Background
People with schizophrenia smoke at higher rates that the general population and are at greater risk for pulmonary and cardiac morbidity and mortality due to their poor diet, sedentary life style and poor preventative medical care1. Additionally, mental health providers are less aggressive in encouraging cessation, either out of fear of causing exacerbation or out of a sense of futility2. Now, use of what is currently thought to be the most effective pharmacologic treatment for tobacco addiction, varenicline3, is hampered by case reports of exacerbations in psychiatric patients taking varenilcine4,5,6. We present here the results of a randomized double blind pilot study that was designed to test the safety, tolerability and efficacy of varenicline in smokers with schizophrenia.
Methods
Stable outpatients were included if they carried a DSM-IV TR7 diagnosis of schizophrenia or schizoaffective disorder for over 3 years, were regular smokers (at least ten cigarettes per day) for one year and scored a total of at least “4” on the Fagerstrom Test for Nicotine Dependency8 (FTND). Other than tobacco, they had no diagnosis of substance abuse in last 3 months or dependence in the past 6 months and were considered psychiatrically stable with no change in medication for 6 months and no change in dose for 3 months. Subjects were excluded, if they had a lifetime history of a suicide attempt or had suicide ideation within 6 months, psychiatric hospitalization within the last 6 months, currently met criteria for a depressive episode, had a Calgary Depression Scale9 total score greater than 10, or if they were currently taking bupropion. During the study, all participants received individual smoking cessation counseling based on the American Lung Association, Freedom from Smoking Program10. The primary outcome measure was smoking cessation, as measured by an end expired carbon monoxide (CO) level of 10 or less11 and subject self report. Secondary measures included the change in CO from baseline to end of study and changes in positive symptoms (measured by the Brief Psychiatric Rating Scale (BPRS), Positive Symptom Items12), depression/anxiety (BPRS Anxiety/Depression Items12) and side-effect measures. Side effects were assessed every week during the treatment phases, using a standard side effect checklist. Following consent, participants were randomized to receive either varenicline (1mg twice daily) or placebo. The study included a 2 week evaluation phase, 2 week premedication phase (individual counseling, only), and a 12 week medication phase (individual counseling plus study medication). The study was approved by the Institutional Review Boards of the University of Maryland and the National Institutes on Drug Abuse.
Results
Nine subjects were randomized (N=4 varenicline, N=5 placebo) and 8 subjects completed. One subject assigned to placebo was withdrawn prior to starting the treatment phase after being diagnosed with cocaine dependence. There were no demographic differences between the groups and no statistical differences were found on the baseline smoking measures or symptoms measures (see Table 1). All subjects were on second generation antipsychotics.
Table 1.
Baseline and Demographic and Clinical Characteristics
| Varenicline (N=4) (mean±SD) |
Placebo (N=4) (mean±SD) |
P value | |
|---|---|---|---|
| Age | 46.3 (9.0) | 44.3 (5.1) | 0.88 |
| Gender | 3 male | 3 male | 1.0 |
| Race | 3 Caucasian | 3 Caucasian | 0.37 |
| Smoking Measures | |||
| Score on FTND | 5.88 (1.31) | 5.75 (2.10) | 0.53 |
| Years smoked | 24.5 (2.38) | 31.0 (2.65) | 0.03 |
| # quit times > 24hr. | 3.5 (1.9) | 2.3 (1.5) | 0.37 |
| Expired CO | 23.87 (19.12) | 26.75 (10.60) | 0.56 |
| Symptom Measures | |||
| BPRS Total | 35.75 (6.34) | 30.25 (4.11) | 0.25 |
| BPRS Positive Item | 11.25 (2.22) | 10.0 (5.94) | 0.56 |
| BPRS Activation | 6.25 (2.87) | 4.25 (1.89) | 0.37 |
| BPRS Anx/ Depr | 5.50 (1.29) | 6.75 (2.06) | 0.30 |
| Calgary Total | 0.75 (0.5) | 0.75 (0.5) | 0.87 |
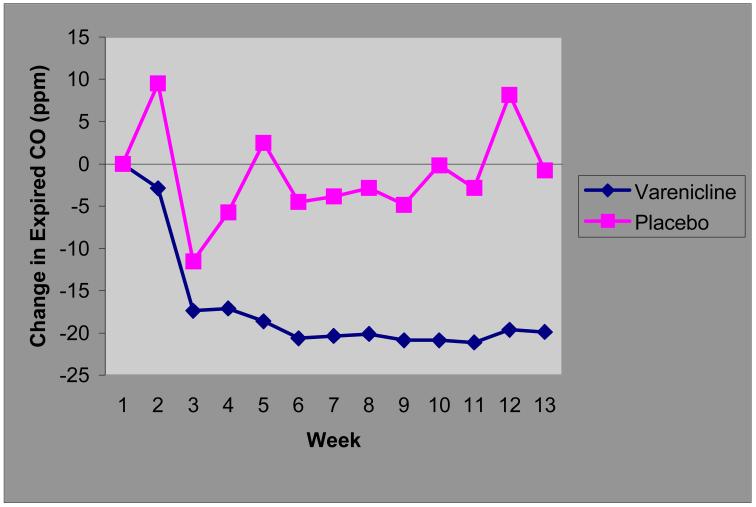
On the primary outcome measure of smoking cessation (defined as expired CO < 10 at each of the last 4 visits), 3 of 4 (75%) varenicline participants achieved sustained abstinence at endpoint and 0/4 of the placebo group were considered abstinent (Fisher’s exact test p = 0.14). Mixed model analysis of covariance found a significant treatment by time interaction (F= 3.28, df=5.1, p= 0.02), with significantly lower CO levels in the varenicline group after week 4 of the Medication Phase (see Figure 1). There was no difference between the groups on the BPRS positive symptom (t=1.7, df=5.1, p=0.29) or anxiety/depression scores (t=0.005, df=4.9, p=0.99). There was a trend toward an increase in the activation item for the varenicline group (mean difference ±s.e. =1.2 ±0.5 2.4, df=5.0, p=0.06). Subjects’ mean total Calgary Depression Scales scores were low by design at baseline and showed little change in either group over the course of the study (t=−0.38, df=4.8, p=0.72). Suicidal ideation (Calgary item 8) was absent in all subjects enrolled at baseline, and remained absent throughout the trial. No subject showed significant exacerbation of psychotic, depressive or other psychiatric symptoms. Overall side effects were low in both groups, though members of the varenicline group tended to report worsening of constipation (2 vs 0), insomnia (3 vs 1), nausea (3 vs 1), all previously reported side effects in the general population.
Figure 1.
Expired Carbon Monoxide (CO) Levels in Varenicline and Placebo Groups
Change in CO levels following the initiation of study medication at week 0.
Participants had 2 sessions of individual counseling before starting study medication at Medication week 0. They were told to quit following one week of study medication (that is at the end of their third counseling session) The average (across visits) varenicline - placebo difference in expired CO was estimated to be −18.8 +/− 5.7 (F=10.76, df=1,5.1, p=0.02); variation in the magnitude of this difference among visits was not significant (F=1.37, df=11,60.2, p=0.21 for treatment × week interaction).
Discussion
To our knowledge this is the first randomized double blind study of varenicline for smoking cessation in people with schizophrenia. Our results in this small sample also support the efficacy of varenicline in people with schizophrenia as observed in naturalistic13 and open label studies14 as well as case series15 and case reports.16,17 Importantly, varenicline was found to be safe and tolerable in the 4 participants randomized to this medication. Those subjects had the expected side effects, including an increase in activation, but this was not associated with any worsening in their psychotic or depressive symptoms. These finding were similar to those of Stapelton et al13 who found that in their mentally ill cohort, varenicline was well tolerated, did not exacerbate psychiatric symptoms and was more effective than nicotine replacement therapy. This study, like ours, had an individual counseling component for all subjects, which might account for the improved cessation rates, as compared to the Smith et al14 open label study, which did not include this component of treatment. Thus, it is apparent that a combined pharmacologic and behavioral approach may be most effective in achieving abstinence in people with schizophrenia.
More research is needed to confirm these preliminary findings, however it is encouraging that this pilot data shows such a robust antismoking effect. In the general population, varenicline has significantly greater efficacy over the currently available alternative agents3 and its use in people with schizophrenia would be an important tool in the fight for smoking cessation in this high risk population. Larger studies are needed to confirm these encouraging results.
Acknowledgments
Supported in part by the National Institutes on Drug Abuse Residential Research Services Contract (N01DA5-9909 Kelly PI).
References
- 1.Kelly DL, McMahon RP, Wehring HJ, Liu F, Mackowick KM, Boggs DL, Warren KR, Feldman S, Shim JC, Love RC, Dixon L. Cigarette Smoking and Mortality Risk in People With Schizophrenia. Schizophr Bull. 2009 Dec 17; doi: 10.1093/schbul/sbp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry. 2003;160(12):2228–2230. doi: 10.1176/appi.ajp.160.12.2228. [DOI] [PubMed] [Google Scholar]
- 3.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008 Jul 16;(3):CD006103. doi: 10.1002/14651858.CD006103.pub3. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration . Public Health Advisory: Important Information on Chantix (varenicline) U S Food and Drug Administration, Center for Drug Evaluation and Research; [Accessed May 4, 2008]. Feb 1, 2008. http://www.fda.gov/cder/drug/advisory/varenicline.htm. [Google Scholar]
- 5.Freedman R. Exacerbation of schizophrenia by varenicline. Am J Psychiatry. 2007 Aug;164(8):1269. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- 6.Popkin MK. Exacerbation of recurrent depression as a result of treatment with varenicline. Am J Psychiatry. 2008 Jun;165(6):774. doi: 10.1176/appi.ajp.2008.07111735. [DOI] [PubMed] [Google Scholar]
- 7.First MB, Spitzer RL, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV Axis Disorders (SCID-IV) Biometrics Research Department, New York State Psychiatric Institute; New York: 1997. [Google Scholar]
- 8.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127.18. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 9.Addington D, Addington J, Matrick-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 10.Freedom from Smoking, Guide for Clinic Facilitators. American Lung Association; 2007. [Google Scholar]
- 11.Henningfield JE, Stitzer ML, Griffiths RR. Expired air carbon monoxide accumulation and elimination as a function of number of cigarettes smoked. Addict Behav. 1980;5:265–272. doi: 10.1016/0306-4603(80)90049-0. [DOI] [PubMed] [Google Scholar]
- 12.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 13.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008 Jan;103(1):146–54. doi: 10.1111/j.1360-0443.2007.02083.x. Epub 2007 Nov 19. [DOI] [PubMed] [Google Scholar]
- 14.Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, Sershen H, Lajtha A. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009 May;110(1-3):149–55. doi: 10.1016/j.schres.2009.02.001. Epub 2009 Feb 28. [DOI] [PubMed] [Google Scholar]
- 15.Evins AE, Goff DC. Varenicline treatment for smokers with schizophrenia: a case series. J Clin Psychiatry. 2008 Jun;69(6):1016. doi: 10.4088/jcp.v69n0620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anghelescu I. Successful smoking cessation and improvement of negative symptoms with varenicline in a stable schizophrenia patient. J Neuropsychiatry Clin Neurosci. 2009 Winter;21(1):102–3. doi: 10.1176/jnp.2009.21.1.102. [DOI] [PubMed] [Google Scholar]
- 17.Fatemi SH. Varenicline efficacy and tolerability in a subject with schizophrenia. Schizophr Res. 2008 Aug;103(1-3):328–9. doi: 10.1016/j.schres.2008.05.002. Epub 2008 Jun 24. [DOI] [PubMed] [Google Scholar]