Abstract
Cysteine thiyl radicals engage in reversible intramolecular hydrogen transfer reactions with amino acid residues in peptides and proteins. These reactions can be experimentally demonstrated through covalent H/D exchange when experiments are carried out in D2O. To this end, hydrogen transfer reactions have been observed between Cys thiyl radicals and Gly, Ala, Ser, Val and Leu in both model peptides and a protein, insulin. The relevance of such reactions for protein oxidation under conditions of oxidative stress is discussed.
Introduction
Protein cysteine (Cys) residues are prominent targets for chemical modification [1], and such modifications can have significant functional and conformational consequences [2, 3]. For example, the reversible modification of Cys through S-nitrosation, S-glutathiolation or sulfenic acid formation plays an important role in signaling processes [2, 3]. Thiyl radicals (RS•) have been implicated in at least one possible mechanism of S-nitrosation, i.e. the radical-radical reaction 1 [4-6]. In theory, thiyl radicals can also serve as origin for S-glutathiolation (reactions 2 and 3, where GS– denotes the thiolate form of glutathione) and sulfenic acid formation (reactions 4 and 5).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Thiyl radicals easily form via hydrogen transfer from Cys to a variety of carbon- and oxygen-centered radicals, or via electron transfer from the thiolate form of Cys to any suitable one-electron acceptor [7]. In radiation biology, and the biology of oxidative stress, in general, hydrogen transfer processes of thiols had been referred to as “repair reactions”, for example “repairing” radicals located on DNA strands [8]. Only later was the reverse reaction, hydrogen abstraction by thiyl radicals, recognized when thiyl radicals were utilized for the inversion at chiral centers in organic molecules [9, 10]. Time-resolved pulse radiolysis experiments provided rate constants for hydrogen abstraction by thiyl radicals (reaction 7) from aliphatic alcohols and ethers, which are on the order of 103-104 M-1s-1 [11, 12]. These are several orders of magnitude slower compared to hydrogen transfer processes from thiols to carbon-centered radicals from alcohols and ethers (reaction -7), which generally proceed with rate constants on the order of 106-108 M-1s-1 [7]. Hence, for aliphatic alcohols and ethers, equilibrium 7 is located far on the left hand side, well accounted for by the differences in the homolytic bond dissociation energies (BDE) between the S-H bond of Cys, BDE(S-H, Cys) = 367 kJ/mol [13], and the C-H bonds of aliphatic alcohols and ethers, e.g., BDE(C-H) = 393 kJ/mol for CH3OH [14]. Nevertheless, several enzymes utilize thiyl radicals for turnover: in ribonucleotide reductase [15-17], benzylsuccinate synthase [18, 19], and glycerol dehydratase [20], thiyl radicals engage in hydrogen transfer reactions with substrates (while in pyruvate formate lyase, thiyl radicals add to the carbonyl group of pyruvate [17, 21]).
| (7-7) |
A more efficient hydrogen abstraction by thiyl radicals would be expected from substrates containing C-H bonds of lower homolytic bond dissociation energies. The αC-H bonds of amino acid residues in peptides and proteins can have significantly lower homolytic bond dissociation energies, depending on peptide and protein conformation. For example, for fully optimized structures of model peptides, BDE(C-H, Gly)opt = 348 kJ/mol [22], while for an α-helix, BDE(C-H, Gly)helix ≈ 361 kJ/mol [22], i.e.values which are smaller than BDE(S-H, Cys) = 367 kJ/mol [13]. Moreover, peptides and proteins offer the opportunity for intramolecular hydrogen transfer reactions. Therefore, the formation of thiyl radicals in peptides and proteins may lead to hydrogen abstraction from various amino acid sites, which ultimately may result in irreversible damage to the peptide or protein. Our laboratory has recently provided evidence for quite efficient hydrogen abstraction reactions by thiyl radicals within peptides and proteins in solution. These experiments are summarized below, followed by a discussion of the potential consequences of these reactions.
Hydrogen abstraction by thiyl radicals from C-H bonds in peptides
The intermolecular reactions of thiyl radicals with amino acids within model peptide structures, N-acetyl amino acid amides and diketopiperazines, proceed with rate constants on the order of k = 103-105 M-1s-1 [23]. Considering the significantly lower BDEs of the αC-H bonds of amino acid residues compared to the C-H bonds of alcohols and ethers, the similar rate constants suggest that hydrogen transfer reactions by thiyl radicals are not controlled by thermodynamics alone. In fact, polar effects promote the reactions of thiyl radicals with alcohols and ethers [10, 24], but may be of lower significance for the reactions of thiyl radicals with amino acid residues in peptides. It has been concluded that the αC-H bonds of amino acid residues are deactivated by inductive effects, especially in reactions with highly oxidizing radicals such as the hydroxyl radical (HO•) and chlorine radical (Cl•), but such inductive effects are less likely to control the reactions of less oxidizing radicals (such as thiyl radicals) [25].
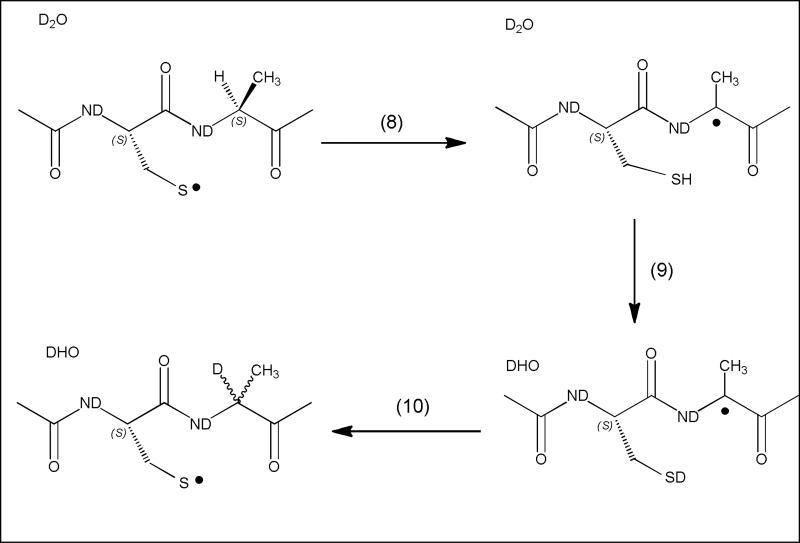
Experimental evidence for intramolecular hydrogen transfer reactions between thiyl radicals and nearby amino acid residues in peptides and proteins was obtained through covalent H/D exchange [26-29], as outlined in Scheme 1 for a model peptide containing the –Cys-Ala-subsequence. Generally, thiyl radicals were generated through the photolytic cleavage of disulfide bonds and the ensuing incorporation of deuterium quantified by mass spectrometry.
Scheme 1.
Reversible intramolecular hydrogen transfer between a Cys thiyl radical and an αC-H bond in a peptide
Reaction 8 represents the initial intramolecular hydrogen transfer from the αC-H bond of Ala in position n+1 relative to the Cys thiyl radical, generating a carbon-centered radical and Cys. In D2O, the S-H bond rapidly converts to an S-D bond (reaction 9), which reacts with the carbon-centered radical (reaction 10). Pulse radiolysis experiments have provided rate-constants for the reversible hydrogen transfer between Cys thiyl radicals and Ala for the model peptide N-Ac-Cys-Ala-Ala-Asp-Ala-Ala-Ala (reactions 10/-10), where k10 ≈ 104 M-1s-1 and k–10 ≈ 105 M-1s-1 [30]. Importantly, equilibrium 10/-10 bears the opportunity for the inversion of the chiral center at the αC-position of Ala, and L-Ala-to-D-Ala conversion was experimentally detected after photolysis of the disulfide bond-containing dipeptide (Leu-Gly-Ala-Cys-Ala-Gly-Leu)2 [29]. Substitution of Ala by Gly in the model peptide N-Ac-Cys-Gly-Gly-Asp-Gly-Gly-Gly resulted in a 10-fold enhancement of both the forward (reaction 11) and reverse (reaction -11) hydrogen transfer compared to that with Ala, i.e. k11 ≈ 105 M-1s-1 and k–11 ≈ 106 M-1s-1 [30]. Hence, K10 (= k10/k-10) ≈ K11 (=k11/k-11), which may be expected considering the rather similar αC-H BDEs of Gly (348-350 kJ/mol [13, 31] and Ala (345 kJ/mol [31]), while the absolute rate constants differ significantly.
| (10-10) |
| (11-11) |
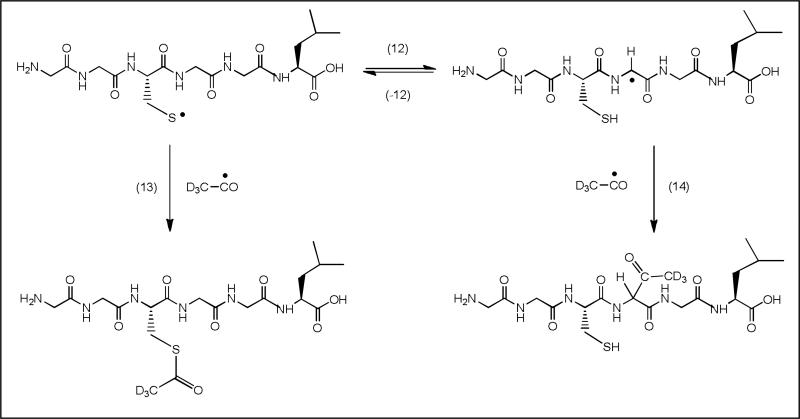
Additional evidence for the intermediary formation of αC• radicals was derived from complementary experiments, where thiyl radicals were generated via the photolysis of acetone in the presence of Cys-containing peptides [29]. The photolysis of acetone (CH3COCH3) yields •CH3 and CH3CO•; when •CH3 and CH3CO• were reacted with Leu-Gly-Ala-Cys-Ala-Gly-Leu in D2O, significant incorporation of deuterium was detected in all amino acids of the subsequence Cys4-Ala5-Gly6. The photolysis of deuterated acetone (CD3COCD3) leads to •CD3 and CD3CO•, which were reacted with Gly-Gly-Cys-Gly-Gly-Leu. Both carbon-centered radicals are expected to react primarily with the Cys thiol group, yielding thiyl radicals, which equilibrate with αC• radicals (Scheme 2; reactions 12/-12). Mass spectrometry analysis revealed a series of radical-radical combination products consistent with equilibrium 12/-12. For example, the fragmentation pattern of a reaction product with m/z 508.2 is rationalized by the reaction of CD3CO• with (i) the thiyl radical of Gly-Gly-Cys-Gly-Gly-Leu (Scheme 2; reaction 13) and (ii) the αC• radical at the Gly residue at position n+1 from Cys (Scheme 2; reaction 14).
Scheme 2.
Radical-radical combination products between peptide radicals and acetyl radicals during the photochemical decomposition of acetone-d6 in the presence of Gly-Gly-Cys-Gly-Gly-Leu.
From the relative intensity of characteristic mass spectrometric fragments of both reaction products we estimate that the ratio of thiyl radical to αC• radical in equilibrium 12 is on the order of ca. (5-10):1, which is good agreement with the equilibrium constant K11 ≈ 0.1, determined for the intramolecular reaction of Cys thiyl radical and Gly in the peptide N-Ac-Cys-Gly-Gly-Asp-Gly-Gly-Gly.
Hydrogen abstraction by thiyl radicals from C-H bonds in proteins
The intramolecular hydrogen abstraction by thiyl radicals in a protein was experimentally studied through the photolysis of insulin [27]. Insulin possesses three disulfide bonds, of which two connect the A- and B-chains, and one is an intrachain disulfide bond located on the A-chain. It should be noted that the photolysis of insulin can generate thiyl radicals not only via direct homolysis of one or more of the disulfide bonds, but also via photo-induced electron transfer from a Tyr residue to the disulfide bond, resulting in cleavage of the disulfide bond into thiyl radical and thiolate. Importantly, when insulin was subjected to photoirradiation in D2O, only six amino acids incorporated significant amounts of deuterium (where the letter and number in parenthesis indicate the location on A- or B-chain, respectively): Leu(B6), Gly(B8), Ser(B9), Val(B18), Gly(B20), and Cys(A20). This rather selective H/D exchange is best rationalized by the secondary structure of insulin, which may (i) limit the access of thiyl radicals to amino acid residues, and (ii) prevent hydrogen transfer reactions through control of C-H BDEs. We note, that the BDE of a Gly αC-H bond is significantly higher when Gly is located in an α-helix (BDE = 361 kJ/mol [22]) or in a β-sheet (BDE = 402 kJ/mol [22]), compared to a Gly residue within a fully optimized structure (BDE = 348 kJ/mol [22]).
More recently, we have extended our studies to radical reactions with Cys residues on (i) glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and (ii) the sarco/endoplasmic reticulum Ca-ATPase (SERCA). In preliminary experiments, both proteins were exposed in D2O to radicals generated through (1) the thermal decomposition of 2,2′-azobis-2-methylpropanimidamide, dihydrochloride (AAPH), and (2) the decomposition of peroxynitrite (ONOO–) in the presence of bicarbonate (HCO3–). These reactions resulted in significant incorporation of deuterium into specific peptide sequences of both GAPDH and SERCA (unpublished data), analogous to our results with model peptides delineated in reactions 8-10 in Scheme 1.
Relevance to the chemistry and biology of protein oxidation
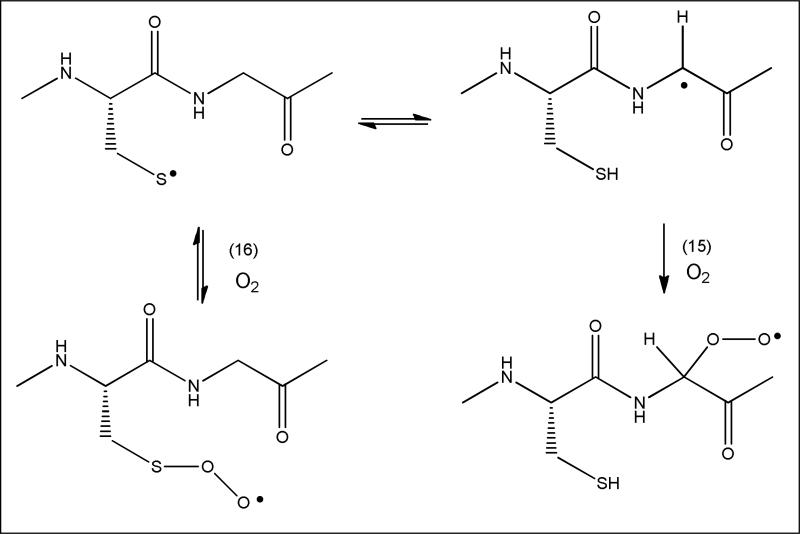
During conditions of oxidative stress, tissue is exposed to a variety of reactive oxygen and nitrogen species, which display significantly different reactivities towards biomolecules [32]. For example, the hydroxyl radical (generated through exposure to ionizing radiation, the reduction of hydrogen peroxide, or the homolysis of peroxynitrous acid) is a strong oxidant which reacts very fast to diffusion-controlled with most amino acids [33]. In fact, the reactivity of amino acids in a protein towards hydroxyl radicals is controlled largely by their surface exposure. On the other hand, superoxide shows very low reactivity towards proteins (except for protein-bound redox-active transition metals). Other radicals of biological significance are NO, NO2, carbon-centered radicals (-•CH-), peroxyl radicals (ROO•), alcoxyl radicals (RO•), phenoxyl radicals (e.g., tyrosyl radicals, TyrO•), and the carbonate radical (CO3•–) [32, 34]. Except NO, all of these radicals have in common, that they oxidize thiols to thiyl radicals. Moreover, among the 20 essential amino acids represented in mammalian proteins, Cys represents the amino acid most easily attacked by all these radicals. In fact, superoxide does not react at a measurable rate with any of these amino acids except Cys, and the rate constant for this reaction is slow (ca. 102-103 M-1s-1) [35]. The same is true for tyrosyl radicals; most carbon-centered radicals react with thiols with rate-constants on the order of 106-108 M-1s-1 [7] while reactions with the other amino acids are comparatively slow. Hence, a protein Cys residue will always represent a preferred target for these radicals, and, except for most carbon-centered radicals, the reactivity of Cys will be enhanced through deprotonation of the thiol. Therefore, especially thiols with low pKa values constitute hot spots for free radical oxidation. Once formed, a peptide or protein thiyl radical has then the opportunity to abstract a hydrogen atom from a nearby aliphatic amino acid. Though these reactions are reversible, the data presented above provide evidence that the resulting carbon-centered radicals can react via additional pathways. In Scheme 2, such possibility is represented by reaction 14, where the carbon-centered radical combines with an acetyl radical. In tissue, carbon-centered radicals may react with oxygen, generating amino acid peroxyl radicals (Scheme 3; reaction 15). Such peroxyl radicals on protein backbones may ultimately lead to protein fragmentation [36]. We note that also thiyl radicals add oxygen, generating thiyl peroxyl radicals (reaction 16). However, oxygen addition to thiyl radicals is reversible with a relatively high rate constant for oxygen elimination (reaction -16; k-16 = 6.3×105 s-1 for thiyl radicals from 2-mercaptoethanol) [37].
Scheme 3.
The reaction of oxygen with peptide radicals
Reaction 17 displays the reaction of a carbon-centered radical with Cys, and reaction 18 the hydrogen abstraction by the ensuing thiyl radical from an amino acid. In this sequence, the thiol is restored, and has functioned as a catalyst for the reaction of the carbon-centered radical with the amino acid. An analogous catalytic function of thiols has been described before for synthetic organic reactions with alcohols and ethers, referred to as “polarity-reversal catalysis” [10].
| (17) |
| (18) |
In proteins, such catalytic role of Cys residues may have significant consequences such as fragmentation and/or aggregation. Moreover, the possible inversion of a chiral center, i.e. the conversion of an L-amino acid to a D-amino acid, such as demonstrated for L-Ala, may be of relevance to protein conformation and immunogenicity. Future experiments must show to what extent especially proteins with low pKa thiols are susceptible to the mechanisms described above. Considering that one possible mechanism for the formation of S-nitrosothiols involves the reaction of protein thiyl radicals with NO [4-6], and that physiologic concentrations of NO are low, the competitive reaction of such thiyl radicals with amino acid C-H bonds must be considered.
Conclusion
The intramolecular reaction of peptide and protein thiyl radicals with C-H bonds of surrounding amino acids is reversible, and leads to intermediary carbon-centered radicals. The extent of such reactions will depend on peptide and protein sequence and structure, and has the potential for irreversible modifications of peptides and proteins such as epimerization and/or fragmentation. Through hydrogen transfer reactions from other amino acids, Cys and Cys thiyl radicals can function as catalysts for protein damage.
Acknowledgement
We gratefully acknowledge financial support from Amgen Inc and the NIH (P01AG12993).
References
- 1.Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic. Biol. Med. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Janssen-Heininger YMW, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: priciples, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J. Biol. Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 5.Madej E, Folkes LK, Wardman P, Czapski G, Goldstein S. Thiyl radicals react with nitric oxide to form S-nitrosothiols with rate constants near the diffusion-controlled limit. Free Radic. Biol. Med. 2008;44:2013–2018. doi: 10.1016/j.freeradbiomed.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Bosworth CA, Toledo JC, Jr., Zmijewski JW, Li Q, Lancaster JR., Jr. Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. USA. 2009;106:4671–4676. doi: 10.1073/pnas.0710416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Sonntag C. Free radical reactions involving thiols and disulfides. In: Chatgilialoglu C, Asmus K-D, editors. Sulfur-Centered Reactive Intermediates in Chemistry and Biology. Plenum Press; New York: 1990. pp. 359–366. [Google Scholar]
- 8.Von Sonntag C. The Chemical Basis of Radiation Biology. Taylor & Francis; London: 1987. pp. 353–374. [Google Scholar]
- 9.Akhlaq MS, Schuchmann H-P, von Sonntag C. The reverse of the “repair” reaction of thiols. The H-abstraction from carbon by the thiyl radical. Int. J. Radiat. Biol. 1987;51:91–102. doi: 10.1080/09553008714550531. [DOI] [PubMed] [Google Scholar]
- 10.Dang H-S, Roberts BP, Tocher DA. Selective radical-chain epimerization at electron-rich chiral tertiary C-H centres using thiols as protic polarity reversal catalysts. J. Chem. Soc., Perkin Trans. 2001;1:2452–2461. [Google Scholar]
- 11.Schöneich Ch., Bonifacić M, Asmus K-D. Reversible H-atom abstraction from alcohols by thiyl radicals: determination of absolute rate constants by pulse radiolysis. Free Radic Res. Commun. 1989;6:393–40. doi: 10.3109/10715768909087923. [DOI] [PubMed] [Google Scholar]
- 12.Schöneich Ch., Asmus K-D, Bonifacić M. Determination of absolute rate constants for the reversible hydrogen-atom transfer between thiyl radicals and alcohols or ethers. J. Chem. Soc. Faraday Trans. 1995;91:1923–1930. [Google Scholar]
- 13.Rauk A, Yu D, Armstrong DA. Oxidative damage to and by cysteine in proteins: an ab initio study of the radical structures, C-H, S-H, and C-C bond dissociation energies, and transition structures for H abstraction by thiyl radicals. J. Am. Chem. Soc. 1998;120:8848–8855. [Google Scholar]
- 14.McMillen DF, Golden DM. Hydrocarbon bond dissociation energies. Ann. Rev. Phys. Chem. 1982;33:493–532. [Google Scholar]
- 15.Stubbe J, van der Donk WA. Protein radicals in enzyme catalysis. Chem. Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 16.Lenz R, Giese B. Studies on the mechanism of ribonucleotide reductases. J. Am. Chem. Soc. 1997;119:2784–2794. [Google Scholar]
- 17.Himo F, Siegbahn PEM. Quantum chemical studies of radical-containing enzymes. Chem. Rev. 2003;103:2421–2456. doi: 10.1021/cr020436s. [DOI] [PubMed] [Google Scholar]
- 18.Himo F. Catalytic mechanism of benzylsuccinate synthase, a theoretical study. J. Phys. Chem. B. 2002;106:7688–7692. [Google Scholar]
- 19.Li L, Marsh ENG. Deuterium isotope effects in the unusual addition of toluene to fumarate catalyzed by benzylsuccinate synthase. Biochemistry. 2006;45:13932–13938. doi: 10.1021/bi061117o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Gallo AA, Florián J, Liu Y-S, Mora S, Xu W. QM/MM (ONIOM) study of glycerol binding and hydrogen abstraction by the coenzyme B12-independent dehydratase. J. Phys. Chem. B. 2010;114:5497–5502. doi: 10.1021/jp910349q. [DOI] [PubMed] [Google Scholar]
- 21.Becker A, Fritz-Wolf K, Kabsch W, Knappe J, Schultz S, Wagner AFV. Structure and mechanism of the glycyl radical enzyme pyruvate formate-lyase. Nature Struct. Biol. 1999;6:969–975. doi: 10.1038/13341. [DOI] [PubMed] [Google Scholar]
- 22.Rauk A, Yu D, Armstrong DA. Toward site specificity of oxidative damage in proteins: C-H and C-C bond dissociation energies and reduction potentials of the radicals of alanine, serine, and threonine residues – an ab initio study. J. Am. Chem. Soc. 1997;119:208–217. [Google Scholar]
- 23.Nauser T, Schöneich Ch. Thiyl radicals abstract hydrogen atoms from the αC-H bonds in model peptides: absolute rate constants and effect of amino acid structure. J. Am. Chem. Soc. 2003;125:2042–2043. doi: 10.1021/ja0293599. [DOI] [PubMed] [Google Scholar]
- 24.Reid DL, Shustov GV, Armstrong DA, Rauk A, Schuchmann MN, Akhlaq S, von Sonntag C. H-atom abstraction from thiols by C-centered radicals. A theoretical and experimental study of reaction rates. Phys. Chem. Chem. Phys. 2002;4:2965–2974. [Google Scholar]
- 25.Watts ZI, Easton CJ. Peculiar stability of amino acids and peptides from a radical perspective. J. Am. Chem. Soc. 2009;131:11323–11325. doi: 10.1021/ja9027583. [DOI] [PubMed] [Google Scholar]
- 26.Mozziconacci O, Sharov V, Williams TD, Kerwin B, Schöneich Ch. Peptide cysteine thiyl radicals abstract hydrogen atoms from surrounding amino acids: the photolysis of a cystine-containing model peptide. J. Phys. Chem. B. 2008;112:9250–9257. doi: 10.1021/jp801753d. [DOI] [PubMed] [Google Scholar]
- 27.Mozziconacci O, Williams TD, Kerwin BA, Schöneich Ch. Reversible intramolecular hydrogen transfer between protein cysteine thiyl radicals and αC-H bonds in insulin: control of selectivity by secondary structure. J. Phys. Chem. B. 2008;112:15921–15932. doi: 10.1021/jp8066519. [DOI] [PubMed] [Google Scholar]
- 28.Mozziconacci O, Kerwin BA, Schöneich Ch. Photolysis of an intrachain peptide disulfide bond: primary and secondary processes, formation of H2S, and hydrogen transfer reactions. J. Phys. Chem. B. 2010;114:3668–3688. doi: 10.1021/jp910789x. [DOI] [PubMed] [Google Scholar]
- 29.Mozziconacci O, Kerwin BA, Schöneich Ch. Reversible hydrogen transfer between cysteine thiyl radical and glycine and alanine in model peptides: evidence for carbon-centered radical through radical-radical reactions and L- to D-Ala conversion. J. Phys. Chem. B. 2010;114:6751–6762. doi: 10.1021/jp101508b. [DOI] [PubMed] [Google Scholar]
- 30.Nauser T, Casi G, Koppenol WH, Schöneich Ch. Reversible intramolecular hydrogen transfer between cysteine thiyl radicals and amino acids in model peptides: absolute rate constants derived by pulse radiolysis and laser flash photolysis. J. Phys. Chem. B. 2008;112:15034–15044. doi: 10.1021/jp805133u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauk A, Yu D, Taylor J, Shustov GV, Block DA, Armstrong DA. Effects of structure on αC-H bond enthalpies of amino acid residues: relevance to H transfers in enzyme mechanisms and protein oxidation. Biochemistry. 1999;38:9089–9096. doi: 10.1021/bi990249x. [DOI] [PubMed] [Google Scholar]
- 32.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 33.Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O–) in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:513–886. [Google Scholar]
- 34.Augusto O, Bonini G, Amanso AM, Linares E, Santos CC, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 35.Jones CM, Lawrence A, Wardman P, Burkitt MJ. Electron paramagnetic resonance spin trapping investigation into the kinetics of glutathione oxidation by the superoxide radical: re-evaluation of the rate constant. Free Radic. Biol. Med. 2002;32:982–990. doi: 10.1016/s0891-5849(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins CL, Davies MJ. Generation and propagation of radical reactions on proteins. Biochim. Biophys. Acta. 2001;1504:196–219. doi: 10.1016/s0005-2728(00)00252-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zhang N, Schuchmann H-P, von Sonntag C. Pulse radiolysis of 2-mercaptoethanol in oxygenated aqueous Solution. Generation and reactions of the thiylperoxyl radical. J. Phys. Chem. 1994;98:6541–6547. [Google Scholar]