Abstract
Purpose
To compare ocular biometric parameters in primary angle closure suspects (PACS), primary angle closure glaucoma (PACG) and acute primary angle closure (APAC).
Methods
This cross-sectional study was performed on 113 patients including 33 cases of PACS, 45 patients with PACG and 35 subjects with APAC. Central corneal thickness (CCT), axial length (AL), anterior chamber depth (ACD) and lens thickness (LT) were measured with an ultrasonic biometer. Lens-axial length factor (LAF), relative lens position, corrected ACD (CACD) and corrected lens position were calculated. The parameters were measured bilaterally but only data from the right eyes were compared. In the APAC group, biometric parameters were also compared between affected and unaffected fellow eyes. Logistic regression analysis was performed to identify risk factors.
Results
No statistically significant difference was observed in biometric parameters between PACS and PACG eyes, or between affected and fellow eyes in the APAC group (P>0.05 for all comparisons). However, eyes with APAC had thicker cornea (P=0.001), thicker lens (P<0.0001), shallower ACD (P=0.009), shallower CACD (P=0.003) and larger LAF (P<0.0001). Based on ROC curve analysis, lower ACD, and larger LT, LAF and CCT values were associated with APAC. In the APAC group, LAF (P<0.0001) and CCT (P=0.001) were significant risk factors.
Conclusion
This study revealed no significant difference in biometric characteristics in eyes with PACS and PACG. However, larger LAF and CCT were predictive of APAC.
Keywords: Primary Angle Closure Glaucoma, Biometry, Pachymetry
INTRODUCTION
Primary angle closure glaucoma (PACG) is a leading cause of blindness. By 2020, there will be 80 million people affected by glaucoma, of whom 26% will have PACG.1 It is estimated that PACG blinds two to five times more individuals than primary open angle glaucoma.2 Early detection by effective screening, and appropriate prophylaxis may prevent blindness from angle closure glaucoma.
A considerable proportion of the population (10.35%) have “occludable angles” which is now termed primary angle closure suspect (PACS) according to more recent definitions.3 An occludable angle may result in acute primary angle closure (APAC) or PACG, however some eyes never develop any sign of glaucoma.4 There is no exact explanation for this observation. One possible mechanism is based on ocular biometric characteristics. Although biometric differences have been reported in different subtypes of PACG,5-7 few studies have evaluated biometrics in eyes with PACS, as compared to eyes with APAC or chronic PACG. The current definition of PACS requires at least 270 degrees of iridotrabecular contact on gonioscopy.4 He et al8 reported ocular biometric parameters in patients with PACS based on the current definition. However, in other studies the definition of PACS varies and none have compared biometric parameters within subtypes of angle closure.3,5,6,9-12 In this study, we compare biometric parameters in eyes with PACS to eyes with APAC and chronic PACG.
METHODS
This cross-sectional comparative study enrolled patients with PACS, APAC and PACG who were referred to the glaucoma clinic of a tertiary eye care center. The study was approved by the local Ethics Committee and informed consent was obtained from all subjects. All patients underwent an ophthalmologic examination including slit lamp biomicroscopy, Goldmann applanation tonometry, indentation gonioscopy with a Sussman 4-mirror goniolens to detect appositional or synechial closure, and stereoscopic assessment of the optic disc with a +78 diopter lens. All ocular examinations and laser iridotomies were performed by one surgeon (MRR) in all groups.
The diagnosis of APAC was based on classic symptoms of acute-onset unilateral ocular pain, blurred vision, headache, nausea, vomiting, halos around lights, acutely elevated intraocular pressure (IOP≥ 35 mmHg) accompanied by red eye, corneal edema, shallow anterior chamber, an unreactive mid-dilated pupil but no glaucomatous optic neuropathy.5 All patients were offered laser peripheral iridotomy (LPI) in both eyes. In the APAC group, LPI was performed after IOP reduction using mannitol serum and antiglaucoma medications, and resolution of corneal edema. Three weeks after LPI, a complete ophthalmologic examination and biometry were performed.
Patients with PACG were characterized by a shallow anterior chamber, a narrow angle with synechiae, and typical glaucomatous optic nerve head cupping and visual field defects. PACG cases with history of APAC were excluded. Subjects were classified as PACS if there was >270 degrees of iridotrabecular contact without peripheral anterior synechiae, glaucomatous optic neuropathy or increased IOP.4
Exclusion criteria consisted of a history of ocular trauma, prior intraocular surgery, any intraocular disorder except for cataract, secondary angle closure glaucoma, presence of the “double-hump” sign on indentation gonioscopy indicating plateau iris configuration, evidence of active keratitis or anterior segment pathology precluding gonioscopy and fundus examination, and the use of miotics or anticholinergics.
Pachymetry was performed on the central cornea using an ultrasonic pachymeter (Paxis, Biovision Inc., Clermont-Ferrand, France). Ten measurements were performed and the lowest reading was used as central corneal thickness (CCT). The lowest reading most likely reflects perpendicular placement of the pachymeter probe and therefore the most accurate measurement.13
Contact (non-immersion) ultrasonic biometry was performed (Echoscan US-800, Nidek Co. Ltd, Gamagori, Japan) to measure axial length (AL), anterior chamber depth (ACD), lens thickness (LT) and vitreous depth (VD). Six measurements were performed per subject; if the standard deviation (SD) of these measurements was 0.12 mm or greater, all six readings were discarded and the process was repeated until the SD was less than 0.12 mm.14 The measured parameters were used to calculate corrected ACD (CACD=ACD-CCT), lens-axial length factor (LAF=LT/AL×10), lens position (ACD+½LT), relative lens position (RLP=[ACD+½LT]/AL×10), and corrected lens position (CLP=CACD+½ LT).11,15
Statistical analysis was performed using the Statistical Package for Social Sciences software version 17 (SPSS v17, IBM Corp., New York, NY, USA). Categorical data were compared using the Chi-square test and numerical data were compared employing the one-way ANOVA and Student t-test. P values less than 0.05 were considered as statistically significant. Mean values were reported with standard deviation (mean±SD). Biometric parameters were measured in both eyes of all patients, but only data from the right eyes were analyzed for intergroup comparisons. In the APAC group, biometric parameters were also compared between affected and unaffected fellow eyes.
Receiver operating characteristic (ROC) curves were plotted for each biometric variable as a predictive factor for APAC. The best sensitivity/specificity relation was tested by definition of different cut-off points. The biometric parameters with statistically significant differences among the study groups were used to build a binary logistic regression analysis model to predict the risk of APAC. Calibration of the model was performed using the Hosmer & Lemeshow test, ROC curve area, and Nagelkerke R square. LAF and CCT variables remained in the model [probability of APAC= 1/(1+e-z)], where z = constant + β1 × LAF + β2 × CCT. The area under ROC curves for predicted probabilities, and the Nagelkerke R square were 0.839 and 0.419, respectively.
RESULTS
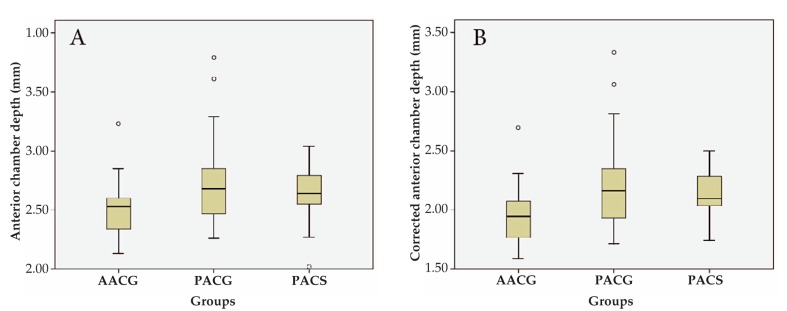
One hundred and forty-eight eyes of 113 eligible subjects were evaluated; these included 33 eyes with PACS, 45 eyes with PACG, 35 eyes with APAC and 35 unaffected fellow eyes of patients with APAC. APAC cases were significantly (P=0.025) younger (54.50±9.67 years) than patients with PACG (60.79±9.03 years) and PACS (58.54±9.03 years). There was no statistically significant differences in terms of gender among the 3 groups (P=0.843). All biometric parameters and CCT values were comparable between affected and fellow eyes in the APAC group (Table 1). Biometric parameters and multiple comparisons among the three study groups are presented in Tables 2 and 3, respectively. The APAC group had statistically significantly thicker cornea (P=0.001) and lens (P<0.0001); and shallower ACD (P=0.009) and CACD (P=0.003) as compared to the PACS and PACG groups (Fig. 1). There was no significant difference in lens thickness between the PACG and PACS groups (P=0.801). LAF was greatest in the APAC group (P=<0.0001) due to thicker lens but comparable axial length as compared to other groups.
Table 1.
Biometric parameters and central corneal thickness in affected and fellow eyes of patients with acute primary angle closure
| Biometric Parameters | Affected Eyes | Fellow Eyes | P value |
|---|---|---|---|
| Central corneal thickness (microns) | 556.81 ± 38.51 | 555.32 ± 22.91 | 0.860 |
| Anterior chamber depth (mm) | 2.52 ± 0.22 | 2.56 ± 0.19 | 0.426 |
| Corrected anterior chamber depth (mm) | 1.96 ± 0.23 | 1.99 ± 0.19 | 0.657 |
| Lens thickness (mm) | 4.86 ± 0.30 | 4.90 ± 0.32 | 0.630 |
| Axial length (mm) | 21.76 ± 1.30 | 21.80 ± 1.21 | 0.898 |
| Vitreous depth (mm) | 14.40 ± 1.26 | 14.35 ± 1.33 | 0.873 |
| Lens position | 4.95 ± 0.23 | 5.02 ± 0.23 | 0.299 |
| Corrected lens position | 4.39 ± 0.24 | 4.46 ± 0.23 | 0.279 |
| Relative lens position | 2.28 ± 0.19 | 2.31 ± 0.21 | 0.615 |
| Lens/Axial length factor | 2.23 ± 0.16 | 2.25 ± 0.21 | 0.702 |
| Intraocular lens power (diopters) | 24.77 ± 4.16 | 24.48 ± 3.87 | 0.774 |
| Mean keratometry (diopters) | 45.31 ± 2.13 | 45.35 ± 1.99 | 0.935 |
mm, millimeters
Values are presented as mean ± standard deviation
Table 2.
Demographic and biometric parameters in the study groups
| Parameter | APAC | PACG | PACS | P value |
|---|---|---|---|---|
| Age (years) | 54.50 ± 9.67 | 60.79 ± 9.03 | 58.54 ± 9.03 | 0.025 |
| Sex (Male/Female) | 8/27 | 12/33 | 7/26 | 0.843 |
| Central corneal thickness (microns) | 556.81 ± 38.51 | 524.75 ± 38.59 | 533.56 ± 26.57 | 0.001 |
| Anterior chamber depth (mm) | 2.52 ± 0.22 | 2.72 ± 0.35 | 2.65 ± 0.21 | 0.009 |
| Corrected anterior chamber depth (mm) | 1.96 ± 0.23 | 2.20 ± 0.37 | 2.13 ± 0.18 | 0.003 |
| Lens thickness (mm) | 4.86 ± 0.30 | 4.54 ± 0.47 | 4.56 ± 0.22 | <0.0001 |
| Axial length (mm) | 21.76 ± 1.30 | 22.09 ± 1.06 | 22.14 ± 0.88 | 0.287 |
| Vitreous chamber depth (mm) | 14.40 ± 1.26 | 14.87 ± 1.00 | 14.90 ± 0.79 | 0.084 |
| Lens position | 4.95 ± 0.23 | 4.97 ± 0.30 | 4.95 ± 0.20 | 0.936 |
| Corrected lens position | 4.39 ± 0.24 | 4.43 ± 0.32 | 4.42 ± 0.20 | 0.796 |
| Relative lens position | 2.28 ± 0.19 | 2.25±0.13 | 2.23 ± 0.08 | 0.432 |
| Lens/Axial length factor | 2.23 ± 0.16 | 2.05 ± 0.23 | 2.06 ± 0.11 | <0.0001 |
| Intraocular lens power (diopters) | 24.77 ± 4.16 | 23.46 ± 3.15 | 23.66 ± 2.39 | 0.194 |
| Mean keratometry (diopters) | 45.31 ± 2.13 | 45.33 ± 2.08 | 45.01 ± 1.72 | 0.751 |
APAC, acute primary angle-closure; PACG, chronic angle-closure glaucoma; PACS, primary angle-closure suspect
mm, millimeters
Values are presented in mean ± standard deviation
Table 3.
Results of least significant difference in age and biometric parameters by post hoc multiple comparisons among the study groups (ANOVA)
| Mean difference (P-value) | |||
|---|---|---|---|
| APAC vs. PACG | APAC vs. PACS | PACG vs. PACS | |
| Age | -6.29545 (0.007) | -4.04545 (0.0097) | 2.25000 (0.291) |
| Central corneal thickness (microns) | 32.05645 (0.000) | 23.24395 (0.010) | -8.81250 (0.295) |
| Anterior chamber depth (mm) | -0.20124 (0.002) | -0.12596 (0.071) | 0.07527 (0.248) |
| Corrected anterior chamber depth (mm) | -0.24142 (0.001) | -0.17060 (0.020) | 0.07081 (0.297) |
| Lens thickness (mm) | 0.32318 (0.000) | 0.30156 (0.001) | -0.02162 (0.801) |
| Axial length (mm) | -0.32908 (0.187) | -0.38310 (0.154) | -0.05402 (0.831) |
| Vitreous depth (mm) | -0.47031 (0.050) | -0.49860 (0.054) | -0.02830 (0.907) |
| Lens position | -0.0151 (0.794) | 0.00505 (0.937) | 0.02055 (0.734) |
| Corrected lens position | -0.04359 (0.502) | -0.02732 (0.687) | 0.01627 (0.798) |
| Relative lens position | 0.03317 (0.326) | 0.04495 (0.218) | 0.01178 (0.731) |
| Lens/Axial length factor | 0.017972 (<0.001) | 0.17363 (<0.001) | -0.00609 (0.887) |
| Intraocular lens power | 1.30448 (0.084) | 1.10448 (0.173) | -0.20000 (0.793) |
| Mean keratometry | -0.02217 (0.961) | 0.30120 (0.537) | 0.32337 (0.483) |
ANOVA, analysis of variances; APAC, acute primary angle closure; PACG, primary angle closure glaucoma
PACS, primary angle closure suspect; mm, millimeters
Figure 1.
Anterior chamber depth (A) and corrected anterior chamber depth (B) in patients with acute primary angle closure glaucoma (APAC), primary angle closure glaucoma (PACG) and primary angle closure suspects (PACS).
DISCUSSION
Ocular biometric studies have shown that eyes with PACG have several different characteristics as compared to normal eyes, such as a shallower anterior chamber,15-18 thicker lens,16,20 more anterior LP16,17,20 and shorter AL.16-18 However, biometric differences between eyes with PACS and PACG, and risk factors for APAC or PACG versus PACS have not been widely evaluated.
As shown in Table 5, the definition for PACS in previous studies has not been uniform or standardized. George et al3 evaluated ocular biometric values in 148 patients with occludable angles (defined as an eye in which less than 180 degrees of the filtering trabecular meshwork was visible before indentation) and 22 patients with angle closure glaucoma (defined as IOP >21 mmHg in the presence of an occludable angle). These authors reported no significant difference in AL, ACD or LT between these groups. It should be emphasized that the current definition of glaucoma differs from what George et al3 proposed in their study. In fact, subjects who had been classified as glaucoma had primary angle closure using current classifications. Sihota et al9 defined PACS when >180 degrees of posterior pigmented trabecular meshwork not visible and reported an ACD of 3.06 mm in 19 patients. This high value for ACD may be due to their definition for PACS.9 The current definition of PACS is applied when ≥270 degrees of the posterior pigmented trabecular meshwork is not visible on gonioscopy. The only study that included PACS patient using current definitions was published by He et al.8 In their study, mean ACD was 2.05, lower than 2.65 in our patients. Additionally, the lens was thicker (4.7 vs. 4.56) and AL was higher (22.5 vs. 22.14) in the report by He et al8 as compared to our study. The differences between our study and He et al’s are likely due to different sets of patients (Chinese vs. Iranian), selecting one best measure from multiple measurements in He et al’s compared to averaging multiple measurements in our study, and using a higher standard deviation (<0.13) than that of our study (<0.12) for selecting measurements. In the present study ACD was lower in PACS as compared to APAC.
In our study, there was no statistically significant difference in AL among the 3 groups. Based on this observation, it seems that either lens thickness or lens position should be the leading factor predisposing to APAC. The shallower ACD in APAC is in part due to thicker and more anterior position of the crystalline lens.16 A thick lens plays an essential role in the pathogenesis of angle closure by causing a decrease in ACD, resulting in angle crowding.3,17 The relative size of the lens is represented by LAF. Although in our study there was no difference in LAF between PACG and PACS, this value was greater in the APAC group. There was no difference in LP (P=0.936) and RLP (P=0.432) among the three groups. However, a significant difference was found in LT (P<0.0001) and LAF (P<0.0001). Considering the lack of significant difference in axial length among the three groups, the main determinant of shallow anterior chamber seems to be lens thickness. The regression model for predicting APAC versus PACS and PACG showed the significant contribution of LAF in our series. However, the association between various lens parameters such as LP, RLP and LAF has not been established conclusively, and there have been conflicting reports on the importance of LP and RLP in angle closure.6,7,9,15-17
It has been well documented that female subjects are more susceptible to angle closure glaucoma.21 Although there were more female patients in all groups in our study, no significant difference in gender was found among the groups. Patients with APAC were significantly younger than those with PACS and PACG. Shallower ACD and CACD, thicker lens and greater LAF may be factors precipitating an acute attack at younger age.
Alsbirk18 and Lee et al19 found no significant difference in anterior chamber biometric parameters comparing eyes with APAC to fellow eyes, or to patients with subtypes of PACG. Two studies have reported that APAC eyes have shallower ACD, more anterior LP and lower RLP values as compared to unaffected fellow eyes.11,22 Merula et al23 evaluated 30 patients with APAC and reported a difference only in LP between affected and fellow eyes. The lens in APAC eyes was located more anteriorly as compared to fellow eyes. Although biometric parameters in the present study were not significantly different between APAC and fellow eyes, values for ACD, CACD, AL, LP and CLP were lower and those for LT and LAF were higher in affected eyes. This may be due to limited sample size or there may be other physiologic factors that are important in triggering an acute attack.
Previous studies have not presented information on CCT. In our patients average CCT in the APAC group (555µ) was significantly larger than PACG (524µ) and PACS (533µ). Hence, CACD in APAC was significantly shallower than the other groups. Considering the fact that CCT was comparable in affected and fellow eyes in the APAC group, the thicker cornea cannot be secondary to an acute attack. According to the ROC curve for CCT, patients with CCT greater than 540.5 µ were at higher risk of APAC with sensitivity of 74.2% and specificity of 70.8%. In the logistic regression model, CCT was a significant predictor (as was LAF) for discriminating APAC. Additionally, CACD less than 2.02mm was associated with a greater risk of developing APAC with sensitivity of 70% and specificity of 68.1%.
Lowe24 and Alsbirk25 reported that PACG was uncommon in eyes with central ACD >2.5 mm. It has been suggested that measurement of ACD can be applied for screening angle closure glaucoma.26,27 The APAC group in our study, had deeper ACD (2.52 vs. 2.25 and 2.11mm respectively) and smaller LT (4.86 vs. 5.1 and 5.01mm respectively) as compared to other reports.5,11 Patients with ACD<2.55 mm and LT>4.66 mm were at higher risk of APAC, with sensitivity of 60% and 67.6%, and specificity of 65.3% and 60.5%, respectively.
Limitations of the current study include performing biometry after LPI in the APAC group, limited sample size, and the drawbacks inherent to a hospital-based study. Although previous studies28-30 have failed to demonstrate significant changes in biometric parameters before and 2 weeks after laser iridotomy, it has been reported that laser iridotomy deepens the peripheral anterior chamber without any effect on central ACD.19 Therefore, the results seem to be comparable to what would have been found prior to iridotomy.
Our study demonstrated that eyes with APAC were characterized by a more crowded anterior segment as compared to eyes with PACG and PACS, but there was no significant biometric difference between eyes with PACG and PACS. LAF, which reflects relative lens thickness, was found to be the main factor associated with APAC. In addition, CCT was greater in APAC and consequently CACD was shallower which appears to play an important role in the development of an acute attack. It is logical to take into account CCT in biometric evaluation of patients with PACS or APAC. The lack of difference in biometric characteristics between PACG and PACS in our study does not preclude the importance of these factors in progressing from PACS to PACG; it is possible that biometric differences were subtle and not detectable using our methods and setting.
Table 4.
The results of receiver operating characteristic curve analysis for each biometric variable
| Variable | Area under curve | Pvalue | Cut-off point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| Corrected anterior chamber depth | 0.716 | 0.001 | <2.028 | 70 | 68.1 |
| Anterior chamber depth | 0.680 | 0.004 | <2.555 | 60 | 65.3 |
| Vitreous depth | 0.621 | 0.044 | <14.765 | 67.6 | 56.6 |
| Lens thickness | 0.726 | <0.0001 | >4.665 | 67.6 | 60.5 |
| Intraocular lens power | 0.575 | 0.203 | ______ | ______ | ______ |
| Axial length | 0.571 | 0.231 | ______ | ______ | ______ |
| Lens axial-length factor | 0.760 | <0.0001 | >2.139 | 73.5 | 71.1 |
| Relative lens position | 0.520 | 0.741 | _______ | _______ | ______ |
| Central corneal thickness | 0.734 | <0.0001 | >540.500 | 74.2 | 70.8 |
| Mean keratometry | 0.488 | 0.845 | ________ | _______ | _______ |
Table 5.
Studies reporting ocular biometrics in angle closure
| Authors | Groups;numbers | Definitions | Mean age(standard deviation) | CCT (µ) | Lensthickness (mm) | Anteriorchamber depth (mm) | Axiallength | Lens/axiallength factor |
|---|---|---|---|---|---|---|---|---|
| He et al7 | PACS; 72 | 270 degrees of posterior pigmented TM not visible | NP | NP | 4.7 | 2.05 | 22.5 | NP |
| Sihota et al8 | PACS; 19 | > 180 of posterior pigmented TM non visible | 37.19(10.5) | 560.98 | 4 | 3.06 | 23.21 | NP |
| ACG; 34 | 49.3 (14.4) | 542.51 | 4.04 | 2.87 | 22.58 | NP | ||
| Ramani et al9 | PACS; 57 | >180degrees of posterior pigmented TM not visible | 52.4 (10.3) | 480 | 4.27 | 2.24 | 22.23 | 2.1 |
| Lan et al4 | AACG; 33 | 65.9 (8.5) | NP | 5.1 | 2.25 | 22.39 | 2.22 | |
| CACG; 41 | 63.9 (9.3) | NP | 4.84 | 2.69 | 23.13 | 2.23 | ||
| Lim et al10 | AACG;73 | 61 (10.9) | NP | 5.01 | 2.11 | 21.86 | 2.1 | |
| George et al3 | Occludableangle; 143 | < 180degrees of posterior pigmented TM not visible | 4.43 (9.23) | NP | 4.4 | 2.53 | 22.07 | 1.99 |
| ACG; 22 | Occludableangle + IOP> 22 mmHg | 57.45 (8.5) | NP | 4.23 | 2.63 | 21.92 | 1.91 | |
| Ramani et al11 | PACS; 82 | >180degrees of posterior pigmented TM not visible | 52.1 (10) | 480 | 4.23 | 2.43 | 22.1 | 2.1 |
| Salmon et al5 | CACG; 46 | 63.26 | NP | 4.73 | 2.24 | 22.43 | 2.11 |
CCT, central corneal thickness; NP, not provided; mm, millimeters; PACS, primary angle closure suspect; ACG, angle closure glaucoma; AACG, acute angle closure glaucoma; CACG, chronic angle closure glaucoma; TM, trabecular meshwork
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster PJ, Oen FT, Machin D, Ng TP, Devereux JG, Johnson GJ, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118:1105–1111. doi: 10.1001/archopht.118.8.1105. [DOI] [PubMed] [Google Scholar]
- 3.George R, Paul PG, Baskaran M, Ramesh SV, Raju P, Arvind H, et al. Ocular biometry in occludable angles and angle closure glaucoma: a population based survey. Br J Ophthalmol. 2003;87:399–402. doi: 10.1136/bjo.87.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster P, He M, Liebmann J. Epidemiology, Classification and Mechanism. In: Weinreb RN, Friedman DS, editors. Angle closure and angle closure glaucoma. Hague, Kugler publication; 2006. [Google Scholar]
- 5.Lan YW, Hsieh JW, Hung PT. Ocular biometry in acute and chronic angle-closure glaucoma. Ophthalmologica. 2007;221:388–394. doi: 10.1159/000107498. [DOI] [PubMed] [Google Scholar]
- 6.Salmon JF, Swanevelder SA, Donald MA. The dimensions of eyes with chronic angle-closure glaucoma. J Glaucoma. 1994;3:237–243. [PubMed] [Google Scholar]
- 7.Markowitz SN, Morin JD. The ratio of lens thickness to axial length for biometric standardization in angle-closure glaucoma. Am J Ophthalmol. 1985;99:400–402. doi: 10.1016/0002-9394(85)90005-4. [DOI] [PubMed] [Google Scholar]
- 8.He M, Friedman DS, Ge J, Huang W, Jin C, Lee PS, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114:494–500. doi: 10.1016/j.ophtha.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Sihota R, Ghate D, Mohan S, Gupta V, Pandey RM, Dada T. Study of biometric parameters in family members of primary angle closure glaucoma patients. Eye (Lond) 2008;22:521–527. doi: 10.1038/sj.eye.6702687. [DOI] [PubMed] [Google Scholar]
- 10.Ramani KK, Mani B, Ronnie G, Joseph R, Lingam V. Gender variation in ocular biometry and ultrasound biomicroscopy of primary angle closure suspects and normal eyes. J Glaucoma. 2007;16:122–128. doi: 10.1097/01.ijg.0000212285.55174.f5. [DOI] [PubMed] [Google Scholar]
- 11.Lim MC, Lim LS, Gazzard G, Husain R, Chan YH, Seah SK, et al. Lens opacity, thickness, and position in subjects with acute primary angle closure. J Glaucoma. 2006;15:260–263. doi: 10.1097/01.ijg.0000212212.10395.76. [DOI] [PubMed] [Google Scholar]
- 12.Ramani KK, Mani B, George RJ, Lingam V. Follow-up of primary angle closure suspects after laser peripheral iridotomy using ultrasound biomicroscopy and A-scan biometry for a period of 2 years. J Glaucoma. 2009;18:521–527. doi: 10.1097/IJG.0b013e318193c12d. [DOI] [PubMed] [Google Scholar]
- 13.Razeghinejad MR, Safavian H. Central corneal thickness in patients with Weill-Marchesani syndrome. Am J Ophthalmol. 2006;142:507–508. doi: 10.1016/j.ajo.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Lee AJ, Saw SM, Gazzard G, Cheng A, Tan DT. Intraocular pressure associations with refractive error and axial length in children. Br J Ophthalmol. 2004;88:5–7. doi: 10.1136/bjo.88.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998;105:2091–2098. doi: 10.1016/S0161-6420(98)91132-0. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson A, Leighton DA. Ocular dimensions in the heredity of angle-closure glaucoma. Br J Ophthalmol. 1973;57:475–486. doi: 10.1136/bjo.57.7.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 1970;54:161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alsbirk PH. Primary angle-closure glaucoma. Oculometry, epidemiology, and genetics in a high risk population. Acta Ophthalmol Suppl. 1976:5–31. [PubMed] [Google Scholar]
- 19.Lee DA, Brubaker RF, Ilstrup DM. Anterior chamber dimensions in patients with narrow angles and angle-closure glaucoma. Arch Ophthalmol . 1984;102:46–50. doi: 10.1001/archopht.1984.01040030030029. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y. Ultrasonic evaluation of the lens thickness to axial length factor in primary closure angle glaucoma. Yan Ke Xue Bao. 1993;9:12–14. [PubMed] [Google Scholar]
- 21.Thapa SS, Paudyal I, Khanal S, Paudel N, van Rens GH. Comparison of axial lengths in occludable angle and angle-closure glaucoma- the Bhaktapur Glaucoma Study. Optom Vis Sci. 2011;88:150–154. doi: 10.1097/OPX.0b013e318205e320. [DOI] [PubMed] [Google Scholar]
- 22.Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin Experiment Ophthalmol. 2000;28:253–258. doi: 10.1046/j.1442-9071.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 23.Merula RV, Cronemberger S, Diniz Filho A, Calixto N. New comparative clinical and biometric findings between acute primary angle-closure and glaucomatous eyes with narrow angle. Arq Bras Oftalmol. 2010;73:511–516. doi: 10.1590/s0004-27492010000600009. [DOI] [PubMed] [Google Scholar]
- 24.Lowe RF. Primary angle-closure glaucoma. Inheritance and environment. Br J Ophthalmol. 1972;56:13–20. doi: 10.1136/bjo.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsbirk PH. Anterior chamber depth and primary angle-closure glaucoma. I. An epidemiologic study in Greenland Eskimos. Acta Ophthalmol (Copenh) 1975;53:89–104. doi: 10.1111/j.1755-3768.1975.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 26.Congdon NG, Youlin Q, Quigley H, Hung PT, Wang TH, Ho TC, et al. Biometry and primary angle- closure glaucoma among Chinese, white, and black populations. Ophthalmology. 1997;104:1489–1495. doi: 10.1016/s0161-6420(97)30112-2. [DOI] [PubMed] [Google Scholar]
- 27.Nolan WP, Baasanhu J, Undraa A, Uranchimeg D, Ganzorig S, Johnson GJ. Screening for primary angle closure in Mongolia: a randomised controlled trial to determine whether screening and prophylactic treatment will reduce the incidence of primary angle closure glaucoma in an east Asian population. Br J Ophthalmol. 2003;87:271–274. doi: 10.1136/bjo.87.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Aung T, Husain R, Chan YH, Lim LS, Seah SK, et al. Choroidal expansion as a mechanism for acute primary angle closure: an investigation into the change of biometric parameters in the first 2 weeks. Br J Ophthalmol. 2005;89:288–290. doi: 10.1136/bjo.2004.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caronia RM, Liebmann JM, Stegman Z, Sokol J, Ritch R. Increase in iris-lens contact after laser iridotomy for pupillary block angle closure. Am J Ophthalmol. 1996;122:53–57. doi: 10.1016/s0002-9394(14)71963-4. [DOI] [PubMed] [Google Scholar]
- 30.Gazzard G, Friedman DS, Devereux JG, Chew P, Seah SK. A prospective ultrasound biomicroscopy evaluation of changes in anterior segment morphology after laser iridotomy in Asian eyes. Ophthalmology. 2003;110:630–638. doi: 10.1016/S0161-6420(02)01893-6. [DOI] [PubMed] [Google Scholar]