Abstract
Stroke is one of the leading causes of death and disability in developed countries. Since protecting neurons alone is not sufficient for stroke therapy, research has shifted to the rescue of multiple cell types in the brain. In particular, attention has focused on the study of how cerebral blood vessels and brain cells communicate with each other. Recent findings suggest that cerebral endothelial cells may secrete trophic factors that nourish neighboring cells. Although data are strongest in terms of supporting endothelial-neuronal interactions, it is likely that similar interactions occur in white matter as well. In this mini-review, we summarize recent advances in the dissection of cell–cell interactions in white matter. We examine two key concepts. First, trophic interactions between vessels and oligodendrocytes (OLGs) and oligodendrocyte precursor cells (OPCs) play critical roles in white matter homeostasis. Second, cell–cell trophic coupling is disturbed under diseased conditions that incur oxidative stress. White matter pathophysiology is very important in stroke. A deeper understanding of the mech- anisms of oligovascular signaling in normal and pathologic conditions may lead us to new therapeutic targets for stroke and other neurodegenerative diseases.
Keywords: oligovascular signaling, white matter injury, stroke, oligovascular niche, oligodendrocyte, neurovascular unit
INTRODUCTION
Over the past several decades, study of central nervous system (CNS) pathologies has focused on intra-neuronal mechanisms. In recent years, however, this neuron-based model has gradually shifted to a more integrated paradigm that emphasizes cell–cell interactions.1) The concept of the “neurovascular unit” emphasizes the importance of multiple brain cell types in many CNS diseases (Fig. 1; the reader is encouraged to seek more detailed reviews on this broad topic.2—7)). Briefly, this neurovascular paradigm is under- scored by dynamic interactions between cerebral endothelial cells, astrocytes, neurons, and the extracellular matrix. Perhaps, the role of the cerebral endothelium is especially important. Traditionally, cerebral endothelial cells have been thought as inert pipes for blood flow to the brain. But, recent findings suggest that cerebral endothelium may be an endocrine organ embedded within the brain itself.1,8,9) Endothelial-derived growth factors may nourish other cell types in the brain. Consequently, diseased conditions may interrupt these neurovascular trophic signals, thus contributing to loss of normal brain function. Hence, elucidating the mechanisms of cell–cell signaling in the brain may be crucial for our ongoing search for effective therapeutics in CNS diseases suchas stroke.
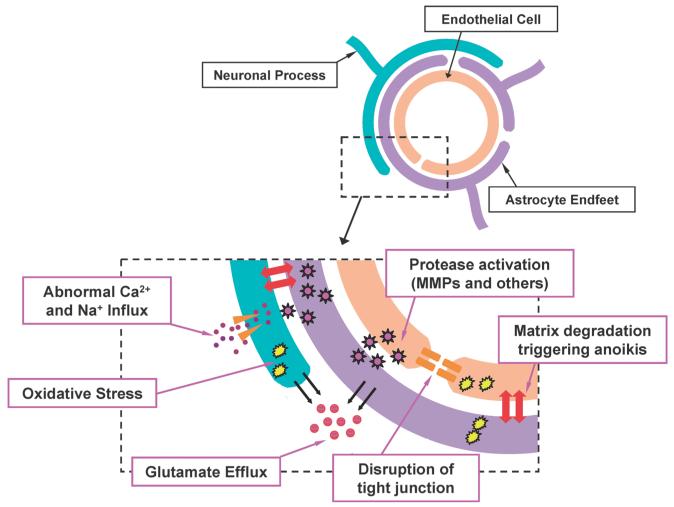
Fig. 1.
Schematic of the Neurovascular Unit under Stroke Conditions Neurons, astrocytes, cerebral endothelial cells, and extracellular matrix comprise the neurovascular unit. This concept emphasizes the functional aspects of cell–cell and cell–matrix signaling. Under normal conditions, these components work together to maintain brain function. After ischemia, neurovascular damage occurs by multiple deleterious pathways, resulting in loss of brain function. The reader is encouraged to seek more detailed reviews describing these events.2—7)
Stroke is the third leading cause of death and a leading cause of adult disability in developed countries.2) Under stroke conditions, brain function is perturbed due to cerebral ischemia (lack of blood supply to the brain) caused by thrombosis/embolism or hemorrhage. In central areas of ischemic regions, blood flow deficits are severe and brain cells die rapidly. In peripheral areas (the so-called penumbra), blood flow deficits are relatively mild, so that therapeutic salvage is theoretically possible.10) Thus far, for acute stroke therapy, only thrombolytic therapy with tissue-plasminogen activator has been approved by the FDA to be effective in targeting the sal- vageable ischemic penumbra11). Although many advances have been made in terms of the basic molecular mechanisms underlying neuronal death, clinically effective neuroprotec- tive drugs in stroke have not yet been discovered.12—14) There are at least two potential issues worth examining further. One is that, as described above, a singular focus on saving neu- rons alone might not be sufficient. Perhaps seeking a broader “neurovascular protection” is required? As shown in Fig. 1, many parallel deleterious events are activated under stroke conditions, resulting in neurovascular damage. A second issue is that most of our research to date is only focused on gray matter. But without considering the oligodendrocytes (OLGs) and their precursor cells (oligodendrocyte precursor cells: OPCs) in white matter, we may not be able to truly save the brain.
In this mini-review, we will briefly explore current knowledge on trophic coupling in white matter under normal and stroke conditions. First, the basic steps involved in OLG maturation will be introduced. OLG is one of the major cell types in the CNS white matter, and OLG differentiation/maturation in adult brain is an important event for white matter maintenance and repair. Next, we will overview the phenomena of white matter damage in stroke. Under ischemic conditions, several deleterious signal cascades are activated and OLGs eventually die. On the other hand, in the penumbra, the number of OPCs may even increase as the brain attempts to repair itself. Finally, we will survey recent data to support the idea that cell-cell trophic coupling in white matter is critical for maintaining white matter homeostasis. We will mainly discuss the interaction between cerebral endothelium and OLG/OPC (i.e., oligovascular signaling). Under stroke conditions, oligovascular coupling is interrupted, and this may contribute to white matter injury. Taken together, understanding the mechanisms of oligovascular signaling in stroke may lead us to new approaches for stroke therapy.
1. OLIGODENDROCYTE LINEAGE AND GROWTH FACTORS
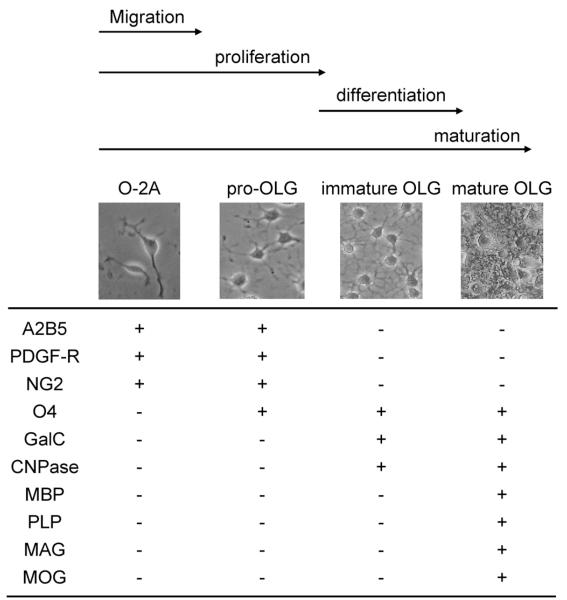
In CNS white matter, oligodendrocytes (OLGs) form myelin sheaths that encircle axonal bundles. OLGs differentiate from their precursor cells (oligodendrocyte precursor cells; OPCs). The maturation of OPCs into mature OLGs occurs in a multiple steps defined by the expression of specific cell-surface receptors.15—17) Overall, there are four stages in this maturation process; oligodendrocyte-type 2 astrocyte (O-2A) cells, pro-OLGs, immature OLGs, and mature OLGs (Fig. 2).
Fig. 2.
Characteristics of OLG Maturation OLG maturation is defined by the expression of specific cell-surface receptors, cell morphology, and proliferative/motility responses. O-2A cells are bipolar cells with high proliferative and motile activities. Pro-OLGs still divide but no longer show motility response. Immature OLGs are one of the two differenciated OLGs, but do not form myelin yet. The other differenciated OLG is mature OLGs, which express the myelin proteins to form the myelin sheath around axons. The reader is encouraged to seek more detailed reviews describing the full complexities of OLG maturation.15—17)
During this complex process of OPC/OLG maturation, multiple growth factors are likely to contribute to proliferation, differentiation, and cytoprotection. Basic fibroblast growth factor (bFGF, also know FGF-2) and platelet-derived growth factor (PDGF) are the most documented growth factors known to promote the proliferation of O-2A cells. bFGF has also been shown to upregulate PDGF-receptor expression in O-2A cells/pro-OLGs, and to block the maturation step from pro-OLGs to immature OLGs.18) Interestingly, pro-OLGs in vitro undergo a transient phenotypic reversion to the less mature stage (O-2A cells) in response to PDGF but not bFGF.19) Insulin-like growth factor 1 (IGF-1) is also a well examined OPC proliferation and OLG myelination factor.20—23) Moreover, it has shown that IGF-1 also protects cul- tured OLGs and their precursor cells from apoptosis induced by tumor necrosis factor (TNF)-alpha, glutamate, and growth factor deprivation.24—26) In addition, other growth factors such as neuregulin, ciliary neurotrophic factor (CNTF), brain-derived growth factor (BDNF), and nerve growth factor may also be involved in the regulation of OLG development/survival. Altogether, a complex network of feedback and interplay between different growth factor may be quite important in the promotion of this OPC/OLG maturation process. The reader is encouraged to seek more detailed reviews describing these various mechanisms.15—17)
If growth factors mediate OPC/OLG survival and maturation during development, it may be reasonable to hypothesize that analogous cell–cell interactions help maintain white matter function even in adult brain. Without support from neighboring cell, OLGs and OPCs may not survive, resulting in white matter dysfunction. Before discussing the trophic coupling between OLG/OPC and other brain cells, we will briefly overview key events of white matter injury under stroke conditions of ischemic stress.
2. WHITE MATTER DAMAGE IN STROKE
Gray matter and white matter are the major two components of the CNS. The ratio between white matter and gray matter in human neocortex is approximately 1. But this ratio is much smaller in rodent neocortex, where white matter only comprises 10—15% of total volume.27) White matter primarily consists of axonal bundles ensheathed with myelin. Myelin is synthesized by OLGs that tend to be arranged in rows parallel to axonal tracts. Just before and after birth, OPCs multiply rapidly and mature into OLGs. The OLGs then develop processes that form the myelin sheaths. Although this standard model emphasizes the central role of OLGs, recent data now suggest that even in adult brain, OPCs may also be involved in white matter maintenance. Subpopulations of OPCs persist throughout the adult brain.28—31) These OPCs are thought to contribute to myelin maintenance and repair by generating new OLGs. Therefore, under pathologic conditions such as stroke, OLG/OPC dysfunction and death may contribute to white matter damage.
White matter is especially susceptible to stroke.32—34) Because white matter blood flow is lower than in gray matter and there is little collateral blood supply in deep white matter, white matter ischemia is typically severe with rapid cell swelling and tissue edema.2) Minor white matter strokes often cause extensive neurological deficits by interrupting the passage of large axonal bundles such as those within the internal capsule.2) Axons contain abundant mitochondria, which is an organelle for a source of reactive oxygen species. In fact, free radical scavenging significantly reduces white matter injury in rodent stroke models.35—38) Furthermore, white matter ischemia activates several kinds of proteases, which weaken the structural integrity of axons and myelin sheath. Neuro- filaments are major structural components of white matter axons, and calpains have been demonstrated to be involved in neurofilament degradation under ischemic conditions.39) Matrix metalloproteinases (MMPs) can directly attack mye- lin components such as myelin-basic protein.40) Ischemia- induced degradation of myelin-basic protein is reduced in MMP-9 knockout mice.41) Importantly, chronic white matter lesions are associated with upregulation of MMPs in autop- sied samples from patients with vascular dementia.42)
Like neurons in gray matter, axons and OLGs are vulnerable to damage by excitatory amino acids, oxidative stress, trophic factor deprivation, and activation of apoptotic pathways.43—45) Because a single OLG myelinates multiple axons, damage to only one OLG can cause dysfunction in many different neuronal pathways (Fig. 3). Therefore, protecting OLGs is a reasonable therapeutic approach for white matter stroke treatment. Thus far, using in vitro and in vivo experimental systems, several pathways have been demonstrated in OLG death/damage under stroke conditions43,45) (Fig. 3). The mechanisms of glutamate-induced excitatory/oxidative stress are well examined. After cerebral ischemia, loss of energy stores induces ionic imbalances, which then promote reversal of the Na -dependent glutamate transporter GLT1, resulting in extracellular glutamate accumulation. In fact, GLT1 blockers threo-beta-benzyloxy aspartate (TBOA) and dihydrokainic acid (DHKA) are both confirmed to protect white matter against ischemic stress in mouse optic nerves.46,47) OLGs express AMPA/kainate receptors, and overactivation of these receptors can also mediate Na and Ca2 influx leading to OLG death.48,49) Furthermore, the cystine-gluta- mate exchange antiporter in OLGs may also contribute to glutamate-induced OLG death. Excessive glutamate blocks the antiporter, which in turn induces glutathione depletion that augments oxidative stress.50) Recently, it has been shown that OLGs also express N-methyl-d-aspartic acid (NMDA) receptors.51—53) NMDA receptors of OLGs are activated by glutamate in white matter ischemia,51) and activation of these receptors can also lead to rise of intracellular Ca2 concen- tration.53) However, the involvement of NMDA receptor in glutamate-induced OLG death is still controversial,47) and therefore further studies are warranted to examine whether the NMDA receptor in OLGs can be a therapeutic target for white matter damage in stroke patients. Since a number of pathways is involved in OLG damage/death under pathological conditions, the reader is encouraged to seek more detailed review regarding these complex mechanisms.43—45,54)
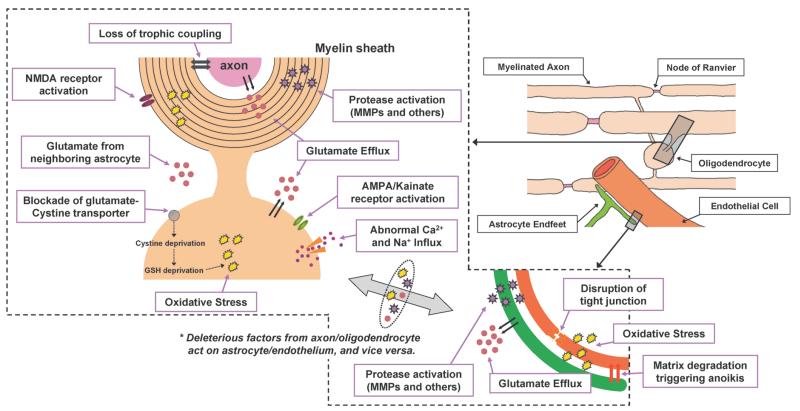
Fig. 3.
Schematic of the Adult White Matter under Stroke Conditions The main components of white matter are the neuronal axon, oligodendrocyte (myelin), astrocyte, and endothelium. Similar to gray matter, cell-cell interactions are important to maintain white matter function. Under ishemic conditions, several deleterious factors/cascades are activated. As in the neurovascular unit, several events occur due to ischemic stress. Glutamate efflux, oxidative stress, and proteinase activation eventually induce cell death. Importantly, deleterious factors secreted by one cell type may affect another cell type. For example, cerebral endothelial cells secrete MMPs after ischemic injury. These MMPs, in turn, damage the myelin sheath produced by oligodendrocytes. Moreover, under ischemic conditions, trophic support from astrocyte/endothelium to myelinated axons is disturbed by oxidative stress. The reader is encouraged to seek more detailed reviews describing the events in white matter ischemia.43—45,54)
In contrast to axonal response, the role of OPCs under stroke conditions in adult brain is mostly unknown. OPCs in culture are susceptible to oxidative stress such as oxygen-glucose deprivation.55,56) Hence it is possible that OPCs are also damaged during acute stroke in vivo. During stroke recovery, OPCs might contribute to myelin repair by generating new OLGs as a source of remyelination and remodeling.57—60) Furthermore, a recent study suggested that OPCs may also serve as a precursor pool for cortical projection neurons in adult brain.61) Therefore, it is reasonable to think that OPCs play critical roles in the recovery phase in stroke. In fact, experimental evidence is now starting to show that myelin repair can occur in peri-infarct areas in rodent stroke models, as judged by upregulated gene expression of prote-olipid protein (PLP) and myelin basic protein (MBP), which are the major protein components of CNS myelin.62,63) Furthermore, using a rat MCAO model, elevations in the number of OPCs and a gradual recovery of OLGs can be found in the peri-infarct area after stroke.64) Taken together, both OLGs and OPCs might comprise interesting targets of the treatment for white matter injury.
3. CELL–CELL INTERACTION IN WHITE MATTER AND NEW CONCEPT OF OLIGOVASCULAR NICHE
Whereas protecting OPCs and OLGs are clearly important per se, it is likely that cell–cell interactions in the white matter may also be extremely important under normal and diseased conditions. In this section, we will briefly examine the recently proposed concept of “oligovascular niche”.
The main cell types comprising white matter are the neuronal axon, OLG (myelin), astrocyte, and endothelial cell (Fig. 3). As in gray matter, there is close anatomical and perhaps functional contact between all these cells. Astrocytes are in close apposition to OLGs within the white matter,65) and couple with OLGs through gap junctions to maintain their functions.66) Furthermore, using astrocyte-OLG co- culture system, astrocytes have been shown to promote OLG survival through an alpha6 integrin-laminin-dependent mechanism.67) Astrocyte-derived soluble factors are also implied to be supportive to both OLGs and OPCs. Conditioned media from astrocyte cultures have been demonstrated to support OLGs/OPCs survival.68—73) In turn, recent findings suggest that OLGs not only myelinate axons but also main- tain their functional integrity and survival thorough OLG- specific proteins and/or trophic factor release.74—76) Thus far, PLP (and its smaller isoform DM20) and 2,3 -cyclic nu- cleotide 3 -phosphodiesterase (CNP) are well documented myelin-associated proteins expressed in OLGs to affect ax- onal functions. Mice with mutations for those genes revealed that axonal support by OLGs is independent of myelin as- sembly.74,75) Myelinating OLGs also provide trophic support for axons by secreting soluble factors. To date, BDNF, NT3, NGF, and PDGF are reported as OLG-axon signaling mediators.76) The reader is encouraged to seek more detailed re- view describing glia-axon signaling in white matter.74—76) Here, we will mainly focus on the cell–cell interactions be- tween cerebral endothelium and OLG/OPC.
Cerebral endothelial cells play central and important roles in the cerebrovascular system both in gray and white matter. These cells work with astrocytes to form the blood-brain barrier (BBB).77—79) Compromised BBB function occurs in many CNS diseases. In the context of stroke, BBB damage leads to cerebral edema and hemorrhage.2) Moreover, beyond outright disruptions of the barrier, damage to cerebral endothelium itself may affect the function and survival of neighboring cells. As discussed previously, under normal conditions, cerebral endothelium secrete trophic factors to support neighboring neurons.1,8,9) This trophic coupling creates a neurovascular niche, wherein cell-cell signaling between cerebral endothelium and neuronal precursor cells help mediate and sustain pockets of ongoing neurogenesis and angiogenesis.4,5,80—83) Therefore, endothelial dysfunction results in not only lack of blood supply to the brain but also loss of trophic coupling in many aspects. Although the concept of the neurovascular unit is usually used to discuss phenomena in the gray matter,2—4,6) it obviously should apply for white matter physiology and pathology as well.
To date, cell–cell interactions between cerebral endothelium and OLG/OPC are not well understood. However, a recent study suggests the existence of an “oligovascular niche”, whereby cerebral endothelial cells support the survival and proliferation of OPCs.55) Cross-talk between the vascular and neuronal compartments in the neurovascular niche is mediated by an exchange of soluble signals, and this phenomenon is partly mediated by the ability of cerebral endothelium to secrete a rich repertoire of trophic factors.8,84,85) Similarly, endothelial-derived growth factors such as BDNF and FGF-2 promote OPC proliferation.55) Importantly, these trophic coupling might be interrupted under pathological conditions. Non-lethal oxidative stress reduces the expression of several growth factors in cultured cerebral endothelial cells.8,55) Also, in a mouse stroke model, BDNF and NGF expression in core infarct areas are significantly decreased.86) Moreover, al- though healthy endothelium can support OPCs even under ischemic conditions, sick endothelium no longer support OPCs.55) In stroke patients and rodent stroke models, en- dothelial dysfunction is often observed by cerebral small- vessel disease in white matter ischemia.87—89) Therefore, dis- ruption of endothelium-OPC/OLG coupling should be one of the major causes for the pathogenesis and progression of white matter lesion in CNS diseases including stroke. Thus far, there is no direct evidence showing OPCs support en- dothelial functions including angiogenesis. Also, endothe- lium-OLG trophic coupling has not been experimentally proved yet. However, it is well known that both cerebral en- dothelium and OPC/OLG secrete many kinds of growth fac- tors.8,9,55,90) Therefore, it is possible that there is two-way trophic coupling between these cells in white matter (Fig. 4).
Fig. 4.
Schematic of the Oligovascular Signaling in Adult White Matter In the oligovascular niche, oligogenesis and angiogenesis might occur to maintain white matter homeostasis. Oligodendrocyte precursor cells (OPCs) are thought to contribute to myelin maintenance and repair by generating new mature oligodendrocytes (OLGs). In addition, trophic coupling may also exist between cerebral endothelial cells and mature OLGs. Under diseased conditions such as stroke, multiple deleterious factors directly attack OLGs and OPCs, which are vulnerable to oxidative stress. Moreover, oxidative stress can disturb the trophic coupling between cerebral endothelial cells and OLGs/OPCs, resulting in further white matter damage.
Compared to the mechanisms of cell–cell interaction in gray matter, trophic coupling in white matter remains relatively understudied and poorly understood. For the neurovascular niche, matrix and trophic interactions between endothelial cells and neurons sustain neurogenesis and angiogenesis83—85) and may also protect neurons against oxidative and metabolic insults.8,9) Analogous interactions within a widely distributed oligovascular niche may provide a similar mechanism for sustaining white matter renewal and integrity. Further studies are warranted to dissect how these mechanisms function in normal brain, and how disruptions in oligovascular signaling may underlie white matter disease and neurodegeneration. Finally, to develop new stroke therapy, one may need to define these mechanisms in aged brains. Aging is the major risk factor for stroke, and it has recently shown that ischemic injury to white matter is an age-dependent process.47,91) How these mechanisms of oligovascular signaling are affected by aging and metabolic disease should be extremely important.
4. CONCLUSIONS
The development of stroke therapies is very challenging. Over the past many decades, a large number of neuroprotection trials have unfortunately failed. Researchers are now changing their research direction from single neuron protection to overall neurovascular protection. In addition to this trend, however, we might also need to take white matter protection into account because white matter damage is a clinically important part of stroke. Needless to say, singular OLG protection may also not be enough. Cell–cell interactions within an oligovascular niche may be crucial for white matter function and dysfunction. In this mini-review, we have surveyed key events in white matter damage in stroke along with the possible role of oligovascular signaling. We have mostly discussed cerebral endothelial cells and OLGs/OPCs in white matter. But clearly, the integrity of interactions within all brain cell types including axonal compartments and astrocytes will also be important in white matter. A systematic dissection of cell–cell trophic signaling in both gray and white matter may provide more novel opportunities for discovering treatments for stroke, and perhaps even other CNS disorders.
Acknowledgments
Supported in part by the Deane Foundation, American Heart Association and NIH grants R37-NS37074, R01-NS48422, R01-NS53560, P50-NS10828 and P01-NS55104.
REFERENCES
- 1).Guo S, Lo EH. Stroke. 2009;40:S4–S7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Lo EH, Dalkara T, Moskowitz MA. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 3).del Zoppo GJ. N. Engl. J. Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 4).Iadecola C. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 5).Zlokovic BV. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6).Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, Lo EH. Neurochem. Res. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 7).Arai K, Jin G, Navaratna D, Lo EH. FEBS J. 2009;276:4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. J. Neurosci. 2008;28:8294–8305. doi: 10.1523/JNEUROSCI.2010-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Lo EH. Nat. Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 11).Hacke W, Brott T, Caplan L, Meier D, Fieschi C, von Kummer R, Donnan G, Heiss WD, Wahlgren NG, Spranger M, Boysen G, Marler JR. Neurology. 1999;53:S3–S14. [PubMed] [Google Scholar]
- 12).Ikonomidou C, Turski L. Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 13).Kennedy TP, Vinten-Johansen J. Curr. Opin. Investig. Drugs. 2006;7:229–242. [PubMed] [Google Scholar]
- 14).Wang CX, Shuaib A. Drugs Aging. 2007;24:537–546. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 15).Pfeiffer SE, Warrington AE, Bansal R. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 16).Miller RH. Trends Neurosci. 1996;19:92–96. doi: 10.1016/s0166-2236(96)80036-1. [DOI] [PubMed] [Google Scholar]
- 17).Butts BD, Houde C, Mehmet H. Cell Death Differ. 2008;15:1178–1186. doi: 10.1038/cdd.2008.70. [DOI] [PubMed] [Google Scholar]
- 18).McKinnon RD, Matsui T, Dubois-Dalcq M, Aaronson SA. Neu- ron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 19).Gard AL, Pfeiffer SE. Dev. Biol. 1993;159:618–630. doi: 10.1006/dbio.1993.1269. [DOI] [PubMed] [Google Scholar]
- 20).Ye P, Carson J, D'Ercole AJ. Neurosci. Lett. 1995;201:235–238. doi: 10.1016/0304-3940(95)12194-3. [DOI] [PubMed] [Google Scholar]
- 21).Carson MJ, Behringer RR, Brinster RL, McMorris FA. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 22).Zumkeller W. Eur. J. Paediatr. Neurol. 1997;1:91–101. doi: 10.1016/s1090-3798(97)80039-6. [DOI] [PubMed] [Google Scholar]
- 23).Goddard DR, Berry M, Butt AM. J. Neurosci. Res. 1999;57:74–85. doi: 10.1002/(SICI)1097-4547(19990701)57:1<74::AID-JNR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24).Cui QL, Zheng WH, Quirion R, Almazan G. J. Biol. Chem. 2005;280:8918–8928. doi: 10.1074/jbc.M414267200. [DOI] [PubMed] [Google Scholar]
- 25).Ye P, D'Ercole AJ. Endocrinology. 1999;140:3063–3072. doi: 10.1210/endo.140.7.6754. [DOI] [PubMed] [Google Scholar]
- 26).Ness JK, Wood TL. Mol. Cell. Neurosci. 2002;20:476–488. doi: 10.1006/mcne.2002.1149. [DOI] [PubMed] [Google Scholar]
- 27).Zhang K, Sejnowski TJ. Proc. Natl. Acad. Sci. U.S.A. 2009;97:5621–5626. doi: 10.1073/pnas.090504197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. J. Neu- rosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Levine JM, Reynolds R, Fawcett JW. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 30).Nishiyama A, Chang A, Trapp BD. J. Neuropathol. Exp. Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 31).Nishiyama A. Hum. Cell. 2001;14:77–82. [PubMed] [Google Scholar]
- 32).Pantoni L, Garcia JH, Gutierrez JA. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- 33).Volpe JJ. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 34).Alix JJ. Eur. Neurol. 2006;56:74–77. doi: 10.1159/000095543. [DOI] [PubMed] [Google Scholar]
- 35).Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Stroke. 2001;32:2149–2154. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 36).Irving EA, Yatsushiro K, McCulloch J, Dewar D. J. Cereb. Blood Flow Metab. 1997;17:612–622. doi: 10.1097/00004647-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 37).Lin S, Rhodes PG, Lei M, Zhang F, Cai Z. Brain Res. 2004;1007:132–141. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 38). Ueno Y, Zhang N, Miyamoto N. Neuroscience. 2009;162:317–327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 39).Stys PK, Jiang Q. Neurosci. Lett. 2002;328:150–154. doi: 10.1016/s0304-3940(02)00469-x. [DOI] [PubMed] [Google Scholar]
- 40).Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Neu- rosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 41).Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. J. Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Rosenberg GA, Sullivan N, Esiri MM. Stroke. 2001;32:1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 43).Dewar D, Underhill SM, Goldberg MP. J. Cereb. Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 44).Bakiri Y, Burzomato V, Frugier G, Hamilton NB, Karadottir R, Attwell D. Neuroscience. 2009;158:266–274. doi: 10.1016/j.neuroscience.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 45).Arai K, Lo EH. Exp. Transl. Stroke Med. 2009 doi: 10.1186/2040-7378-1-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Tekkok SB, Ye Z, Ransom BR. J. Cereb. Blood Flow Metab. 2007;27:1540–1552. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- 47).Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. J. Neurosci. 2008;28:1479–1489. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. J. Neurosci. 2003;23:9519–9528. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Sanchez-Gomez MV, Matute C. Neurobiol. Dis. 1999;6:475–485. doi: 10.1006/nbdi.1999.0264. [DOI] [PubMed] [Google Scholar]
- 50).Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ. J. Neurosci. 1993;13:1441–1453. doi: 10.1523/JNEUROSCI.13-04-01441.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Karadottir R, Cavelier P, Bergersen LH, Attwell D. Nature (London) 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Salter MG, Fern R. Nature (London) 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 53).Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. Nature (London) 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 54).Karadottir R, Attwell D. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Arai K, Lo EH. J. Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Zhang J, Li Y, Zheng X, Gao Q, Liu Z, Qu R, Borneman J, Elias SB, Chopp M. J. Neurosci. Res. 2008;86:1501–1510. doi: 10.1002/jnr.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Gensert JM, Goldman JE. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 58).Keirstead HS, Blakemore WF. J. Neuropathol. Exp. Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 59).Watanabe M, Toyama Y, Nishiyama A. J. Neurosci. Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- 60).Dawson MR, Polito A, Levine JM, Reynolds R. Mol. Cell. Neu- rosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 61).Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. Nat. Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Mandai K, Matsumoto M, Kitagawa K, Matsushita K, Ohtsuki T, Mabuchi T, Colman DR, Kamada T, Yanagihara T. Neuroscience. 1997;77:849–861. [PubMed] [Google Scholar]
- 63).Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Exp. Brain Res. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- 64).Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Brain Res. 2003;989:172–179. doi: 10.1016/s0006-8993(03)03317-1. [DOI] [PubMed] [Google Scholar]
- 65).Butt AM, Ibrahim M, Ruge FM, Berry M. Glia. 1995;14:185–197. doi: 10.1002/glia.440140304. [DOI] [PubMed] [Google Scholar]
- 66).Orthmann-Murphy JL, Abrams CK, Scherer SS. J. Mol. Neu- rosci. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Corley SM, Ladiwala U, Besson A, Yong VW. Glia. 2001;36:281–294. doi: 10.1002/glia.1116. [DOI] [PubMed] [Google Scholar]
- 68).Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Nature (London) 1998;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 69).Yonezawa M, Back SA, Gan X, Rosenberg PA, Volpe JJ. J. Neurochem. 1996;67:566–573. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- 70).Gard AL, Burrell MR, Pfeiffer SE, Rudge JS, Williams WC., 2nd Development. 1995;121:2187–2197. doi: 10.1242/dev.121.7.2187. [DOI] [PubMed] [Google Scholar]
- 71).Pang Y, Cai Z, Rhodes PG. J. Neurosci. Res. 2000;62:510–520. doi: 10.1002/1097-4547(20001115)62:4<510::AID-JNR5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 72).Albrecht PJ, Enterline JC, Cromer J, Levison SW. Neurochem. Res. 2007;32:263–271. doi: 10.1007/s11064-006-9151-6. [DOI] [PubMed] [Google Scholar]
- 73).Bartlett WP, Knapp PE, Skoff RP. Glia. 1998;1:253–259. doi: 10.1002/glia.440010404. [DOI] [PubMed] [Google Scholar]
- 74).Griffiths I, Klugmann M, Anderson T, Thomson C, Vouyiouklis D, Nave KA. Microsc. Res. Tech. 1998;41:344–358. doi: 10.1002/(SICI)1097-0029(19980601)41:5<344::AID-JEMT2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 75).Nave KA, Trapp BD. Annu. Rev. Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 76).Rosenberg SS, Ng BK, Chan JR. Brain Pathol. 2006;16:288–294. doi: 10.1111/j.1750-3639.2006.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Hawkins BT, Davis TP. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 78).Iadecola C, Nedergaard M. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 79).Abbott NJ, Ronnback L, Hansson E. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 80).Zacchigna S, Lambrechts D, Carmeliet P. Nat. Rev. Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 81).Chopp M, Zhang ZG, Jiang Q. Stroke. 2007;38:827–831. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- 82).Greenberg DA, Jin K. Nature (London) 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 83).Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 84).Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Mol. Cell. Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 85).Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 86).Higuchi S, Hayakawa K, Irie K, Kataoka A, Katurabayashi S, Takasaki K, Mishima K, Iwasaki K, Fujiwara M. Annual Meeting of The Pharmaceutical Society of Japan. 28P-pm201; Kyoto. 2009. [Google Scholar]
- 87).Knottnerus IL, Ten Cate H, Lodder J, Kessels F, van Oostenbrugge RJ. Cerebrovasc. Dis. 2009;27:519–526. doi: 10.1159/000212672. [DOI] [PubMed] [Google Scholar]
- 88).Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, Barkhof F, Fazekas F. Neurology. 2004;63:139–144. doi: 10.1212/01.wnl.0000132635.75819.e5. [DOI] [PubMed] [Google Scholar]
- 89).Hainsworth AH, Markus HS. J. Cereb. Blood Flow Metab. 2008;28:1877–1891. doi: 10.1038/jcbfm.2008.91. [DOI] [PubMed] [Google Scholar]
- 90).Du Y, Dreyfus CF. J. Neurosci. Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 91).Baltan S. Neuroscientist. 2009;15:126–133. doi: 10.1177/1073858408324788. [DOI] [PubMed] [Google Scholar]