Abstract
Warfarin-associated intracerebral hemorrhage (W-ICH) is a severe type of stroke. There is no consensus on the optimal treatment for W-ICH. Using a mouse model, we tested whether the rapid reversal of anticoagulation using human prothrombin complex concentrate (PCC) can reduce hemorrhagic blood volume. Male CD-1 mice were treated with warfarin (2 mg/kg over 24 h), resulting in a mean (±s.d.) International Normalized Ratio of 3.5±0.9. First, we showed that an intravenous administration of human PCC rapidly reversed anticoagulation in mice. Second, a stereotactic injection of collagenase was administered to induce hemorrhage in the right striatum. Forty-five minutes later, the animals were randomly treated with PCC (100 U/kg) or saline IV (n = 12 per group). Twenty-four hours after hemorrhage induction, hemorrhagic blood volume was quantified using a photometric hemoglobin assay. The mean hemorrhagic blood volume was reduced in PCC-treated animals (6.5±3.1 μL) compared with saline controls (15.3±11.2 μL, P = 0.015). In the saline group, 45% of the mice developed large hematomas (i.e., > 15 μL). In contrast, such extensive lesions were never found in the PCC group. We provide experimental data suggesting PCC to be an effective acute treatment for W-ICH in terms of reducing hemorrhagic blood volume. Future studies are needed to assess the therapeutic potential emerging from our finding for human W-ICH.
Keywords: anticoagulation, intracerebral hemorrhage, mouse model, warfarin
Introduction
Warfarin-associated intracerebral hemorrhage (W-ICH) is a particularly deadly form of stroke with a short-term mortality rate of > 50% (Aguilar et al, 2007; Rosand et al, 2004; Sjoblom et al, 2001). Along with current demographic trends, the burden of the disease is likely to grow within the next decade (Go, 2005; Go et al, 2001). As randomized trials are not available, treatment guidelines for W-ICH are mostly based on expert opinions and on results from small clinical case series (Aguilar et al, 2007; Steiner et al, 2006). The paramount aim of acute W-ICH therapy is to achieve a rapid reversal of anticoagulation to prevent the ongoing hematoma expansion (Aguilar et al, 2007; Foerch et al, 2008). Potential treatment options include vitamin K, fresh frozen plasma, coagulation factor VII concentrate, and prothrombin complex concentrate (PCC) (Aguilar et al, 2007; Steiner et al, 2006). Plasma-derived PCC contains concentrated amounts of vitamin K-dependent coagulation factors II, VII, IX, and X. The liver synthesis of active forms of these factors is diminished during warfarin therapy (Ostermann et al, 2007). Prothrombin complex concentrate can be used for rapidly reversing warfarin anticoagulation in humans (Lorenz et al, 2007; Pabinger et al, 2008; Preston et al, 2002).
We have recently developed a mouse model of W-ICH. In this model, anticoagulation within the therapeutic range used in humans led to a 2.5-fold increase in hemorrhagic blood volume in the brain 24 h after hemorrhage induction, and to a correspondingly worsened neurologic outcome compared with that in non-anticoagulated mice (Foerch et al, 2008). In this explorative study, we used this mouse model to ask whether PCC can reduce hemorrhagic blood volume in W-ICH.
Materials and methods
Animals
All experiments were carried out after an institutionally approved protocol in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. For the entire study, male CD-1 mice aged 12 to 16 weeks were used.
Warfarin Administration
Following a recently established protocol, we administered warfarin by oral uptake through bottled drinking water. In brief, a 5-mg Coumadin Tablet (crystalline warfarin sodium, Bristol Myers Squibb, New York City, NY, USA) was dissolved in 375-mL of tap water. Assuming a body weight of 40 g and water consumption of 15 mL/100 g per 24 h, this dosage corresponds to a warfarin uptake of 0.08 mg (2 mg/kg) per mouse per day. After 24 h of such warfarin treatment, mean (±s.d.) International Normalized Ratio (INR) values were shown to increase to 3.5±0.9 (Foerch et al, 2008). Although different from the modus of application used in humans, the uptake through drinking water is known to result in more flattened INR kinetics with stable values for at least 6 h after warfarin withdrawal (Foerch et al, 2008).
Acute Reversal of Anticoagulation
In our first experimental series, we investigated whether human PCC (Beriplex, CSL Behring, Germany) can be used to acutely reverse warfarin anticoagulation in mice. Beriplex contains concentrated amounts of human coagulation factors II, VII, IX, and X, as well as the anticoagulant proteins C and S. Detailed data on factor concentrations and pharmacokinetics of this preparation can be found elsewhere (Ostermann et al, 2007).
The protocol was as follows: On day 1, the mice were placed on a heated pad under isoflurane anesthesia (1.5% to 2%) with spontaneous respiration in a nitrous oxide–oxygen mixture. The right jugular vein was surgically prepared without opening the vessel. After closing the skin, the mice were returned to their cages. On day 2, a 24-h period of warfarin feeding was started as described above. On day 3, the right jugular vein was reexposed under anesthesia by bluntly reopening the suture. Using a 30-G needle, PCC (100 U/kg, n = 6) or an equal amount of saline (i.e., 0.16 mL, n = 4) was injected. After removing the needle, the vessel was ligated, the skin closed, and the animals were returned to their cages. Fifteen minutes later, the mice were deeply anesthetized, and a peritoneal midline incision was made. Using a 1-mL syringe and a 25-G needle, 0.6-mL of blood was drawn from the inferior caval vein. Blood was transferred to glass tubes (BD vacutainers, Becton Dickinson, Franklin Lakes, NJ, USA) containing 66.6 μL of 3.2% citrate and was gently mixed immediately. Specimens were centrifuged promptly to obtain platelet-poor plasma. Measurements of INR values and prothrombin time were performed on an MDA coagulation analyzer (Trinity Biotech, Berkeley Heights, NJ, USA) in the Hematology Laboratory of the Massachusetts General Hospital, using Simplastin HTF (Trinity Biotech) as the reagent. Reference values for prothrombin time in the CD-1 mice have been published elsewhere (Lemini et al, 2007). Factor activities of coagulation factors II, VII, IX, and X were determined using human factor-deficient plasmas (Precision Biologic, Dartmouth, Nova Scotia, Canada) with an activated partial thromboplastin time reagent (Platelin L, Trinity Biotech) for factor IX, and Simplastin HTF for the other factors on an MDA coagulation analyzer in the Coagulation Laboratory at the Massachusetts General Hospital (Vainieri and Wingard, 1977).
Determining the Time Point for Prothrombin Complex Concentrate Administration
In our second experimental series, we tried to define an appropriate time point of treatment administration, i.e., to assure that PCC or saline, respectively, was administered after the hemorrhage had started to occur in the brain.
Mice with a normal status of coagulation (n = 5), as well as those treated with warfarin for 24 h (n = 5) underwent hemorrhage induction according to the following protocol:(Clark et al, 1998) Under anesthesia, a small borehole was drilled, and a 32-gauge 0.5-μL microinjection needle (Hamilton, 7000 series, Hamilton, Reno, NV, USA) was slowly lowered into the right striatum at the following stereotactic coordinates from the bregma: 0.0 mm anterior, 2.0 mm lateral, and 3.5 mm depth. Over a period of 5 mins, 0.5 μL of saline containing 0.075 U of collagenase VII-S (Sigma-Aldrich, St Louis, MO, USA) was injected. The needle was left in place for 10 mins, and then slowly removed over a period of 5 mins. Thereafter, the borehole was sealed with bone wax and the scalp closed. Finally, the mice were allowed to wake up (i.e., ~22 mins after hemorrhage induction) and were returned to their cages. A heat lamp was used to maintain animal body temperatures during the surgical procedure.
Forty-five minutes after hemorrhage induction, the mice were killed under deep anesthesia. Their brains were removed and cut in slices using a 1-mm matrix to visually identify the location and size of the intracerebral hemorrhage (ICH). The maximum diameter of hematoma in the center area was determined on photographs using the ImageJ software (http://www.nih.gov).
Prothrombin Complex Concentrate Study
The PCC study was designed as a randomized and blinded trial to assess the efficacy of PCC versus saline in reducing hemorrhagic blood volume.
Rather than administering PCC peripherally through the tail vein in an unsure manner, we decided to use the right jugular vein as the site of injection. The surgical protocol was as follows: On day 1, the right jugular vein was surgically exposed without opening the vessel. On day 2, a 24-h period of warfarin feeding was started. On day 3, before the induction of ICH, the jugular vein was bluntly reexposed by opening the suture. After ligating the distal part of the vessel, a PE-10 tube was inserted into the jugular vein and moved forward in the proximal direction. Immediately after properly fixing the tube at the skin and closing the suture, the animals underwent the procedure of hemorrhage induction (as described above). Forty-five minutes after hemorrhage induction, PCC (100 U/kg) or an equal amount of saline (i.e., 0.16 mL) was injected through the catheter. The correct intravenous position of the catheter was double-checked by drawing some blood into the tube after the injection. The catheter was then ligated and cut near the skin.
Outcome Assessment
Twenty-four hours after hemorrhage induction, neurologic deficits were rated on a simple 5-point scale (0 = no apparent deficit; 1 = slight deficit, e.g., extension deficit of the right forepaw or slight instability during walking, but no circling; 2 = circling to the right with at least some straight movements and some covering of distance; 3 = heavy circling to the right without straight movements or no movements at all; 4 = death) (Arumugam et al, 2006; Foerch et al, 2008).
Measuring Hemorrhagic Blood Volume
Directly after the outcome assessment, the mice underwent transcardial perfusion with 30 mL of phosphate buffered saline under deep anesthesia. Their brains were removed, separated into left and right hemispheres, and placed in glass tubes containing 3 mL of phosphate buffered saline. After 30 secs of homogenization, ultrasound was applied for 1 min to lyse erythrocytic cell membranes. After centrifugation for 30 mins (13,000 r.p.m, 4°C), 250 μL of supernatant was added to 1,000 μL of Drabkin's reagent. Using a photometer, absorption rates were determined at 540 nm, and hemorrhagic blood volumes were calculated for the entire brain (both the hemispheres) using a standard curve. The mice that were found dead in their cages 24 h after hemorrhage induction were not eligible for transcardial perfusion before measurements. In these cases, we subtracted 2.1 μL from the total hemorrhagic blood volume that was determined for these brains. This value was the mean difference in hemorrhagic blood volume between three unperfused and three perfused brain samples (data not shown).
Statistical Analysis
We used SPSS15.0 for statistical analysis. Hemorrhagic blood volume was compared between groups using the t-test. The activity of coagulation factor before and after anticoagulation, and after PCC treatment was compared using the Mann–Whitney U-test. Statistical analysis of categorical data was performed using the χ2-test.
Results
Acute Reversal of Anticoagulation
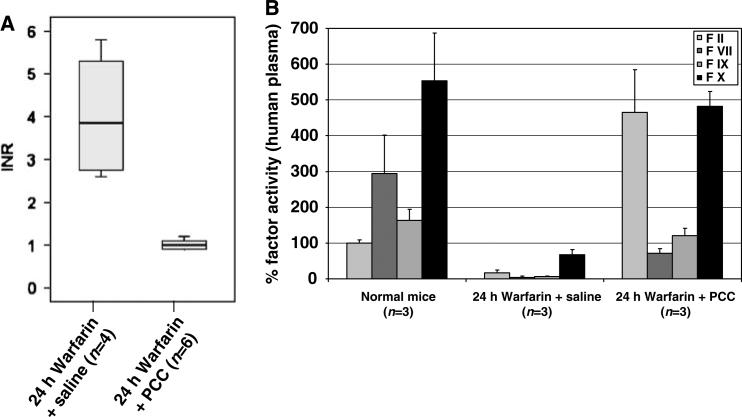
In our first experimental series, we determined whether human PCC can be used to acutely reverse warfarin anticoagulation in mice. As expected, warfarin-anticoagulated mice receiving a control amount of saline had elevated INR values at 15 mins after the injection (mean±s.d.: 4.0±1.5, range: 2.6 to 5.8, n = 4). In contrast, the intravenous administration of 100 U/kg PCC in anticoagulated mice resulted in normalized INR values at 15 mins after the injection (mean±s.d.: 1.0±0.1, range: 0.9–1.2, n = 6; Figure 1A). Warfarin treatment led to a significant decrease in the plasma activity of all four vitamin K-dependent coagulation factors (i.e., factors II, VII, IX, and X; for all comparisons, P≤0.05), indicating that our model properly reflects the status of a full warfarin anticoagulation. However, treating anticoagulated mice with human PCC significantly elevated the plasma activity of these coagulation factors (for all comparisons, P≤0.05, Figure 1B).
Figure 1.
(A) Mice were treated with warfarin (2 mg/kg over 24 h). Fifteen minutes after an intravenous administration of saline or PCC (100 U/kg body weight), respectively, INR values were measured. The INR values were normalized by human PCC (P = 0.001). The box indicates the 25th, 50th, and 75th percentile of the distribution, and the whiskers the 10th and 90th percentile, respectively. (B) The graph displays mice coagulation factor activity (FII, FVII, FIX, FX; measured with human factor deficient plasma) in normal mice, anticoagulated mice, and in anticoagulated mice that received PCC treatment. Given are mean values and s.d.. For all factors, both the decrease during warfarin treatment and the increase after PCC therapy were significant (for all comparisons, P≤0.05).
Determining the Time Point for Prothrombin Complex Concentrate Administration
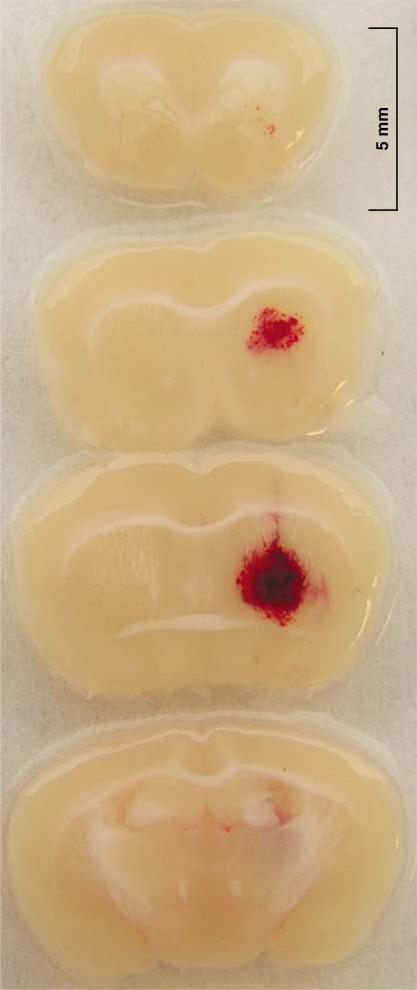
In the second series of experiments, we assessed the state of ICH in the collagenase model at the time of our planned PCC therapy. Forty-five minutes after hemorrhage induction, a striatal hematoma was visible in all the investigated mice (n = 10). Figure 2 shows a representative brain sample with a striatal hematoma. The mean (±s.d.) diameter of hematomas in the central brain sections was 2.5±0.6 mm (range: 1.1 to 3.1). This shows that collagenase-induced ICH has been reliably initiated at this time point, thus allowing us to ask whether PCC can reduce further hematoma expansion in this model.
Figure 2.
Representative sections of a striatal hematoma obtained 45 mins after hemorrhage induction by collagenase injection into the right striatum. Well-defined hematomas were found in all mice.
Prothrombin Complex Concentrate Study
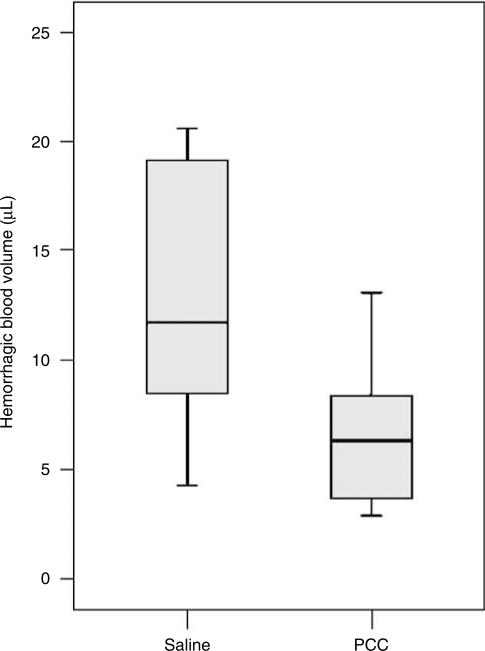
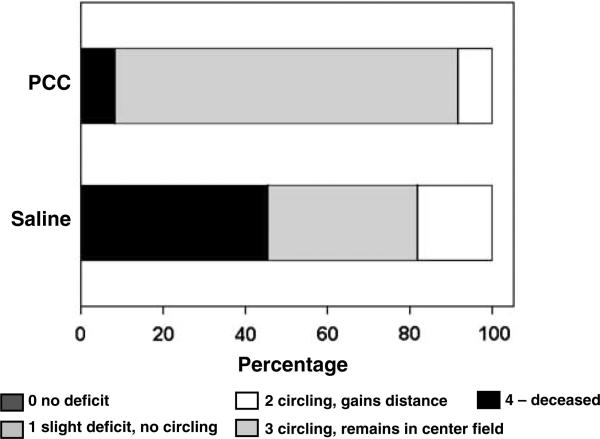
In the third series of experiments, we examined the effects of PCC therapy on hemorrhagic blood volume and—as secondary outcome measures—on survival and neurologic outcomes. Anticoagulated mice were randomly assigned to either saline or PCC treatment 45 mins after hemorrhage induction (n = 12 per group). One animal in the saline group had to be excluded, because of a catheter-associated rupture of its jugular vein. Prothrombin complex concentrate therapy decreased hemorrhagic blood volumes (mean±s.d.: 6.5±3.1 μL, range: 2.9 to 13.1) compared with saline-treated controls (15.3±11.2 μL, range: 4.3 to 44.3, P = 0.015; Figure 3). The survival rate at 24 h after hemorrhage induction was higher in PCC-treated animals (92%, i.e., 11 out of 12) versus saline controls (55%, i.e., 6 out of 11; P = 0.043, Figure 4). All surviving animals showed a moderate-to-severe functional deficit 24 h after hemorrhage induction (i.e., circling behavior, outcome scale: 2 to 3). When focusing on surviving mice only, there was no difference in the neurologic outcome between PCC-treated animals and controls. However, in the surviving mice, hemorrhagic blood volume showed a strong trend of being lower in PCC-treated mice (6.4±3.3 μL) compared with that in saline-treated controls (9.9±4.0 μL; P = 0.070).
Figure 3.
Hemorrhagic blood volume in PCC- and saline-treated animals determined 24 h after hemorrhage induction. PCC therapy 45 mins after hemorrhage induction significantly decreased blood volumes (P = 0.015). The box and whisker graphs are explained in the legend of Figure 1.
Figure 4.
Mortality and neurologic outcome 24 h after hemorrhage induction as derived from the PCC trial. Animals were rated on a simple 5-point scale (see Materials and methods). The mortality rate was significantly lower in the PCC group (P = 0.043), but the neurologic outcome in surviving animals was not different between groups.
Discussion
Our study provides experimental data suggesting that PCC may be an effective treatment for W-ICH in terms of reducing hemorrhagic blood volume.
It has been shown that PCC rapidly reverses oral anticoagulation with coumarines in humans (Lorenz et al, 2007; Pabinger et al, 2008; Preston et al, 2002). We investigated whether human PCC can be used in the experimental setting to rapidly reverse warfarin anticoagulation in mice. In our earlier study, we identified a 24-h warfarin-feeding period through bottled water to result in mean INR values of 3.5±0.9, thus properly reflecting the therapeutic range of anticoagulation used in humans (Foerch et al, 2008). This study further characterizes this model by adding information on the plasma activity of the vitamin K-dependent coagulation factors. After a 24-h period of warfarin feeding, all four factors were reduced to low levels, indicating a status of full anticoagulation. As a limitation, measurements of factor activities were performed using human (and not mouse)-deficient plasma. Thus, we are restricted to analyze the relative changes in factor activities between anticoagulated and non-anticoagulated mice (Vainieri and Wingard, 1977). Our results indicate that an intravenous administration of human PCC (100 U/kg body weight) in anticoagulated mice leads to a significant increase in factor activity for all the four vitamin K-dependent factors 15 mins after treatment. We were not able to identify other publications implementing human PCC in a mouse model. However, one study showed that an intravenous human PCC application (50 U/kg) reverses anticoagulation in phenprocoumon-treated rats (Dickneite, 2007). It is known that the molecular components of the blood coagulation cascade are highly preserved during mammalian evolution, thus explaining why human factors may constitute a functional substitute for the warfarin-dependent depletion of autologous factors in mice (Davidson et al, 2003). Relatedly, human patients with severe factor VIII deficiency due to antifactor VIII auto-antibodies have been successfully treated with factor VIII from pigs.
We tried to define an appropriate time point of PCC administration in our W-ICH model. To closely mirror the human condition, we aimed to administer PCC after collagenase-induced ICH had started to occur in the brain. Earlier publications have shown striatal hematomas to occur early (i.e., within 1 h) after collagenase application (Del Bigio et al, 1996). Indeed, our study confirmed these findings. Forty-five minutes after hemorrhage induction, a striatal hematoma was clearly visible in all the tested animals (i.e., in five anticoagulated and five non-anticoagulated mice). Thus, our W-ICH model appropriately reflects the condition of hematoma formation under an impaired status of coagulation followed by PCC administration during ongoing bleeding.
We then performed a randomized and blinded trial to assess the efficacy of PCC versus saline in reducing hemorrhagic blood volume. We showed earlier that an additional treatment with vitamin K was not necessary because of the rapid normalization of the coagulation status in mice after warfarin depletion (Foerch et al, 2008). As compared with that in the saline group, the mean hemorrhagic blood volume was significantly reduced in PCC-treated animals (by 58%). Although a high proportion of mice in the saline group (45%) developed large and very large hematomas (i.e., > 15 μL), these extensive lesions were never found in the PCC group. The considerable reduction in hemorrhagic blood volume in the PCC group was accompanied by a significantly increased survival rate in comparison to saline-treated animals (92% versus 55%). However, within the limitations of our mouse testing methods and the short follow-up period, we were unable to detect any significant differences in the neurologic outcome between the two groups when analyzing surviving animals only, despite a strong but nonsignificant trend toward reduced hemorrhagic blood volumes in the PCC group.
In the experimental setting, we have shown earlier that anticoagulation within the therapeutic range used in humans led to a 2.5-fold increase in hemorrhagic blood volume 24 h after hemorrhage induction (Foerch et al, 2008). Furthermore, it was shown that hematoma expansion was still ongoing even at 2 h after hemorrhage induction in these anticoagulated mice. Our study suggests that a rapid reversal of anticoagulation using PCC does prevent excessive hematoma formation, most likely by reducing the intensity of bleeding and by terminating the otherwise prolonged process of hematoma expansion. In the future, our model of W-ICH may be used to directly compare the therapeutic efficacy of PCC, coagulation factor VII, and fresh frozen plasma in reducing hemorrhagic blood volume and in improving functional outcome (although the application of fresh frozen plasma may be limited in the mouse model because of the high amount of volume needed to restore coagulation). In addition, our study may be reperformed in mice having higher INR values, which may be obtained from increasing the period of warfarin feeding.
In human W-ICH, a higher rate of hematoma enlargement after a certain time point was reported, which is likely to be a major contributor to excessive hematoma formation and worse functional outcome (Flibotte et al, 2004; Rosand et al, 2004; Yasaka et al, 2003). (Huttner et al, 2006) emphasized a rapid normalization of coagulation status to be of utmost importance in preventing hematoma enlargement rather than a specific form of treatment. To date, there are only retrospective studies available comparing different treatment options for human W-ICH, including vitamin K, fresh frozen plasma, coagulation factor VII concentrate, and PCC (Fredriksson et al, 1992; Huttner et al, 2006; Sjoblom et al, 2001). They failed to identify any treatment form being superior to another. However, it has been shown that PCC can be used for rapidly reversing oral anticoagulation in humans (Lorenz et al, 2007; Pabinger et al, 2008; Preston et al, 2002), and PCC is known to reverse anticoagulation faster than fresh frozen plasma (Fredriksson et al, 1992; Makris et al, 1997). Randomized controlled trials may clarify whether PCC therapy in human W-ICH is safe and leads to both a reduced hematoma volume and a better functional outcome (Kohler et al, 1998; Steiner et al, 2006). In spontaneous ICH in non-anticoagulated individuals, the efficacy of administration of coagulation factor VII was recently tested in randomized multicenter trials. Despite a reduced hematoma size in the treatment group, a positive effect on mortality and severe disability on day 90 was not apparent (Mayer et al, 2005). However in W-ICH, the time window for hemostatic treatment may be considerably longer due to prolonged hematoma expansion.
Our study has shortcomings, such as: (1) Using a collagenase model (and not a blood injection model) seems to be indispensable for meeting the requirements of an experimental study of W-ICH. However, we have to keep in mind that collagenase may not accurately reproduce the local arteriolar rupture that is typical for deep ICHs in humans. Rather unselectively, collagenase dissolves the extracellular matrix in different types of blood vessels. We do not exactly know what coagulation mechanisms take place during collagenase-induced ICH formation. On the basis of our earlier study (Foerch et al, 2008) and these results, it seems justified to assume that the plasmatic coagulation cascade (including factors II, VII, IX, and X) is of relevance in this scenario, but we have to emphasize that the efficacy of PCC therapy in reducing hemorrhagic blood volume may not necessarily be the same in anticoagulated animals or humans. (2) We used the right jugular vein to reliably administer PCC therapy into a central vein. However, for this procedure, the vessel was permanently ligated, which may have increased the venous pressure in the brain and may have worsened the vasogenic cerebral edema and functional outcome. (3) Administering PCC in mice may result in abnormally high amounts of coagulation factors (e.g., in our model, factor II). Subsequently, this may cause thrombosis and embolism, including cerebral ischemic events, which may have a negative effect on survival and functional outcome per se. Long-term investigations are needed to evaluate these parameters.
In summary, our study provides experimental data that identifies the rapid reversal of anticoagulation using PCC to reduce hemorrhagic blood volume in W-ICH. Prothrombin complex concentrate treatment seems to be particularly capable in reducing the number of large and very large hematomas. Further studies are needed to clarify whether PCC is of a similar benefit in human W-ICH.
Acknowledgments
Christian Foerch received the medication used in this study (the Prothrombin Complex Concentrate ‘Beriplex’) as a donation from CSL Behring, Marburg, Germany. This study was in part funded by the ‘Deutsche Forschungsgemeinschaft’ (DFG) and by NIH-grants R37-NS37074, R01-NS48422, R01-NS56458, R01-NS3560 and P01-NS55104.
Footnotes
All the other authors declare no conflicts of interest.
References
- Aguilar MI, Hart RG, Kase CS, Freeman WD, Hoeben BJ, Garcia RC, Ansell JE, Mayer SA, Norrving B, Rosand J, Steiner T, Wijdicks EF, Yamaguchi T, Yasaka M. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–3. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–40. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- Davidson CJ, Tuddenham EG, McVey JH. 450 million years of hemostasis. J Thromb Haemost. 2003;1:1487–94. doi: 10.1046/j.1538-7836.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Yan HJ, Buist R, Peeling J. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke. 1996;27:2312–9. doi: 10.1161/01.str.27.12.2312. discussion 2319–2320. [DOI] [PubMed] [Google Scholar]
- Dickneite G. Prothrombin complex concentrate versus recombinant factor VIIa for reversal of coumarin anticoagulation. Thromb Res. 2007;119:643–51. doi: 10.1016/j.thromres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Flibotte JJ, Hagan N, O'Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–64. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, Lo EH. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson K, Norrving B, Stromblad LG. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke. 1992;23:972–7. doi: 10.1161/01.str.23.7.972. [DOI] [PubMed] [Google Scholar]
- Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61. doi: 10.1111/j.1076-7460.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Huttner HB, Schellinger PD, Hartmann M, Kohrmann M, Juettler E, Wikner J, Mueller S, Meyding-Lamade U, Strobl R, Mansmann U, Schwab S, Steiner T. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hellstern P, Lechler E, Uberfuhr P, Muller-Berghaus G. Thromboembolic complications associated with the use of prothrombin complex and factor IX concentrates. Thromb Haemost. 1998;80:399–402. [PubMed] [Google Scholar]
- Lemini C, Jaimez R, Franco Y. Gender and interspecies influence on coagulation tests of rats and mice. Thromb Res. 2007;120:415–9. doi: 10.1016/j.thromres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Lorenz R, Kienast J, Otto U, Kiehl M, Schreiter D, Haertel S, Barthels M. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565–70. doi: 10.1097/MBC.0b013e3282010d7a. [DOI] [PubMed] [Google Scholar]
- Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–80. [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98:790–7. [PubMed] [Google Scholar]
- Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H. Prothrombin complex concentrate (Beriplex((R)) P/N) for emergency anticoagulation reversal. J Thromb Haemost. 2008;6:622–31. doi: 10.1111/j.1538-7836.2008.02904.x. [DOI] [PubMed] [Google Scholar]
- Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116:619–24. doi: 10.1046/j.0007-1048.2001.03295.x. [DOI] [PubMed] [Google Scholar]
- Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- Sjoblom L, Hardemark HG, Lindgren A, Norrving B, Fahlen M, Samuelsson M, Stigendal L, Stockelberg D, Taghavi A, Wallrup L, Wallvik J. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567–74. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–62. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- Vainieri H, Wingard LB., Jr Effect of warfarin on the kinetics of the vitamin K-dependent clotting factors in rats. J Pharmacol Exp Ther. 1977;201:507–17. [PubMed] [Google Scholar]
- Yasaka M, Minematsu K, Naritomi H, Sakata T, Yamaguchi T. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89:278–83. [PubMed] [Google Scholar]