):227–232 [DOI] [PubMed] [Google Scholar]
- 34. Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001; 358(9291):1389–1399 [DOI] [PubMed] [Google Scholar]
- 35. Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011; 18(4):385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pollan M, Pastor-Barriuso R, Ardanaz E, et al. Recent changes in breast cancer incidence in Spain, 1980–2004. J Natl Cancer Inst. 2009; 101(22):1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lacroix AZ, Chlebowski RT, Manson JE, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011; 305(13):1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placbeo-controlled trial. Lancet Oncol. 2012; 13(5):476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]