Abstract
Purpose
To evaluate the effect of a single dose of intravitreal diclofenac on best- corrected visual acuity (BCVA) and central macular thickness (CMT) in patients with refractory uveitic cystoid macular edema (CME).
Methods
In this prospective non-comparative case series, 8 eyes of 8 patients with refractory CME secondary to chronic intermediate uveitis received a single intravitreal injection of diclofenac (500 µg/0.1ml) in addition to other systemic (oral prednisolone and methotraxate) and topical (betamethasone) remission maintaining drugs. Outcome measures were changes in BCVA and CMT after treatment.
Results
Mean BCVA remained relatively unchanged at 12, 24 and 36 weeks (0.69, 0.70 and 0.64 LogMAR, respectively) as compared to baseline (0.71 LogMAR). Mean CMT, however, decreased from 488 µm at baseline to 416 and 456 µm at 24 and 36 weeks, respectively. None of the changes were statistically significant.
Conclusion
In eyes with refractory uveitic CME, intravitreal injection of diclofenac insignificantly reduced CMT but this was not associated with visual improvement.
Keywords: Cystoid Macular Edema, Diclofenac Sodium, Intravitreal Injection, Visual Acuity, Uveitis
INTRODUCTION
Cystoid macular edema (CME) is the most common cause of visual impairment due to uveitis.1 Treatments that decrease intraocular inflammation can also reduce CME. In some cases, CME persists even after suppressing the inflammation and further treatments are needed to improve visual acuity (VA). These include non-steroidal anti-inflammatory drugs (topical and systemic), corticosteroids (topical, periocular, intravitreal, and systemic) and systemic carbonic anhydrase inhibitors.1-6
Intravitreal triamcinolone (IVT) can improve visual acuity and decrease macular edema in patients with uveitis.1-3 However, IVT entails complications such as increased intraocular pressure (IOP) in vulnerable patients and induced cataract.1-6 Additionally, the short-term effect of IVT therapy necessitates repeated injections.4-6 Anti-vascular endothelial growth factor (VEGF) therapy such as intravitreal bevacizumab (IVB) has recently been shown to improve VA and decrease CME in uveitis.7,8
Only one study in the literature has reported the effect of intravitreal diclofenac (IVD) on CME of different causes in which we reported that IVD may be effective for treatment of CME due to various etiologies including uveitis.9 To the best of our knowledge, no study has evaluated the effect of IVD on uveitic CME. We therefore designed this study to assess the effect of IVD on refractory uveitic CME.
METHODS
In this prospective, interventional case series, 8 eyes of 8 patients with refractory CME secondary to chronic intermediate uveitis received IVD (500 µg/0.1 ml). CME in these patients was recalcitrant to oral treatment as well as intravitreal and periocular injection of corticosteroids, oral immunosuppressive agents (methotrexate) or intravitreal bevacizumab. These eyes had no periocular or intravitreal injections of corticosteroids or bevacizumab over the past six months. CME was confirmed by angiography and optical coherence tomography (OCT). Visual acuity ranged from 5/200 to 20/50. Patients with history of other retinal diseases causing macular edema (such as diabetes or vascular occlusions), monocular patients, candidates for intraocular surgery, subjects with cataracts and media opacities, and patients with history of vitrectomy were excluded.
All patients were first informed of the possible complications of treatment and provided written informed consent. Following a complete history and eye examination, OCT and fluorescein angiography were performed prior to IVD injection. All other medications required for control of uveitis were continued.
The injections were performed on an outpatient basis after anesthesia with topical tetracaine. After the lid speculum was inserted, a few drops of povidone-iodine 5% were instilled in the eye for disinfection. Then 500 µg (0.1 ml) diclofenac was injected intravitreally using a 30-gauge needle on an insulin syringe in the superotemporal quadrant (3.75 mm and 3.25 mm posterior to the limbus for phakic eyes and pseudophakic eyes, respectively).
Follow-up examinations were performed one day and one week after injection taking particular attention to anterior chamber reaction and IOP. Complete examinations were performed by a masked examiner 12, 24 and 36 weeks after IVD injection. These examinations included measurement of best-corrected visual acuity (BCVA) (measured with a Snellen chart), anterior chamber inflammation, lens opacity, macular status, IOP and use of antiglaucoma medications. Serious injection-related complications such as retinal detachment, intravitreal hemorrhage, endophthalmitis and retinal tear were also recorded. Retinal thickness was measured with a spectral domain OCT (3D OCT-1000, Topcon Corporation, Tokyo, Japan) at baseline and at weeks 12, 24 and 36 post-injection.
Main outcome measures were changes in BCVA and central macular thickness (CMT). Wilcoxon signed test was used to compare pre- and post-treatment values. Correlation between CMT and BCVA was tested with Spearman’s coefficient (r).
RESUlTS
Mean age of the patients was 47 years. Three patients had history of cataract surgery with intraocular lens implantation. History of IVT, IVB, and trans-septal steroid injections was present in 2, 3, and 1 cases, respectively. Three cases were on oral prednisolone and 3 others, on oral methotrexate both of which were continued during the study period. Table 1 shows baseline characteristics of the patients and study outcomes including BCVA and CMT at weeks 12, 24 and 36 after IVD injection.
Table 1.
Demographic and ocular characteristics of patients before treatment and 12, 24 and 36 weeks after intravitreal diclofenac injection
| BCVA (LogMAR) | CMT (microns) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Sex | Eye | Age | Diagnosis | Baseline | Week 12 | Week 24 | Week 36 | Change from baseline to week 36 | Baseline | Week 12 | Week 24 | Week 36 | Change from baseline to week 36 |
| 1 | M | OD | 56 | Intermediate non-granulomatousuveitis | 0.60 | 0.18 | 0.18 | 0.18 | -0.42 | 484 | 270 | 254 | 245 | -239 |
| 2 | F | OS | 58 | " | 0.54 | 0.54 | 0.54 | 0.70 | +0.16 | 437 | 423 | 412 | 407 | -30 |
| 3 | F | OD | 57 | " | 0.90 | 1.10 | 1.2 | 1.0 | +0.1 | 345 | 294 | 187 | 223 | -122 |
| 4 | F | OD | 41 | " | 0.78 | 0.78 | 1.0 | 0.60 | -0.18 | 584 | 756 | 780 | 646 | +62 |
| 5 | F | OS | 36 | " | 0.40 | 0.50 | 0.40 | 0.30 | -0.1 | 518 | 522 | 373 | 288 | -230 |
| 6 | F | OS | 35 | " | 0.40 | 0.30 | 0.40 | 0.40 | 0 | 476 | 465 | 437 | 416 | -60 |
| 7 | F | OD | 47 | " ‘ | 1.30 | 0.78 | 0.78 | 0.90 | -0.4 | 390 | 294 | 452 | 705 | +315 |
| 8 | F | OS | 43 | Intermediate granulomatous uveitis | 0.78 | 1.30 | 1.10 | 1.00 | +0.22 | 672 | 490 | 437 | 721 | +49 |
| Mean | - | - | 47 | - | 0.71 | 0.69 | 0.70 | 0.64 | -0.08 | 488 | 439 | 416 | 456 | -32 |
| (SD) | (0.30) | (0.38) | (0.37) | (0.32) | (0.24) | (105) | (161) | (175) | (207) | (180) | ||||
| P-value forchange from baseline (Wilcoxon Signed rank test) | 0.916 | 0.686 | 0.446 | 0.123 | 0.208 | 0.575 | ||||||||
BCVA, best-corrected visual acuity; CMT, central macular thickness; M, male; F, female; OD, right eye; OS, left eye; SD, standard deviation
BCVA improved in 3 patients up to week 12; in 2 patients BCVA continued to improve as compared to baseline towards the end of the follow-up. BCVA deteriorated in 2 patients and remained unchanged in 3 patients over 36 weeks of follow-up. Mean BCVA remained relatively stable at 12, 24 and 36 weeks (0.68, 0.70 and 0.64 logMAR, respectively) as compared to baseline (0.71 logMAR). Mean change in BCVA was -0.08±0.24 logMAR from baseline at 36 weeks post-injection. The above mentioned changes failed to reach statistical significance.
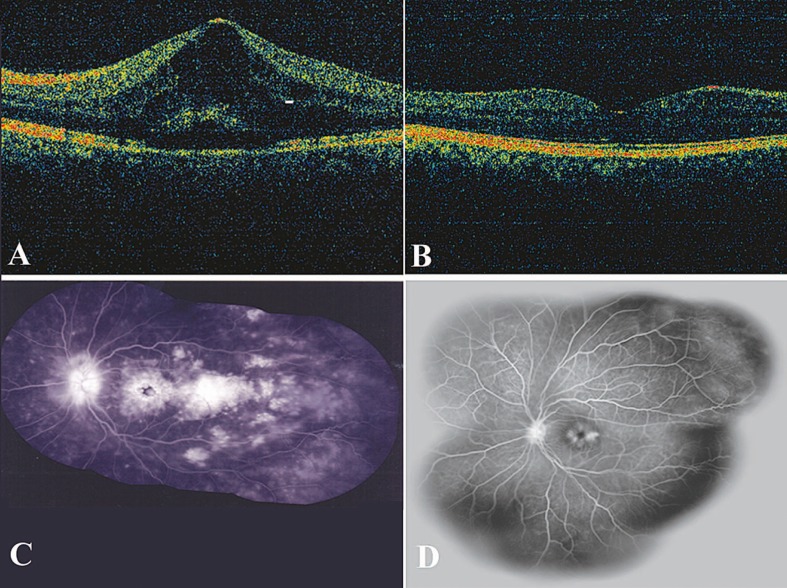
CMT decreased from baseline values in 6 eyes after 12 weeks and in 5 eyes after 36 weeks. Mean CMT decreased up to 24 weeks and slightly increased at week 36; however, it remained lower than baseline. Mean changes in CMT from baseline to 36 weeks was -32±180 microns. However none of these changes was statistically significant. Figure 1 shows OCT and angiography images of a patient before and 12 weeks after treatment.
Figure 1.
A and B. Optical coherence tomography images of a patient before and 2 weeks after intravitreal diclofenac injection. C and D. Fluorescein angiography before and 12 weeks after the injection.
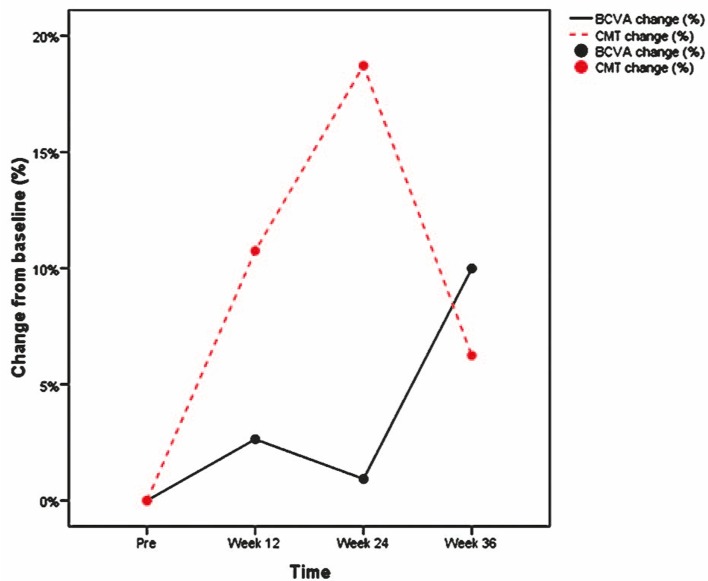
There was no significant correlation between changes in CMT and BCVA at any time point (r=0.25, -0.17 and 0.21 at weeks 12, 24 and 36, respectively). As shown in Figure 2, changes in BCVA did not parallel CMT changes.
Figure 2.
Correlation between percent of changes in best-corrected visual acuity (BCVA) and central macular thickness (CMT) during follow-up.
Mean IOP showed no significant change from baseline (13.4 mmHg) to week 12 (11.8 mmHg) and week 36 (13.8 mmHg). There were no cases of endophthalmitis, vitreous hemorrhage, retinal detachment or other complications.
DISCUSSION
In our series of 8 eyes undergoing IVD injection, BCVA improved in four cases 36 weeks after injection, however mean BCVA showed no significant change up to 36 weeks. CMT improved in 5 patients up to 36 weeks; mean CMT decreased at all follow-up visits as compared to baseline, but these changes also failed to reach statistical significance.
The pathophysiology of CME may help understand the rationale for employing IVD in this study. The inflammatory response to tissue damage results in release of arachidonic acid (AA) from cell membrane phospholipids. AA is converted to prostaglandins (PG) and thromboxane by cyclooxygenase enzymes (COX1 and COX2), and to leukotrienes by 5-lipooxigenase. PGE2 induces the production of VEGF and basic fibroblast growth factor (b-FGF) in cultured Muller cells.10 Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause vascular regression in chronic granulomatous inflammation by suppressing the COX enzymes, which in turn decrease PGE1 and PGE2.11 Diclofenac sodium is an NSAID which blocks both the cyclooxygenase and lipoxygenase pathways similar to corticosteroids.12
In the present study, BCVA improved in 4 cases 36 weeks after the injection which is comparable to our previous report on IVD for CME of various etiologies.9 Mean BCVA improvement in the present study was 0.07 LogMAR at 36 weeks. This beneficial effect of IVD on refractory uveitic CME does not seem to be as significant as other intravitreal agents including triamcinolone (visual improvement of -0.32±0.32 LogMAR) or bevacizumab (visual improvement of -0.3±0.45 LogMAR).9 Topical NSAIDs have also been shown to be effective in such cases.13,14 For instance, nepafenac 0.1% resulted in BCVA improvement of 0.36±0.20 LogMAR in six eyes with refractory uveitis.15
CMT was decreased in most of our cases at all follow-up visits; however, this change was small in some eyes and at some follow-up visits which could be attributed to inter-operator variability or to the sensitivity of the OCT machine. In addition, none of the eyes demonstrated a dry macula at any visit explaining the failure of notable visual improvement. As we demonstrated in our previous study, despite CMT reduction, BCVA improvement is not always expected.9
Diclofenac is a drug with a small molecule (318.13 Daltons) and has a short half-life in the vitreous (2.87 hours).16 Rapid elimination of diclofenac sodium from the vitreous necessitates several intravitreal injections which may be associated with complications such as retinal detachment and endophthalmitis.17,18
Methods of increasing the half-life of diclofenac in the vitreous should focus on its pharmacokinetic model and lipophilicity, as well as the delivery system.16 An animal study revealed that a less soluble form of the drug, such as diclofenac acid rather than diclofenac sodium salt, will remain in the vitreous cavity for up to 24 days, potentially resulting in therapeutic levels in posterior segment tissues for a few months.19 An effective slow-release delivery system for intravitreal diclofenac is a practical solution to achieve sustained therapeutic levels with the goal of providing prolonged clinical benefit. Moreover, this system helps control the delivered dose and the rate of dosing.20 Studies have shown that liposomal encapsulation of some intravitreal drugs increases activity, prolongs efficacy and reduces toxicity.20,21 Many other drug delivery systems such as biodegradable and non-biodegradable implants, microspheres, nanoparticles, gels and transporter-targeted prodrugs have been proposed to maximize the effects of ophthalmic drugs in the posterior segment. The safety and efficacy of these techniques need to be verified.22 Currently, there is no available study on implementation of these drug delivery strategies for intravitreal diclofenac.
Our intervention was not associated with major complications and systemic side effects. Kim et al23 showed that diclofenac at a dose of 500 µg is not toxic for the rabbit eye based on electroretinographic and histopathologic studies. We also found no toxic effect of 500µg (0.1ml) intravitreal diclofenac in human eyes.9
The present study has some shortcomings such as small number of patients and lack of a control group. One should also consider the effects of other systemic medications (steroids and methotrexate) which were taken by our patients and the self-subsiding nature of CME over time. Considering the short half life of diclofenac in the vitreous, not performing an earlier CMT measurement (before week 12) is another limitation of our study. Our outcomes suggest a possible beneficial effect from IVD in uveitic CME. However, the outcomes of our study warrant further evaluation with a larger sample size and a randomized clinical trial.
Acknowledgments
We would like to thank Mehdi Yaseri, PhD for his statistical assistance.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005;112:1916. doi: 10.1016/j.ophtha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Angunawela RI, Heatley CJ, Williamson TH, Spalton DJ, Graham EM, Antcliffe RJ, et al. Intravitreal triamcinalone acetonide for refractory uveitic cystoid macular oedema: longterm management and outcome. Acta Ophthalmol Scand. 2005;83:595–599. doi: 10.1111/j.1600-0420.2005.00438.x. [DOI] [PubMed] [Google Scholar]
- 3.Sallam A, Comer RM, Chang JH, Grigg JR, Andrews R, McCluskey PJ, et al. Short-term safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema in children. Arch Ophthalmol. 2008;126:200–205. doi: 10.1001/archophthalmol.2007.59. [DOI] [PubMed] [Google Scholar]
- 4.Hogewind BF, Zijlstra C, Klevering BJ, Hoyng CB. Intravitreal triamcinolone for the treatment of refractory macular edema in idiopathic intermediate or posterior uveitis. Eur J Ophthalmol. 2008;18:429–434. doi: 10.1177/112067210801800318. [DOI] [PubMed] [Google Scholar]
- 5.Conti SM, Kertes PJ. The use of intravitreal corticosteroids, evidence-based and otherwise. Curr Opin Ophthalmol. 2006;17:235–244. doi: 10.1097/01.icu.0000193107.00089.ee. [DOI] [PubMed] [Google Scholar]
- 6.Das-Bhaumik RG, Jones NP. Low-dose intraocular triamcinolone injection for intractable macular oedema and inflammation in patients with uveitis. Eye (Lond) 2006;20:934–937. doi: 10.1038/sj.eye.6702063. [DOI] [PubMed] [Google Scholar]
- 7.Soheilian M, Rabbanikhah Z, Ramezani A, Kiavash V, Yaseri M, Peyman GA. Intravitreal bevacizumab versus triamcinolone acetonide for refractory uveitic cystoid macular edema: a randomized pilot study. J Ocul Pharmacol Ther. 2010;26:199–206. doi: 10.1089/jop.2009.0093. [DOI] [PubMed] [Google Scholar]
- 8.Cordero Coma M, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology. 2007;114:1574–9. doi: 10.1016/j.ophtha.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Soheilian M, Karimi S, Ramezani A, Peyman GA. Pilot study of intravitreal injection of diclofenac for treatment of macular edema of various etiologies. Retina. 2010;30:509–515. doi: 10.1097/IAE.0b013e3181bdfa43. [DOI] [PubMed] [Google Scholar]
- 10.Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- 11.Alam CA, Seed MP, Willoughby DA. Angiostasis and vascular regression in chronic granulomatous inflammation induced by diclofenac in combination with hyaluronan in mice. J Pharm Pharmacol. 1995;47:407–411. doi: 10.1111/j.2042-7158.1995.tb05820.x. [DOI] [PubMed] [Google Scholar]
- 12.Kothari HV, Lee WH, Ku EC. An alternate mechanism for regulation of leukotriene production in leukocytes: studies with an anti-inflammatory drug, sodium diclofenac. Biochim Biophys Acta. 1987;921:502–511. doi: 10.1016/0005-2760(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 13.Warren KA, Fox JE. Topical nepafenac as an alternate treatment for cystoid macular edema in steroid responsive patients. Retina. 2008;28:1427–1434. doi: 10.1097/IAE.0b013e31817e7ead. [DOI] [PubMed] [Google Scholar]
- 14.Rothova A. Inflammatory cystoid macular edema. Curr Opin Ophthalmol. 2007;18:487–492. doi: 10.1097/ICU.0b013e3282f03d2e. [DOI] [PubMed] [Google Scholar]
- 15.Hariprasad SM, Akduman L, Clever JA, Ober M, Recchia FM, Mieler WF. Treatment of cystoids macular edema with the new-generation NSAID nepafenac 0.1%. Clin Ophthalmol. 2009;3:147–154. doi: 10.2147/opth.s4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durairaj C, Shah JC, Senapati S, Kompella UB. Prediction of vitreal half-life based on drug physicochemical properties: quantitative structure-pharmacokinetic relationships (QSPKR). Pharm Res. 2009;26:1236–1260. doi: 10.1007/s11095-008-9728-7. [DOI] [PubMed] [Google Scholar]
- 17.Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21:178–183. doi: 10.1097/ICU.0b013e328338679a. [DOI] [PubMed] [Google Scholar]
- 18.Jager RD, Aiello LP, Patel SC, Cunningham ET. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Durairaj C, Kim SJ, Edelhauser HF, Shah JC, Kompella UB. Influence of dosage form on the intravitreal pharmacokinetics of diclofenac. Invest Ophthalmol Vis Sci. 2009;50:4887–4897. doi: 10.1167/iovs.09-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishman PH, Peyman GA, Lesar T. Intravitreal liposome-encapsulated gentamicin in a rabbit model. Prolonged therapeutic levels. Invest Ophthalmol Vis Sci. 1986;27:1103–1106. [PubMed] [Google Scholar]
- 21.Claro C, Ruiz R, Cordero E, Pastor MT, López-Cortés LF, Jiménez-Castellanos M, et al. Determination and pharmacokinetic profile of liposomal foscarnet in rabbit ocular tissues after intravitreal administration. Exp Eye Res. 2009;88:528–534. doi: 10.1016/j.exer.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Adams NA, Toma HS, Belair ML, Thorne JE, Green WR, et al. Safety of intravitreal ketorolac and diclofenac: an electroretinographic and histopathologic study. Retina. 2008;28:595–605. doi: 10.1097/IAE.0b013e31815e98a5. [DOI] [PubMed] [Google Scholar]