Abstract
Purpose
To determine the clinical outcomes of simultaneous penetrating keratoplasty (PK), cataract removal and intraocular lens implantation (triple procedure), and to compare the safety and efficacy of two different cataract extraction techniques during the course of PK.
Methods
This retrospective comparative study was conducted on patients who had undergone a triple procedure. The technique of cataract extraction was either open- sky extracapsular cataract extraction (ECCE) or phacoemulsification (PE). In the ECCE group, the posterior chamber intraocular lens (PCIOL) was implanted in the ciliary sulcus, while in the PE group PCIOLs were fixated within the capsular bag. Outcome measures included best spectacle corrected visual acuity (BSCVA), refractive results, graft clarity and complications.
Results
Seventy-six eyes of 69 consecutive patients with mean age of 61.4±14.2 years were enrolled. Mean follow-up period was 61.4±37.2 months over which mean BSCVA was significantly improved from 1.40±0.68 to 0.44±0.33 LogMAR (P<0.001). Mean postoperative spherical equivalent refractive error was -2.13±3.02 D, which significantly differed from the target refraction (-0.73±0.29 D, P=0.004). At final follow-up, 89.5% of the corneal grafts remained clear.
Conclusion
The triple procedure is a safe and effective approach to restore vision in patients with coexisting corneal pathologies and cataracts. However, unacceptable postoperative refractive error can be anticipated.
Keywords: Corneal Graft, Cataract, Triple Procedure, Penetrating Keratoplasty, Extracapsular Cataract Extraction, Phacoemulsification
INTRODUCTION
Corneal pathologies may coincide with cataracts especially in the elderly. Combined surgery including penetrating keratoplasty (PK), cataract extraction, and intraocular lens (IOL) implantation (often termed the triple procedure) is a well-established and effective surgical treatment in such cases.1-13 This approach could also be indicated for subjects who are likely to develop corneal decompensation following cataract surgery, or in whom corneal surgery may accelerate cataract formation.14
The greatest advantage of the triple procedure is that it can obviate the expense and inconvenience of a second procedure, which is of great benefit for the elderly.2,5-7 Furthermore, a single procedure can reduce the risk of damage to the donor endothelium during cataract surgery.15 A major drawback to the triple procedure, however, is inaccuracy in IOL power prediction. Accurate power calculation requires valid and reproducible biometric data pertaining to corneal curvature, anterior chamber depth and axial length, which could be significantly altered after corneal transplantation.1-6,14-17
On the contrary, cataract extraction and IOL implantation after PK may yield better refractive outcomes.15 Additionally, this sequence provides a chance to correct post- keratoplasty astigmatism by implanting a toric IOL or performing arcuate keratotomies with or without compression sutures. In order to take advantage of these privileges, cataract extraction should be postponed at least one year after PK procedure, until all sutures are removed and graft curvature is stable. This latter approach however, entails the drawback of delayed visual rehabilitation.1,18,19 Other disadvantages of a second surgical procedure include the cost and risks of a second anesthesia, endophthalmitis, and expulsive bleeding, in addition to damage to the graft endothelium.12,15
Despite the advantages associated with both approaches, there is no consensus over the superiority of either one with respect to the conflicting results of different studies.12,15 Nevertheless, triple surgery appears to be more popular among anterior segment surgeons.20
During the combined approach, cataracts can be removed using open-sky extracapsular cataract extraction (ECCE) or phacoemulsification (PE) depending on the severity of the corneal opacity and surgeon’s skill. The main objective of the present study is to report the clinical outcomes of the triple procedure employing ECCE or PE.
METHODS
In this retrospective study, data from all consecutive patients undergoing the triple procedure between September 1995 and March 2009 were compiled. All sutures were removed at least three months before the final examination. Exclusion criteria consisted of pre- existing uveitis, retinal abnormalities and ocular surgery before the triple operation, except for previous corneal transplantation. If any further intervention was performed during follow-up to reduce the refractive error, examinations prior to that intervention were considered for the purpose of the study. The Ethics Committee of the Ophthalmic Research Center approved the study.
A thorough ophthalmic examination, including uncorrected visual acuity (UCVA), manifest refraction and best spectacle corrected visual acuity (BSCVA) in LogMAR notations, slit lamp biomicroscopy, applanation tonometry, dilated fundus examination, and keratometry readings (when possible), was performed preoperatively. Cataracts were categorized based on the Lens Opacity Classification System III (LOCS III). IOL power was calculated with an A-scan ultrasound device (Nidek, US-800, Tokyo, Japan) using the SRK-T formula and a standard constant keratometry value of 44 D. Target refraction was set at an average value of -0.73±0.29 (range: -1.92 to -0.19) D.
All eyes were operated on by a single experienced anterior segment surgeon (MAJ) under general or retrobulbar anesthesia. In subjects with adequate media clarity, PE was performed while open-sky extracapsular cataract extraction was employed when the cornea was opaque.
Extracapsular Cataract Extraction
The pupil was dilated preoperatively with tropicamide 1% eye drops (Mydriacyl; Alcon- Couvreur, Puurs, Belgium). The risk of elevated vitreous pressure was reduced by careful patient positioning, preoperative external ocular massage, administration of hyperosmotic agents, proper eyelid speculum selection, and occasionally lateral canthotomy. A scleral (Flieringa) ring was sutured to the recipient eye using four 7/0 silk fixation sutures before trephination of the recipient cornea to prevent globe collapse after cataract extraction.
After trephination, a large can-opener capsulotomy was created using a 27-gauge needle, and the nucleus was delivered by applying gentle pressure on the sclera. The remaining cortex was aspirated manually and after instillation of a dispersive ophthalmic viscosurgical device (Coatel, Bausch & Lomb, Waterford, Ireland), a 7 mm polymethyl methacrylate (PMMA) posterior chamber IOL (PCIOL) was inserted into the ciliary sulcus.
Phacoemulsification
A single-plane 2.8 mm incision, starting behind the surgical limbus and intended to be 1.5 to 2.0 mm in length, was created. The incision was made to enter the anterior chamber outside the trephination site. The site of the main incision was superotemporal in right eyes and superonasal in left eyes. After injection of a dispersive ophthalmic viscosurgical device (Coatel, Bausch & Lomb, Waterford, Ireland), a 5.0 to 5.5 mm central continuous capsulorhexis was created and phacoemulsification was performed applying the divide and conquer technique. This step was followed by cortical cleanup and implantation of a PCIOL within the capsular bag. Before proceeding to PK, the main and side incisions were closed using 10/0 nylon sutures (Sharpoint, Angiotech, Vancouver, Canada), and the anterior chamber was reformed.
Corneal Transplantation
The recipient cornea was cut with a Hessburg- Barron suction trephine (Katena Products, Denville, NJ, USA) until perforation occurred. The diameter of recipient trephines was selected on the basis of vertical corneal diameter and the extent of corneal pathologies. Subsequently, excision was completed using right and left transplantation scissors. The donor cornea, preserved in Optisol GS (Bausch & Lomb, Rochester, NY, USA), was punched out from the endothelial side with the Barron donor punch (Katena Products, Denville, NJ, USA). Donor- recipient disparity was 0.25 mm in keratoconic eyes and 0.50 mm in other conditions. After placing four cardinal sutures, the donor button was fixed to the recipient using either separate (34 eyes) or running (42 eyes) 10/0 nylon sutures (Sharpoint, Angiotech, Vancouver, Canada). Intraoperative keratoscopy was performed and suturing forceps were used to adjust suture tension and corneal astigmatism. At the end of the operation, subconjunctival injections of 50 mg cefazolin and 4 mg betamethasone were given.
Postoperative Course
Follow-up examinations were performed on days 1, 3, 7, and 30, and then every 3 to 4 months. The patients received topical sulfacetamide 10% every 6 hours for 30 days and topical betamethasone 0.1% every 6 hours tapered over 3 to 4 months. UCVA, BSCVA, keratometry, manifest refraction, slit lamp examination, and intraocular pressure (IOP) were re-evaluated at all follow-up examinations. Postoperatively, if keratometric astigmatism exceeded 4.0 D, tension of the running sutures was adjusted after 1 to 2 months, and selective removal of separate sutures began after 2 months. An episode of epithelial or endothelial graft rejection was managed with frequent topical betamethasone 0.1%.
Statistical Analysis
If any eye required further surgical interventions such as graft refractive surgery to reduce post-PK astigmatism, results prior to those interventions were analyzed. Data, including age, sex, past ocular history, time of operation, pre- and postoperative BSCVA, pre- and postoperative refractive error and biometric values (keratometry readings and axial length measurements), postoperative target refraction, trephine size, implanted IOL power, follow-up duration, and complications were collected and analyzed using SPSS statistical software version 13 (SPSS Inc., Chicago, IL, USA). Normality was evaluated by using the Kolmogorov-Smirnov test and normally distributed data were described in mean ± standard deviation. The paired t-test was employed to compare pre- and postoperative visual acuity, keratometry values, and refraction. Associations between achieved BSCVA (dependent factor) and explanatory factors including patient age, preoperative BSCVA, indications, technique of cataract extraction, graft size, and follow-up duration, were investigated by multiple linear regression analysis. The Kaplan-Meier method and log- rank test were used to calculate the cumulative incidence of rejection-free graft survival as well as graft survival in the two subgroups. The level of statistical significance was set at 0.05.
RESUlTS
Seventy-six (38 right) eyes from 69 (41 male) patients were enrolled in the study. Mean age at the time of operation was 61.4±14.2 (range, 17 to 80) years and patients were followed for 61.4±37.2 (range, 17 to 158) months. ECCE was performed in 46 (60.5%) eyes while PE was employed in the remaining 30 (39.5%) eyes. The two groups were comparable with respect to the severity of lens opacity (P>0.05). Table 1 summarizes the indications for corneal transplantation.
Table 1.
Comparison of demographic, operative and postoperative characteristics between study subgroups
| Characteristics | ECCE group | PE group |
|---|---|---|
| (n=46) | (n=30) | |
| Age (years) | 65.1±12.7 | 54.6±14.3 |
| Follow-up (months) | 66.4±39.4 | 51.3±30.4 |
| Preoperative BSCVA (LogMAR) | 1.70±0.64 | 1.0±0.49 |
| Postoperative BSCVA (LogMAR) | 0.79±0.62 | 0.35±0.32 |
| Preoperative diagnosis (n; %) | ||
| Fuchs’ endothelial dystrophy | 19 (41.3) | 5 (16.7) |
| Keratoconus | 3 (6.5) | 13 (40.0) |
| Regraft | 4 (8.7) | 8 (26.7) |
| Trachoma | 8 (17.4) | 1 (3.3) |
| Scar | 6 (13.0) | 2 (6.7) |
| Recipient trephine size (mm) | 7.58±0.23 | 7.82±0.23 |
| Donor trephine size (mm) | 8.06±0.22 | 8.15±0.18 |
| Axial length (mm) | 23.58±2.15 | 24.59±2.04 |
| Intraocular lens power (D) | 19.94±5.79 | 17.80±5.46 |
| Target refraction (D) | -0.66±0.27 | -0.80±0.31 |
| Postoperative spherical equivalent(D) | -2.84±3.20 | -1.17±2.54 |
| Absolute difference between targetand spherical equivalent refraction (D) | 2.81±1.98 | 2.01±1.52 |
| Postoperative mean keratometry (D) | 45.70±2.27 | 45.10±2.23 |
| Keratometric astigmatism (D) | 5.52±3.60 | 4.38±3.85 |
| Postoperative refractive astigmatism(D) | 4.23±1.99 | 3.37±2.0 |
ECCE, extracapsular cataract extraction; PE, phacoemulsification; BSCVA, best spectacle corrected visual acuity
LogMAR, logarithm of the minimum angle of resolution; D, diopter
Mean recipient and donor trephine sizes were 7.68±0.26 (range, 7.0 to 8.5) mm and 8.10±0.21 (range, 7.5 to 8.75) mm in the ECCE and PE groups respectively. Preoperatively, keratometry and refractive error were measurable in 33 eyes (mean keratometry 49.53±5.77; range, 43.0 to 65 D) and 22 eyes (spherical equivalent -3.13±5.95; range, -16.0 to +5.0 D), respectively; average preoperative keratometry was steeper than postoperative values (45.31±2.06; range, 42.0 to 49.5 D), (P=0.009). Table 1 demonstrates refractive outcomes at final follow-up.
The mean difference between target and postoperative spherical equivalent refractive error was -1.22±2.82 (range, -6.55 to +3.78 D, P=0.004) while mean absolute error was 2.46±1.81 (range, 0.04 to 6.55) D. Postoperative refractive error was within ±1.0 D of target refraction in 26 (34.2%) eyes and within ±2.0 D in 35 (46.05%) eyes.
Preoperatively, BSCVA was 1.40±0.68 (range, 0.18 to 2.90) LogMAR, which was significantly (P<0.001) improved to 0.44±0.33 (range, 0.10 to 1.70) LogMAR postoperatively. At final follow-up, BSCVA ≤ 0.2 LogMAR was observed in 31 (40.8%) eyes. There was no significant difference among different suturing techniques in terms of visual and refractive outcomes (P>0.05).
Multiple linear regression analysis demonstrated that postoperative BSCVA had a significant association with preoperative BSCVA (R=0.56, P<0.001), preoperative indication for PK (R=0.47, P=0.035), technique of cataract extraction (R=0.39, P=0.001), and recipient (R=0.35, P=0.009) and donor trephine graft size (R=0.88, P=0.04). Accordingly, the best results were observed in the keratoconus group with phacoemulsification technique and a larger trephine size. Nevertheless, postoperative BSCVA was independent of patient age (P=0.37) and duration of follow-up (P=0.81).
In one eye in the ECCE group, vitreous loss occurred following IOL implantation due to positive vitreous pressure which necessitated automated anterior vitrectomy and exchange of the IOL with an angle-supported anterior chamber IOL. Other patients, however, had uneventful operations.
Persistent corneal epithelial defects taking longer than 14 days to heal were observed in 5 (6.6%) eyes which improved in all cases by administration of frequent topical lubricants and/or bandage contact lenses. Elevated IOP (10 eyes; 13.2%) was controlled medically in all cases, except for one patient in the ECCE group, who required glaucoma shunt implantation. Other complications encountered in this series were postoperative uveitis (1 eye; 1.3%), vascularization of the suture tracts (7 eyes; 9.2%), recurrence of herpes simplex keratitis (HSK) in the graft (3 eyes; 3.9%), and posterior capsular opacification (22 eyes; 28.9%).
Thirty (39.5%) eyes experienced at least one episode of graft rejection (endothelial rejection in 18, epithelial rejection in 5, and both epithelial and endothelial rejection in 7 eyes) including ten eyes with two or more episodes of rejection. All eyes but 5 recovered from the rejection episodes using frequent topical steroids.
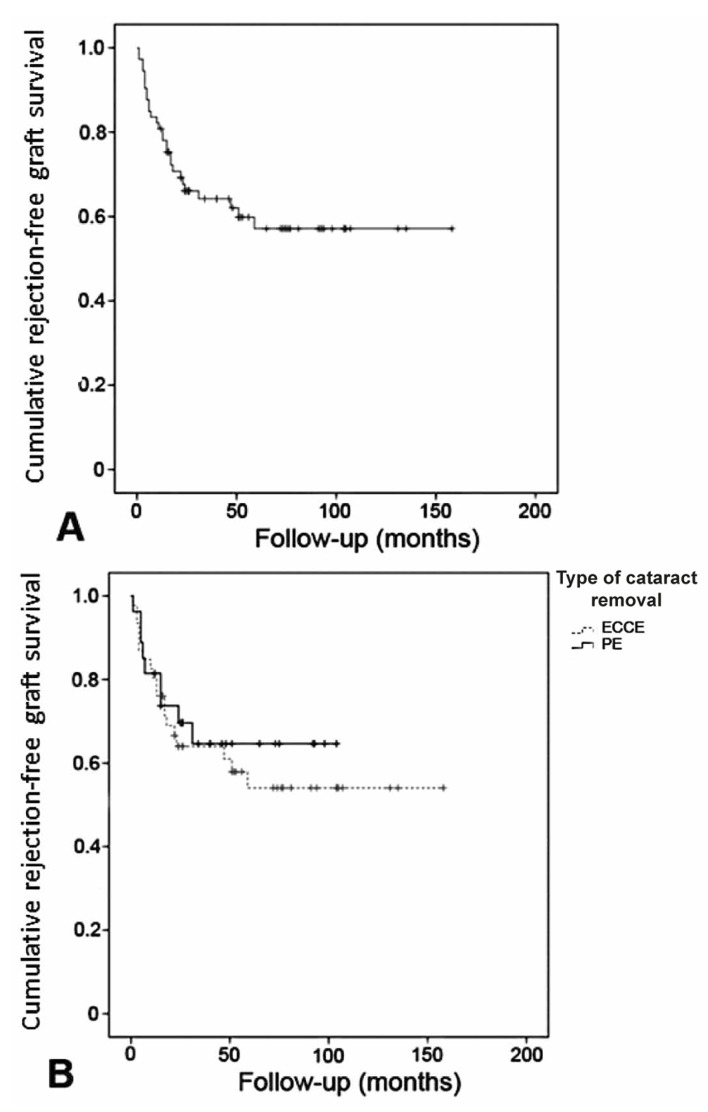
The mean rejection-free graft survival period was 98.2 (95% confidence interval [CI], 81 to 115) months. Figure 1 demonstrates that cumulative rejection-free graft survival was 59.8% at month 56.
Figure 1.
The Kaplan-Meier method reveals cumulative rejection-free graft survival in the whole study population (A), as well as in each group of cataract removal (B). (ECCE, extracapsular cataract extraction; PE, phacoemulsification)
At final follow-up, corneal grafts remained clear in 68 (89.5%) eyes, while failed grafts were observed in 8 (10.5%) eyes with preoperative diagnoses of Fuchs’ endothelial dystrophy (2 eyes), corneal scar (3 eyes), previously failed grafts (2 eyes), and glaucomatous bullous keratopathy due to Axenfeld-Rieger syndrome (1 eye). Out of these, 5 graft failures were due to endothelial graft rejection; other reasons for graft failure included recurrent HSK (1 eye), uncontrolled IOP (1 eye), and graft ulceration (1 eye).
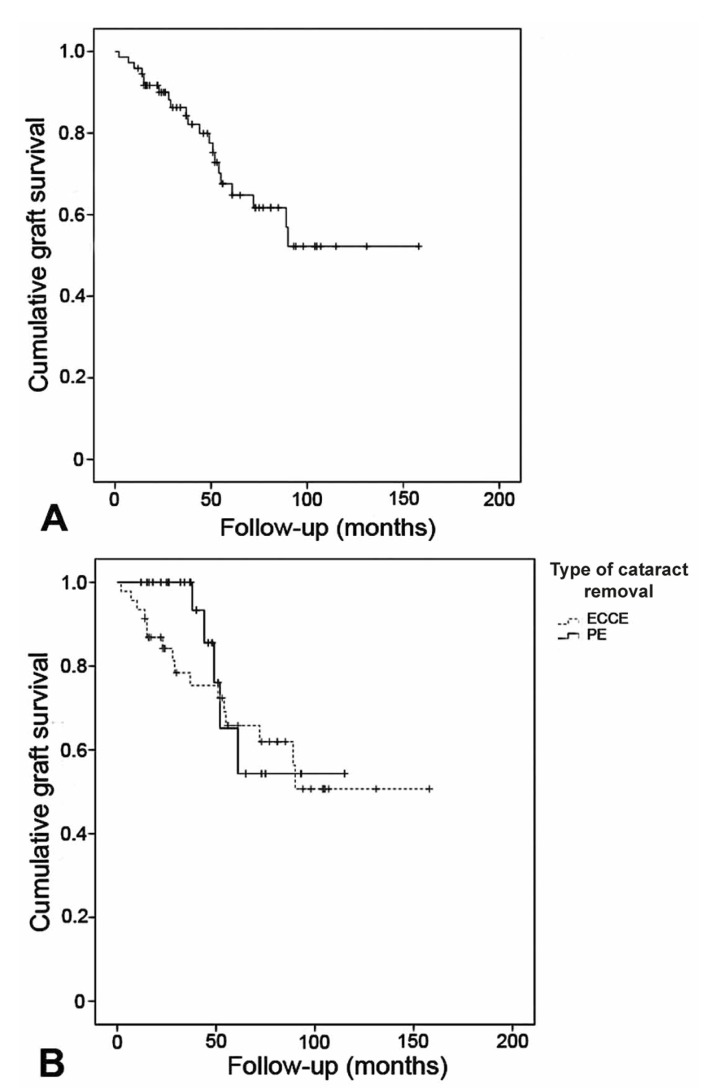
Mean graft survival in the present study was 106.2 (95% CI, 89 to 123) months. Figure 2 demonstrates that cumulative graft survival rate in the whole study population was 61.7% at month 56 (median of follow-up).
Figure 2.
The Kaplan-Meier method demonstrates cumulative graft survival in the whole study population (A), as well as in each group of cataract removal (B). (ECCE, extracapsular cataract extraction; PE, phacoemulsification)
The most prevalent surgical intervention in the current series (11 eyes; 14.5%) was graft refractive surgery, consisting of relaxing incisions with or without counter-quadrant compression sutures for high graft astigmatism, followed by laser capsulotomy in 8 (10.5%) eyes, which was performed at a mean of 57.5±42 months after transplantation. Resuturing was performed in 1 (1.3%) eye due to wound overriding after suture removal.
DISCUSSION
The triple procedure is a well-established surgical option which provides good visual outcomes in patients with coexisting corneal pathologies and cataracts.1-13 In this series, BSCVA significantly improved by 10 lines on the Snellen chart, and 40.8% of eyes gained BSCVA≥20/40. The majority of previous studies have reported comparable outcomes, with BSCVA≥20/40 ranging from 38% to 64% of eyes.1,3-12 Graft survival after the triple procedure has been found to range from 60% to 100% in the literature.2,6,8,11-13
In this series, 89.5% of the grafts remained clear, which can be attributed to including a high percentage of patients with inherently low-risk indications for graft rejection, such as Fuchs’ endothelial dystrophy (FED) and keratoconus. Indications for corneal transplantation have a significant effect on graft survival. Two studies on patients with FED and cataract, reported 96% and 100% graft survival estimates, respectively.11,12
The most important challenge following the triple procedure is the high rate of postoperative refractive error, which stems from inaccuracy in IOL power calculation, adding to problems with high astigmatism commonly observed after corneal transplantation (up to 17.0 D in this series).1-6,14-17 In the current study, a postoperative spherical equivalent within ±2.0 D of target refraction was observed in 35 (46.05%) eyes. This finding is in line with previous studies, reporting 26% to 68% of eyes achieving ±2 D of target refraction, and refractive errors varying from −14.7 to +8.0 D.2-8,10,16,17,21
To accurately predict IOL power, reliable keratometry readings, anterior chamber depth and axial length measurements are essential. In contrast to cataract surgery alone, all of these parameters may alter following the triple procedure, increasing the risk of unanticipated refractive errors.1-6,14-17 The use of preoperative keratometric (K) readings from the affected or fellow eye, multiple regression analysis with surgeon-specific values, individualized A-constants, or fixed values for keratometry are among the different adopted strategies to reduce the risk.3,11,15,22,23Katz and Forster3 noted the lowest postoperative refractive error following the triple procedure to occur with the use of preoperative keratometric readings of the operative eye. The results of the present study, which includes keratoconic eyes, also reveal that in a subgroup of patients with measurable preoperative keratometry, these values were significantly steeper than postoperative readings. Shimmura et al15 employed keratometry of the affected eye, keratometry of the healthy eye (when it was not measurable in the affected eye), or a fixed value of 7.60 mm (when both corneas were unfit for keratometry) and reported spherical equivalent refraction of -2.8±4.2 D with an average absolute error from target refraction of 3.2±3.0 D. K readings from the fellow eye, however, may not provide reliable measurements.3 Serdarevic et al11 hypothesized that a correlation exists between the preoperative dioptric power of peripheral recipient corneas and the postoperative central power of the grafts, and applied videokeratoscopic analysis of peripheral recipient corneas to determine IOL power. The current study indicates that using surgeon-specific postoperative average K readings (44 D) for IOL calculations could be an acceptable method for triple surgery. Meanwhile, both the surgeon and the patient should anticipate unacceptable refractive error, necessitating further refractive surgery.
No significant difference in terms of intraoperative complications was observed between the two subgroups of cataract surgery (ECCE versus PE) in the current study. Moreover, the rate of vitreous loss was 1.3%, in our study, which is lower than that reported by Borderie et al (10.0%).24
Phacoemulsification, performed to remove a cataract through a small incision before trephination; reduces potential intraoperative complications encountered during open-sky surgery namely, shallow anterior chamber, iris prolapse, forward movement of the lens-iris diaphragm resulting in peripheral extension of the capsulorhexis, posterior capsular bulging and tearing, difficulty in cortex removal and IOL insertion, vitreous loss, and expulsive hemorrhage.10 However, this method requires good visualization and is not applicable in corneas that are too cloudy. This study establishes that when adequate measures to control vitreous pressure are performed, open-sky extracapsular cataract surgery could be as safe as phacoemulsification; these preventive approaches may include careful patient positioning, appropriate eyelid speculum selection and positioning, preoperative external ocular massage and administration of hyperosmotic agents to dehydrate and decrease vitreous volume and hence soften the globe, scleral support by suturing a Flieringa ring, and occasionally lateral canthotomy.
By applying multiple linear regression analysis, we observed a significant association between cataract extraction technique and postoperative BSCVA. Several factors may have contributed to this difference as also supported by the results of the current study. Better preoperative visual acuity, indications for corneal transplantation such as keratoconus, and larger graft size have been found to influence visual outcomes after the triple procedure.25
Coexisting ocular pathologies may explain the influence of preoperative BSCVA and indications for corneal transplantation. Besides the cornea, other intraocular structures may have been affected by the condition leading to the corneal pathology. Another influential factor on visual outcome was recipient trephine size. Irregular astigmatism can be lower when a larger trephine is used which explains why a significant association was found between postoperative BSCVA and larger trephine size.25
Apart from the above-mentioned factors, postoperative visual outcomes could be influenced by the position of the IOL. Evaluating the consequence of IOL placement on the clinical results of the triple procedure, Borderie et al24 concluded that in-the-bag IOL placement yields better results in terms of visual acuity as compared to ciliary sulcus implantation. Nevertheless, the results of the current study indicate that IOL position has no effect on the rate of graft rejection reactions, an observation in contradiction to what was reported by Borderie et al.24
In conclusion, the triple procedure using either ECCE or PE is a safe and effective approach to restore visual acuity in patients with both corneal pathology and cataract.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Davis EA, Azar DT, Jakobs FM, Stark WJ. Refractive and keratometric results after the triple procedure: experience with early and late suture removal. Ophthalmology. 1998;105:624–630. doi: 10.1016/S0161-6420(98)94015-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee JR, Dohlman CH. Intraocular lens implantation in combination with keratoplasty. Ann Ophthalmol. 1977;9:513–518. [PubMed] [Google Scholar]
- 3.Katz HR, Forster RK. Intraocular lens calculation in combined penetrating keratoplasty, cataract extraction and intraocular lens implantation. Ophthalmology. 1985;92:1203–1207. doi: 10.1016/s0161-6420(85)33884-8. [DOI] [PubMed] [Google Scholar]
- 4.Binder PS. Intraocular lens powers used in the triple procedure. Effect on visual acuity and refractive error. Ophthalmology. 1985;92:1561–1566. doi: 10.1016/s0161-6420(85)33823-x. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DM, Stern AL, McDonald P. The triple procedure: 2 to 10 year follow-up. Trans Am Ophthalmol Soc. 1986;84:221–249. [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford GJ, Stulting RD, Waring GO, Van Meter WS, Wilson LA. The triple procedure. Analysis of outcome, refraction, and intraocular lens power calculation. Ophthalmology. 1986;93:817–824. doi: 10.1016/s0161-6420(86)33673-x. [DOI] [PubMed] [Google Scholar]
- 7.Busin M, Arffa RC, McDonald MB, Kaufman HE. Combined penetrating keratoplasty, extracapsular cataract extraction, and posterior chamber intraocular lens implantation. Ophthalmic Surg. 1987;18:272–275. [PubMed] [Google Scholar]
- 8.Meyer RF, Musch DC. Assessment of success and complications of triple procedure surgery. Am J Ophthalmol. 1987;104:233–240. doi: 10.1016/0002-9394(87)90410-7. [DOI] [PubMed] [Google Scholar]
- 9.Musch DC, Meyer RF. Prospective evaluation of a regression-determined formula for use in triple procedure surgery. Ophthalmology. 1988;95:79–85. doi: 10.1016/s0161-6420(88)33224-0. [DOI] [PubMed] [Google Scholar]
- 10.Baca LS, Epstein RJ. Closed-chamber capsulorrhexis for cataract extraction combined with penetrating keratoplasty. J Cataract Refract Surg. 1998;24:581–584. doi: 10.1016/s0886-3350(98)80249-6. [DOI] [PubMed] [Google Scholar]
- 11.Serdarevic ON, Renard GJ, Pouliquen Y. Videokeratoscopy of recipient peripheral corneas in combined penetrating keratoplasty, cataract extraction, and lens implantation. Am J Ophthalmol. 1996;122:29–37. doi: 10.1016/s0002-9394(14)71961-0. [DOI] [PubMed] [Google Scholar]
- 12.Pineros OE, Cohen EJ, Rapuano CJ, Laibson PR. Triple vs nonsimultaneous procedures in Fuchs’ dystrophy and cataract. Arch Ophthalmol. 1996;114:525–528. doi: 10.1001/archopht.1996.01100130517002. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DM, Khaliq A, Maxwell R. Keratoplasty and intraocular lenses: current status. Ophthalmology. 1979;86:242–255. doi: 10.1016/s0161-6420(79)35523-3. [DOI] [PubMed] [Google Scholar]
- 14.Claoué C, Ficker L, Kirkness C, Steele A. Refractive results after corneal triple procedures (PK+ECCE+IOL). Eye (Lond) 1993;7:446–451. doi: 10.1038/eye.1993.90. [DOI] [PubMed] [Google Scholar]
- 15.Shimmura S, Ohashi Y, Shiroma H, Shimazaki J, Tsubota K. Corneal opacity and cataract: triple procedure versus secondary approach. Cornea. 2003;22:234–238. doi: 10.1097/00003226-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Viestenz A, Seitz B, Langenbucher A. Intraocular lens power prediction for triple procedures in Fuchs’ dystrophy using multiple regression analysis. Acta phthalmol Scand. 2005;83:312–315. doi: 10.1111/j.1600-0420.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 17.Binder PS. The triple procedure. Refractive results. 1985 update. Ophthalmology. 1986;93:1482–1488. doi: 10.1016/s0161-6420(86)33533-4. [DOI] [PubMed] [Google Scholar]
- 18.Filatov V, Alexandrakis G, Talamo JH, Steinert RF. Comparison of suture-in and suture-out postkeratoplasty astigmatism with single running suture or combined running and interrupted sutures. Am J Ophthalmol. 1996;122:696–700. doi: 10.1016/s0002-9394(14)70489-1. [DOI] [PubMed] [Google Scholar]
- 19.Mader TH, Yuan R, Lynn MJ, Stulting RD, Wilson A, Waring GO. Changes in keratometric astigmatism after suture removal more than one year after penetrating keratoplasty. Ophthalmology. 1993;100:119–126. doi: 10.1016/s0161-6420(93)31705-7. [DOI] [PubMed] [Google Scholar]
- 20.Burdon MA, McDonnell P. A survey of corneal graft practice in the United Kingdom. Eye (Lond) 1995;9:6–12. [PubMed] [Google Scholar]
- 21.Pradera I, Ibrahim O, Waring GO. Refractive results of successful penetrating keratoplasty, intraocular lens implantation with selective suture removal. Refract Corneal Surg. 1989;5:231–239. [PubMed] [Google Scholar]
- 22.Mattax JB, McCulley JP. The effect of standardized keratoplasty technique on IOL power calculation for the triple procedure. Acta Ophthalmol Suppl. 1989;192:24–29. doi: 10.1111/j.1755-3768.1989.tb07091.x. [DOI] [PubMed] [Google Scholar]
- 23.Flowers CW, McLeod SD, McDonnell PJ, Irvine JA, Smith RE. Evaluation of intraocular lens power calculation formulas in the triple procedure. J Cataract Refract Surg. 1996;22:116–122. doi: 10.1016/s0886-3350(96)80280-x. [DOI] [PubMed] [Google Scholar]
- 24.Borderie VM, Touzeau O, Bourcier T, Carvajal-Gonzalez S, Laroche L. The triple procedure: in the bag placement versus ciliary sulcus placement of the intraocular lens. Br J Ophthalmol. 1999;83:458–462. doi: 10.1136/bjo.83.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas JB, Rank RM, Budde WM, Sauder G. Factors influencing visual outcome after penetrating keratoplasty combined with intraocular lens implantation. Eur J Ophthalmol. 2003;13:134–138. doi: 10.1177/112067210301300203. [DOI] [PubMed] [Google Scholar]