The eye contains highly vascularized and completely avascular cell layers in apposition to one another. Such tissue disposition/organization involves tight coordination of vascular growth and vascular arrest. In particular, retinal vascularization, which occurs during the final embryonic stages of development in humans, emerges as a result of coordinated interactions/ cross talks among astrocytes, endothelial cells and pericytes through balanced production of guidance, chemotactic, and pro- and anti- angiogenic regulatory factors. However, the entire repertoire of the key factors involved and their precise role in the process is not fully known. During postnatal life, damage to blood vessels as a result of trauma, hyperoxia, diabetes, aging, dyslipidemia, or a number of other diseases can result in arrest of vascular development, vaso-obliteration and/or vascular occlusion as in retinopathy of prematurity, diabetic retinopathy, age-related macular degeneration, as well as a large number of other eye conditions. The resulting ischemia, which rapidly impairs oxygen levels within surrounding tissue, leads to excessive aberrant compensatory blood vessel growth invading the normally avascular vitreous, eventually causing retinal detachment and blindness. The expression or lack of multiple blood-borne and/or stroma-derived cytokines and chemokines, including those of the vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF) and angiopoietin systems, has been implicated in dysregulation of growth, migration, adhesion and differentiation of retinal cells in retinal vascular diseases.1,2 Interestingly, a novel signaling system with profound effects on retinal vascular development and disease has emerged from the study of a subset of extracellular matrix (ECM) proteins known as Cysteine-rich protein 61/connective tissue growth factor/Novel overexpressed (CCN).3
CCN1/Cyr61 is the leading member of the CCN family of proteins, which also comprises CCN2/CTGF (connective tissue growth factor), CCN3/NOV (nephroblastoma overexpressed), and CCN4-6/WISP1-3 (Wnt- inducible secreted proteins). These proteins also named matricellular proteins, do not subserve a structural/physical role in the extracellular environment but mainly function as upstream regulators of the constitutively expressed ECM proteins and influence cell fate and function.4 Overall, CCN proteins exhibit distinct tissue distribution and function but commonly have a high content and an absolute conservation of the position of 38 cysteine residues in their primary sequences. CCN1/Cyr61 and CCN2/CTGF, the most ubiquitously expressed CCN molecules, share approximately 56% amino acid sequence homology and 4 typical domains including (i) insulin-like growth factor-binding protein (IGFBP), (ii) von Willebrand factor type-C repeat (vWC), (iii) thrombospondin type-1 (TSP1), and (iv) C-terminal (CT) (Fig. 1). The overall functions of the full-length proteins are thought to be the result of either combinatorial or independent actions of their constitutive domains. However, CCN1/Cyr61 and CCN2/CTGF are functionally distinct. CCN2/CTGF regulates primarily epithelial-mesenchymal transition associated tissue development and fibrotic reactions associated with fibroproliferative disorders. CCN1/Cyr61 physically interacts with integrin and non-integrin receptors and modulates vascular cell motility, adhesion, proliferation, predisposition to apoptosis and production of endothelial basement membrane components. As such, this molecule appeared as a prime candidate for modulating normal and aberrant formation of blood vessels characteristic of proliferative retinopathies.
Figure 1.
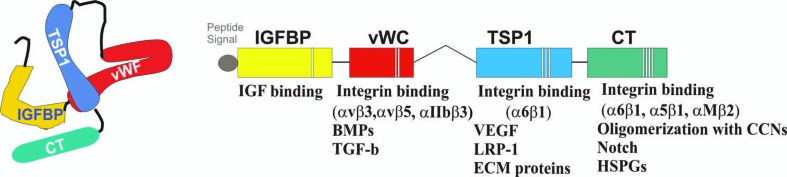
Multimodular structure of the CCN1 protein. Proteins that physically interact with each of the 4 constitutive modules of the CCN1 protein are indicated. IGFBP, insulin-like growth factor (IGF) binding protein; vWC, von Willebrand factor type-C repeat; TSP1, thrombospondin type-1 repeat; CT,: C-terminal; BMPs, bone morphogenic proteins; TGF-b, transforming growth factor-beta; VEGF, vascular endothelial growth factor; LRP-1, low density lipoprotein receptor-related protein-1; ECM, extracellular matrix; HSPGs, heparan sulfate proteoglycans
The importance of CCN1 in the retina has been underscored by its expression pattern during vascular development and its role in ocular angiopathies. During mouse development, CCN1 gene expression peaks as the chorioallantoic plate is invaded by fetal blood vessels from the allantois and its expression continues throughout the developmental stages of the cardiovascular system.5 Interestingly, CCN1-deficient mouse embryos die prematurely due to severe vascular defects including placental vascular insufficiency and hemorrhaging blood vessels which appear with disorganized vascular cells and absence of a basement membrane.6 As CCN1-deficient mice die prematurely before development of retinal vessels which occurs postnatally in mice, the vascular phenotype in the retina of these animals could not be assessed. Using transgenic mice expressing the green fluorescent protein (GFP) gene under control of the CCN1 promoter, we found that CCN1 was dynamically expressed postnatally in the mouse eye and that its expression was largely confined to retinal vasculature (Figure 2). CCN1 levels peaked between P2 and P4, when budding of superficial vessels begins and between P8 and P12 when the secondary deep layer of retinal vasculature starts extending and growing radially outward toward the inner nuclear and outer plexiform layers.7 CCN1 expression then progressively declined and became barely detectable when the adult vasculature was completely established. Taken together with the CCN1 knockout phenotype, these observations indicate that CCN1 expression is required for normal blood vessel development and stabilization notwithstanding the presence of other notable angiogenic factors of the VEGF, IGF and fibroblast growth factor (FGF) families.
Figure 2.
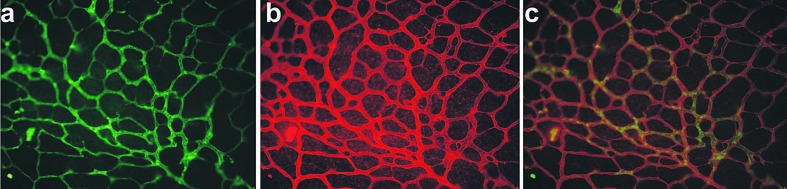
Vascular localization of CCN1 in a flat mounted retina of a transgenic mouse expressing the green fluorescent protein (GFP) gene under the control of a 5.4-kb-promoter of the CCN1 gene. The retina from a mouse pup on postnatal day 7 was fixed and stained with type 4 collagen (a) characteristic of retinal vessel basement membrane and GFP (b) antibodies. Overlay of (a) and (b) is shown in (c). GFP expression pattern recapitulates that of CCN1 with endothelial cells and pericytes as the primary sources of CCN1.
The physiopathological relevance of CCN1 expression in the retinal vasculature was further examined in an acute mouse model of proliferative oxygen-induced retinopathy (OIR). In this model, abnormal retinal angiogenesis is induced through exposure of mouse pups on postnatal day 7 (P7) to hyperoxia (75% oxygen) and characterization following 5 days of normoxia at P17; the time at which a maximal neovascular response can be observed. Under these conditions, CCN1 expression was rapidly repressed and its transcript levels remained low throughout the course of hyperoxia. Unlike other angiogenic factor genes of the VEGF and IGF family which become abnormally upregulated following hyperoxia, CCN1 gene expression was not significantly increased during the subsequent hypoxic phase associated with abnormal new vessel growth and most neovascular tufts did not express the CCN1 protein.8 Thus, CCN1 expression was not associated with abnormal vessel formation. Interestingly, forced expression of the CCN1 gene through lentiviral gene transfer in OIR eyes significantly reduced vaso-obliteration following hyperoxia. Pre-retinal neovascular tufts, which can be seen abundantly in the central and mid-peripheral retina, were minimally present in eyes following ectopic expression of the CCN1 gene. Clearly, re-expression of the CCN1 gene provided the retinal niche with an important regulatory and protective factor critical for vaso-stabilization and/or normalization of the retinal vasculature during and after hyperoxic injury.
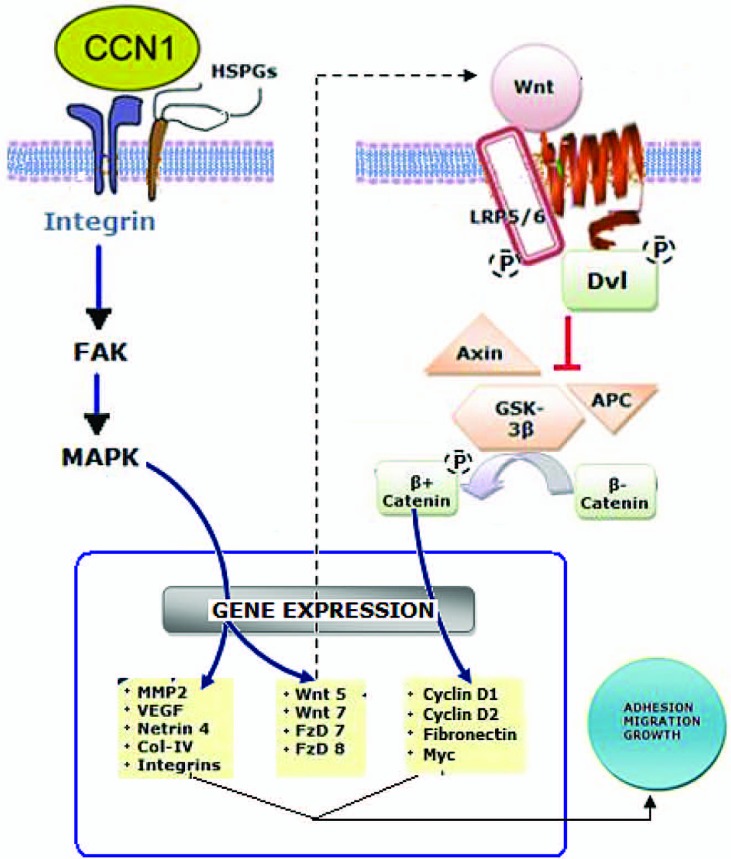
At the molecular level, CCN1 binds to integrin receptors (e.g., α4β1 and α6β1), activates signaling cascades involving focal adhesion kinases and mitogen-activated protein kinases, and stimulates the expression of paracrine factors of the Wnt signaling pathway (Figure 3). These effects contribute, at least in part, to vascular activities of CCN1 in the retina.8 Wnt- signaling influences many aspects of branching tubulogenesis in various species and organ systems, often in coordination with Notch signaling. Numerous studies have identified selective and overlapping roles for multiple Wnt ligands, receptors and gene targets and alternative downstream signaling pathways in the regulation of distinct events in vessel development and pathology.9
Figure 3.
Molecular activities of the CCN1 protein. CCN-1-induced integrin- and Wnt-dependent gene expression culminates in cell growth, adhesion and/or migration. HSPGs, heparan sulfate proteoglycans; FAK, focal adhesion kinase; MAPK, mitogen-activated protein kinase; Wnt, Wingless integration; LRP5/6, low density lipoprotein receptor-related protein 5/6; Dvl, disheveled; GSK-3b, glycogen synthase kinase 3 beta; APC, adenomatous polyposis coli; MMP-2, matrix metalloproteinase-2; VEGF, vascular endothelial growth factor Col-IV, type 4 collagen; FzD, Frizzled
However, other molecular activities of CCN1 may account for its ability to reduce the formation of retinal new vessels. (i) CCN1 has the potential to override the activity of VEGF on new vessel growth. This effect may involve a direct physical and/or functional interaction between CCN1 and either VEGF or VEGF receptors and modulate angiogenesis in an antagonistic, additive, or synergistic manner. In particular, VEGF164 which is required for pathological angiogenesis can directly interact with the TSP1 domain of the CCN proteins and hinder VEGF- mediated neoangiogenesis.10 (ii) CCN1 induces selective modulation of tumor necrosis factor alpha (TNF-α) activity which is particularly important in vascular tuft regression.11 This provides an environment that is conducive for more efficient and rapid vascular recovery.
Meanwhile, clinical studies involving analyses of fluids and tissue biopsies from human specimens have shown increased levels of CCN1 in several ocular vascular complications including proliferative diabetic retinopathy (PDR), proliferative vitreoretinopathy (PVR) and active Graves’ ophthalmopathy.12-14 CCN1 levels were similarly increased in other cardiovascular conditions including ischemic cardiomyopathy and atherosclerotic plaque formation.15,16 Similarly, CCN1 protein levels were markedly increased in retinal blood vessels in the early stages of diabetes and in late stages of vitreoproliferative disorders in mouse models of diabetic and ischemic angiopathies.17 These changes in CCN1 expression are viewed as part of a stress response given that the CCN1 gene is rapidly induced and responsive to changes in environmental stimuli, including those associated with inflammation and tissue repair.18-20 However, as a fetal gene, CCN1 expression in pathological disorders is expected to recapitulate biological events characteristic of earlier developmental stages. Instead of CCN1 re- enacting its vaso-formative and vaso-stabilizing effects observed during embryonic development, its expression in vascular disorders was rather concomitant with vascular remodeling. Potential explanations for these observations include the following. The effects of CCN1 are cell type- , dose- and context-dependent in that they depend on the expression and availability of receptors and binding partners in a given tissue/ cell type at a particular time point. Thresholds of CCN1 levels are also required to induce cell type-specific effects. In this regards, excessive accumulation of CCN1 in the ECM initiates de- adhesion of vascular cells such as pericytes by competing with the constitutively expressed ECM proteins for integrin binding and subsequently converting strong cell-ECM interactions into weak ones.21 This results in death of pericytes by anoikis, a form of apoptosis by loss of cell- matrix interactions which contributes to vaso- obliteration seen at the early stages of diabetic retinopathy. Another explanation may be that the CCN1 protein is susceptible to proteolytic degradation, a form of posttranslational regulation that generates CCN1 truncated variants that convey biological activities different from those of the full-length protein (Choi and Chaqour, Personal Communication). Indeed, CCN1 has been validated as a substrate of matrix metalloproteinases and its partial or total degradation is likely in the highly inflamed protease-rich diabetic environment.22,23 Vitreous fluids of patients with proliferative vitreopathy were found to be highly enriched with truncated forms of CCN proteins containing fewer modules suggesting that posttranslational alterations of CCN1 occur under pathological conditions.24 Further studies are needed to examine the post- translational alterations of the CCN1 protein but also to unravel the function of cryptic domains of this protein. The generation of proteolysis- resistant forms of the CCN1 protein will then be useful in a therapeutic purpose.
In conclusion, CCN1 protein is a non- structural protein which bridges the functional divide between structural ECM proteins and growth factors, cytokines and other related proteins by virtue of its ECM-like structural features and its multifaceted molecular activities including modulation of cell motility, adhesion, proliferation, predisposition to apoptosis and remodeling of ECM components. The complexity of the function of this protein was not predicted when the CCN1 protein was described two decades ago. However, a lesson learned from CCN1-related studies performed so far is that the CCN1 function can only be fully and accurately appreciated from in vivo studies in the context of the whole organism. Studies aimed at unraveling the CCN1 interactome will also certainly be valuable to build a model of the molecular and functional interactions of this interesting protein in normal and pathological angiogenesis. The unique molecular structure and biological activities of the CCN1 domains can then be exploited and used as templates to engineer highly efficient therapeutic agents useful in angiogenic diseases of the eye.
Acknowledgments
This work was supported by grants from the National Eye Institute of the National Institutes of Health EY022091-01 and EY019387-01A1.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc Res. 2007;74:131–144. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Kim LA, D’Amore PA. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am J Pathol. 2012;181:376–379. doi: 10.1016/j.ajpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 4.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mo FE, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circ Res. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caballero S, Yang R, Grant MB, Chaqour B. Selective blockade of cytoskeletal actin remodeling reduces experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2011;52:2490–2496. doi: 10.1167/iovs.10-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasan A, Pokeza N, Shaw L, Lee HS, Lazzaro D. The matricellular protein cysteine-rich protein 61 (CCN1/Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy. J Biol Chem. 2011;286:9542–9554. doi: 10.1074/jbc.M110.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 11.Kociok N, Radetzky S, Krohne TU, Gavranic C, Joussen AM. Pathological but not physiological retinal neovascularization is altered in TNF-Rp55- receptor-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:5057–5065. doi: 10.1167/iovs.06-0407. [DOI] [PubMed] [Google Scholar]
- 12.Lantz M, Vondrichova T, Parikh H, Frenander C, Ridderstrale M, Asman P, et al. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005;90:4784–4791. doi: 10.1210/jc.2004-2275. [DOI] [PubMed] [Google Scholar]
- 13.You JJ, Yang CH, Chen MS, Yang CM. Cysteine- rich 61, a member of the CCN family, as a factor involved in the pathogenesis of proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3447–3455. doi: 10.1167/iovs.08-2603. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Yu W, Dong F. Cysteine-rich 61 (CYR61) is up-regulated in proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250:661–668. doi: 10.1007/s00417-011-1882-7. [DOI] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper EJ, de Smet MD, van Meurs JC, Tan HS, Tanck MW, Oliver N, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124:1457–1462. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- 18.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna M, Liu H, Amir J, Sun Y, Morris SW, Siddiqui MA, et al. Mechanical regulation of the proangiogenic factor CCN1/CYR61 gene requires the combined activities of MRTF-A and CREB- binding protein histone acetyltransferase. J Biol Chem. 2009;284:23125–23136. doi: 10.1074/jbc.M109.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R, Amir J, Liu H, Chaqour B. Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiol Genomics. 2008;36:1–14. doi: 10.1152/physiolgenomics.90291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce deadhesion and anoikis of retinal pericytes. Endocrinology. 2008;149:1666–1677. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 22.Butler GS, Dean RA, Morrison CJ, Overall CM. Identification of cellular MMP substrates using quantitative proteomics: isotope-coded affinity tags (ICAT) and isobaric tags for relative and absolute quantification (iTRAQ). Methods Mol Biol. 2010;622:451–470. doi: 10.1007/978-1-60327-299-5_26. [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 24.Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, et al. Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2004;27:758–764. doi: 10.2337/diacare.27.3.758. [DOI] [PubMed] [Google Scholar]