Abstract
Recent studies have found that baseline inflammatory status affected the response of the lipid profile to diet intervention. The goal of this study was to determine whether baseline inflammatory status, as reflected in C-reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) affected the lipid and insulin response to a weight loss intervention. A second goal was to determine whether inflammatory markers were related to traditional metabolic risk factors, such as lipids and insulin, in our sample of 190 overweight [body mass index (BMI) 27–30 kg/m2], pre-menopausal women. Body composition, fat distribution, serum lipids, insulin sensitivity (Si), and markers of inflammation were assessed at baseline, and after weight loss to BMI < 25 kg/m2. All measurements were taken after a 4-week period of weight maintenance. Mixed-model, repeated-measures analysis was used to determine whether the interaction of baseline inflammatory status and time was significant in determining the changes in metabolic risk factors (Si and lipids) with weight loss. Weight loss was associated with significant reductions in total cholesterol, lowdensity lipoprotein cholesterol (LDL-C), triglycerides, and insulin, and increases in high-density lipoprotein cholesterol (HDL-C) and Si. Triglycerides were higher (P = 0.054), and insulin sensitivity lower (P = 0.057), with increasing C-reactive protein tertile. The interaction of baseline inflammatory status and time was not significant for any outcome variable of interest. These results do not support the hypothesis that baseline inflammatory status affects the lipid and insulin response to a weight loss intervention. However, in these young, healthy women, weight loss had a beneficial impact on both inflammatory status and risk factors for chronic metabolic disease.
Keywords: C-reactive protein, inflammation, obesity, weight loss, insulin sensitivity, triglycerides
INTRODUCTION
Chronic inflammation is increasingly being recognized as a risk for cardiovascular disease (CVD) (1–3) and type 2 diabetes (4–6). Particularly, proinflammatory cytokines have been reported to induce the production of interleukin-6 (IL-6) which in turn stimulates the production of C-reactive protein (CRP) from the liver. High circulating levels of CRP would then increase the concentration of circulating cell adhesion molecules and tissue factors as well as mediate the uptake, by macrophages, of low-density lipoprotein cholesterol (LDL-C) (1) leading to atherosclerotic plaque. CRP can thus be considered a major risk factor in the progression of CVD and has been recognized as an emerging risk factor by the National Cholesterol Education Program Adult Treatment Panel III (7).
However, markers of inflammation also may more broadly reflect metabolic status. In diet intervention studies, baseline CRP concentration was associated with the nature and degree of diet-induced changes in triglycerides (TG) (8). For example, men with low CRP concentrations at baseline had a reduction in fasting TG whereas those with high baseline CRP had an increase in TG with a low-fat diet. With a high monounsaturated fat diet, men with high CRP had greater reductions in total cholesterol (TC) and low-density lipoprotein cholesterol (LDLC) than those with low baseline CRP. Similar interactive relationships between CRP and diet on lipids have been reported (9–11).
Previous studies have thus shown that an individual’s lipid response to dietary treatment may be dependent on his/her CRP levels at diet onset. We therefore designed this study to determine if the lipid and insulin responses to a weight loss intervention would differ depending on baseline inflammatory status. The objective of this study was to examine whether baseline inflammatory status would modulate the effects of a weight loss intervention on CVD risk factors in overweight, pre-menopausal women. We hypothesized that subjects with high baseline levels of inflammatory markers would have greater reductions in lipid levels and a greater improvement in insulin sensitivity than subjects with low baseline levels of inflammatory markers. A second objective of this study was to examine the association between inflammatory markers and traditional metabolic risk factors, such as lipids and insulin, in overweight women. We hypothesized that women who had higher insulin and lipid levels would also have higher levels of inflammatory markers.
SUBJECTS & METHODS
Subjects included 213 Black and White pre-menopausal women between the ages of 20 and 41 y recruited for a weight loss intervention study designed to examine the long term effects of exercise on weight loss and body composition. Subjects were recruited at a body mass index (BMI, in kg/m2) of 27–30 and had a family history of overweight (BMI >27) in at least one first-degree relative. Classification of Black or White included subjects’ report that both parents and grandparents were of that race. Normal glucose tolerance was documented by an oral glucose tolerance test. Subjects were non-smokers and were not taking medications known to affect energy expenditure, fuel utilization, insulin concentration, heart rate or thyroid status. The study was approved by The University of Alabama at Birmingham (UAB) Institutional Review Board.
Prior to testing, subjects underwent a 4-week outpatient energy balance period, during which body weight was measured at the General Clinical Research Center (GCRC) 3 times weekly during the first 2 weeks and 5 times weekly during the final 2 weeks. Throughout this period, the energy content of the meals was adjusted to achieve energy balance. At the end of the 4-week period, subjects were admitted to the GCRC for testing. Testing included a frequently sampled intravenous glucose tolerance test (FSIGT) for determination of insulin sensitivity (Si), blood sampling in the fasting state for determination of fasting lipids, glucose, and inflammatory markers, and body composition measurements (anthropometrics, dual energy X-ray absorptiometry [DXA], and computed tomography [CT]). Details of the measurement methods are given below.
After the 4-week energy balance period, subjects were randomized to three weight loss groups: 1) weight-loss by diet alone; 2) weight-loss by diet and aerobic exercise; and 3) weight-loss by diet and resistance exercise. Subjects assigned to the exercise groups participated in three supervised training sessions per week throughout the active weight loss phase. The active weight loss period ended when the subject had achieved a BMI of < 25.
At the end of the active weight loss period, subjects were again placed on a 4-week energy balance period. Baseline measurements were repeated at the end of this post-weight loss energy balance period. Subjects assigned to the exercise groups continued to participate in the exercise sessions throughout this period. The subjects included in this study are those who successfully lost > 10 kg and reached a BMI of < 25.
Body composition measurements
Body composition was assessed by DXA and CT scanning. A whole-body DXA scan (DXA; Prodigy, GE-Lunar, Madison, WI) was performed at baseline and after weight loss. Intraabdominal adipose tissue (IAAT) was assessed via CT scanning at the level of the L4-L5 vertebrae. CT scans were performed at baseline and after weight loss using a HiLight/HTD Advantage scanner (General Electric Co., Milwaukee, WI). The scanner was set at 120 peak kV and 40 mA, as previously described (12). All scans were read by the same trained research assistant.
Collection of sera and frequently-sampled, intravenous, glucose tolerance test (FSIGT)
At approximately 7:00 am, after a 12-hour fast, flexible intravenous catheters were placed in the subject’s antecubital spaces of both arms. Three blood samples were drawn over a 40-min period, and sera subsequently separated and pooled for analysis of lipids and markers of inflammation. Three additional blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time “0", glucose (50% dextrose; 11.4 g/m2) was administered intravenously. At minute 20 following glucose administration, subjects received an intravenous bolus of insulin (0.02 units/kg). Blood samples were collected at the following times relative to glucose administration at 0 min: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, and 240 min. Sera were analyzed for glucose and insulin, and values were entered into the MINMOD computer program (version 3.0, © Richard N. Bergman) for determination of insulin sensitivity (Si) and the acute insulin response to glucose (AIRg) (13–15). AIRg is the integrated incremental area under the curve for insulin during the first 10 minutes of the test.
Assay of glucose, insulin, and lipids
Analyses were performed in the Core Laboratory of the GCRC and the Clinical Nutrition Research Center at UAB. Glucose was measured in 10 µL sera using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY). In the Core Laboratory, this analysis has a mean intra-assay coefficient of variation (c.v.) of 0.61%, and a mean inter-assay c.v. of 1.45%. Insulin was assayed in duplicate 100 µL aliquots with Linco Research Products Inc. (St. Charles, MO) reagents. In the Core Laboratory, this assay has a sensitivity of 3.35 µIU/mL, a mean intra-assay c.v. of 3.49%, and a mean interassay c.v. of 5.57%. Commercial quality control sera of low, medium, and high insulin concentration are included in every assay to monitor variation over time. Total cholesterol, HDL-cholesterol (HDL-C), and TG were measured with the Ektachem DT II System. With this system, HDL-C is measured after precipitation of LDL-C and very-low-density lipoprotein cholesterol with dextran sulfate and magnesium chloride. Control sera of low and high substrate concentration are analyzed with each group of samples, and values for these controls must fall within accepted ranges before samples are analyzed. The DT II is calibrated every 6 months with reagents supplied by the manufacturer. LDL-C was estimated using the Friedewald formula (16).
Assays of markers of inflammation
All markers of inflammation were assessed using enzyme-linked immunosorbent assays (ELISA). TNF-α was assessed using a high sensitivity ELISA kit, (Quantikine HSTA00C, R&D Systems, Minneapolis, MN). This assay requires 200 μL sera per test; sensitivity is 0.5 pg/mL; mean intra-assay CV is 9.0%; mean inter-assay CV is 19.0%. IL-6 was assayed using the Quantikine HS600B (R&D Systems). This assay requires 100 µL sera per test; sensitivity is 0.156 pg/mL; mean intra-assay CV is 12%; mean inter-assay CV is 14.5%. CRP was assayed using the high sensitivity ELISA kit 030-9710s (ALPCO, Windham, NH). CRP samples were diluted in a 1:100 ratio prior to analysis. This assay requires 100 uL diluted sera per test (sera diluted 1:100); sensitivity is 0.124 ng/mL; mean intra-assay CV is 13%; mean inter-assay CV is 12.6%.
Statistical Methods
Baseline characteristics are presented as means and standard deviations. Distributions of TG, AIRg, Si and insulin were skewed and were transformed to the log10 scale for analysis. To evaluate the relationship between baseline inflammatory markers (CRP, IL-6, TNF-α) and the lipid and insulin outcome variables, the inflammatory markers were divided into clinically relevant categories. CRP was categorized as <1.0, 1.0 to 3.0, >3.0 mg/L as described by the Centers for Disease Control and the American Heart Association (17). Because there is no established guideline for risk assessment cutpoints for IL-6 and TNF-α, baseline concentrations were analyzed both as continuous and as categorical variables. As categorical variables, IL-6 and TNF-α were divided into tertiles: ≤1.16, 1.17–1.7, >1.7 pg/mL for IL-6 and ≤0.5, 0.51–0.85, >0.85 pg/mL for TNF-α. Results for IL-6 and TNF-α were similar whether data were analyzed as continuous or categorical. Thus, results were presented as categorical to be consistent with those for CRP.
In all analyses, women with CRP levels of >10.0 mg/L were excluded (n=16) as suggested by the Centers for Disease Control and Prevention and the American Heart Association as such high CRP levels may represent acute inflammation or infection (17). Seven women in the 2 exercise intervention groups were excluded because they were less than 70% adherent to their regimens. Therefore 190 subjects are included in this investigation.
Relationships between baseline inflammatory marker concentrations and lipid and insulin concentrations were examined by the MIXED procedure in SAS (SAS Institute, Inc., Cary, NC, Version 9.1) for repeated measurements with adjustment for changes in IAAT, and controlling for time (before or after weight loss measurement period), race (White vs. Black), and baseline age in years. Predicted mean concentrations of lipids and fasting insulin and insulin sensitivity were plotted by level of baseline inflammatory marker. Mean levels across categories were compared using the Tukey adjustment. Baseline inflammatory marker by visit interactions were tested in each multivariate model. These interactions of inflammatory marker on lipids, glucose, and insulin variables were our main outcomes of interest. The impact of race on these interactions was tested but was found not to be significant. All relationships were considered significant at P < 0.05.
RESULTS
There was no difference between treatment groups in weight, BMI, waist circumference, lipid parameters, glucose, insulin, AIRg, Si, and markers of inflammation across the weight loss period. Consequently, treatment groups were combined for subsequent analyses. Table 1 shows the anthropometric characteristics of the subjects before and after weight loss as well as their serum concentrations of lipids and markers of inflammation. Per study protocol, all women lost weight and had reductions in BMI, waist circumference and body fat during the study. With the exception of HDL-C, which increased, all plasma lipid parameters decreased as well as AIRg, fasting insulin, and IL-6 concentrations. Insulin sensitivity was increased after weight loss.
Table 1.
Anthroprometric characteristics, serum lipids and cytokines in women before and after weight loss (n = 190).
| Overweight | Weight-reduced | P for overweight vs weight- reduced |
|
|---|---|---|---|
| Body weight, kg | 76.5 (7.1) | 65.1 (6.3) | < 0.0001 |
| BMI, kg/m2 | 28.3 (1.4) | 23.9 (1.0) | < 0.0001 |
| Waist circumference, cm | 86.9 (6.6) | 75.9 (5.1) | < 0.0001 |
| Total body fat, % | 44.8 (4.0) | 34.4 (5.1) | < 0.0001 |
| Intraabdominal adipose tissue, cm2 |
79.6 (32.1) | 48.9 (21.7) | < 0.0001 |
| Total cholesterol, mg/dL | 157.2 (31.9) | 151.0 (27.8) | 0.005 |
| LDL cholesterol, mg/dL | 99.5 (29.2) | 92.5 (23.8) | < 0.0001 |
| HDL-cholesterol, mg/dL | 39.7 (10.5) | 45.0 (11.8) | < 0.0001 |
| Triglycerides, mg/dL 2 | 89.3 (40.6) | 67.7 (27.0) | < 0.0001 |
| Fasting glucose, mg/dL | 87.5 (6.7) | 86.3 (7.3) | 0.29 |
| Fasting insulin, μIU/mL 2 | 11.8 (4.0) | 8.4 (3.3) | < 0.0001 |
| AIRg, μIU/mL × 10 min | 777.1 (544.4) | 580.2 (437.2) | < 0.0001 |
| Si3, μIU/mL × 10−4 min−1 | 3.0 (1.8) | 4.6 (2.2) | < 0.0001 |
| CRP, mg/L | 2.1 (1.9) | 1.9 (6.2) | < 0.0001 |
| IL-6, pg/mL 2 | 1.7 (1.2) | 1.3 (0.8) | 0.001 |
| TNF-α, pg/mL | 1.10 (2.44) | 0.94 (0.75) | 0.06 |
Values are means (SD).
Statistical analyses were performed on log-transformed values.
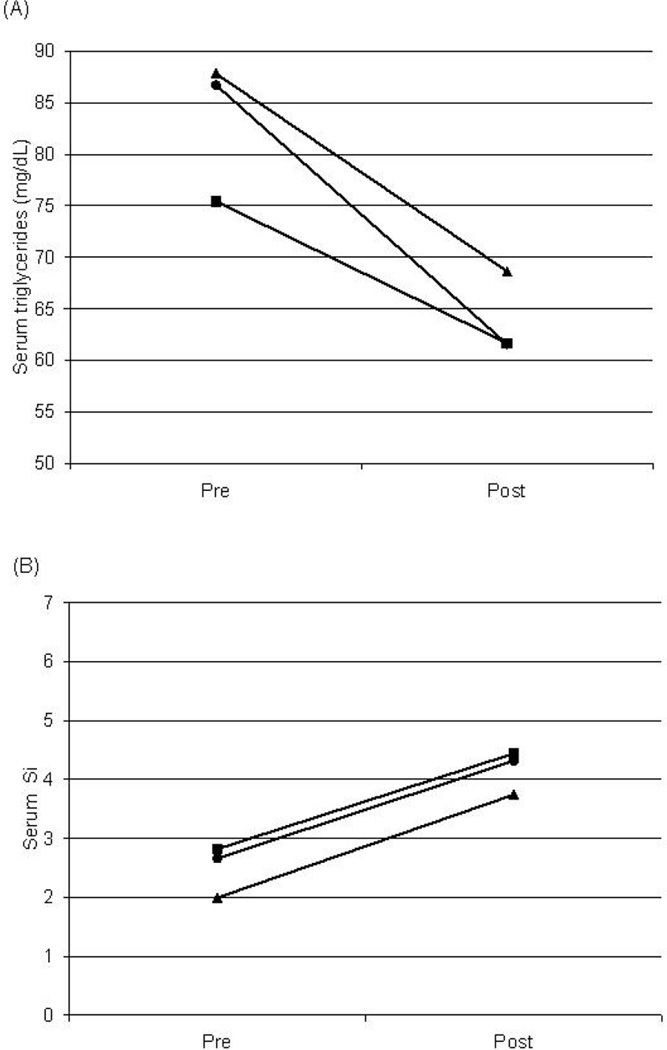
Regression models testing the relationship between baseline concentrations of inflammatory markers and lipid and insulin responses to weight loss are shown in Table 2, Table 3 and Table 4. The baseline CRP-by-time interaction was not significant for any dependent variable (TC, LDL-C, HDL-C, TG, fasting insulin, or Si). Baseline CRP concentration was not a significant predictor of any dependent variable examined, but there was a trend towards significance for TG (P = 0.0549, Figure 1A) and Si (P = 0.0573, Figure 1B). Women in the highest CRP category had higher TG concentrations than those in the lowest CRP group (P < 0.05), and women in the lowest CRP group had higher Si than those in the highest CRP group (P < 0.05).
Table 2.
Relationship between change in plasma lipids and baseline CRP levels during weight loss1
| Factor | Total Cholesterol | LDL-C | HDL-C | Triglycerides (log10) | Insulin (log10) | Si (log10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | |
| Age (yrs) | 0.7460 (0.3729) |
0.0468 | 0.5368 (0.3414) |
0.1176 | 0.1100 (0.1264) |
0.3855 | 0.0027 (0.0002) |
0.1338 | −0.0035 (0.0018) |
0.0518 | 0.0087 (0.0026) |
0.0009 |
| IAAT | 0.0576 (0.0629) |
0.3614 | 0.0603 (0.0538) |
0.2679 | −0.0471 (0.0227) |
0.0393 | 0.001 (0.0004) |
0.0040 | 0.0012 (0.0004) |
0.0012 | −0.0026 (0.0005) |
<0.0001 |
| Baseline | ||||||||||||
| CRP (mg/L) <1 |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| 1–3 | 9.4315 (7.5335) |
0.4097 | 7.4770 (6.4830) |
0.4100 | −3.4807 (2.7894) |
0.3985 | 0.1247 (0.0527) |
0.0549 | 0.03476 (0.0551) |
0.1778 | −0.0352 (0.0847) |
0.0573 |
| >3 | 8.7315 (8.8797) |
8.5566 (7.6266) |
−3.4226 (3.2949) |
0.0969 (0.0624) |
0.1204 (0.0648) |
−0.2335 (0.1002) |
||||||
| Race | ||||||||||||
| (White vs.Black) |
6.6353 (4.5714) |
0.1673 | 4.3868 (4.1751) |
0.2947 | −3.8676 (1.5543) |
0.0137 | 0.1461 (0.0225) |
<0.0001 | −0.0487 (0.0220) |
0.0280 | 0.2185 (0.0317) |
<0.0001 |
| Visit | −4.1992 (4.0171) |
0.0482 | −4.0074 (3.3423) |
0.0327 | 2.0481 (1.5338) |
0.0033 | −0.0601 (0.0298) |
<0.0001 | −0.0794 (0.0312) |
<0.0001 | 0.0987 (0.0474) |
<0.0001 |
| Baseline | ||||||||||||
| CRP (mg/L) *visit <1 |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| 1–3 | −4.5970 (4.6711) |
0.5832 | −3.6591 (3.8121) |
0.5994 | 2.4321 (1.8282) |
0.4140 | −0.0718 (0.0377) |
0.1595 | −0.0204 (0.039) |
0.3599 | 0.0149 (0.0616) |
0.4255 |
| >3 | −0.5982 (5.7058) |
−0.4942 (4.6610) |
1.4910 (2.2302) |
−0.0234 (0.0457) |
−0.0686 (0.0479) |
0.0949 (0.0745) |
||||||
Abbreviations: CRP, C-reactive protein; IAAT, intraabdominal adipose tissue.
Table 3.
Relationship between change in plasma lipids and baseline IL-6 tertiles during weight loss1
| Factor | Total Cholesterol | LDL-C | HDL-C | Triglycerides (log10) | Insulin (log10) | Si (log10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | |
| Age (yrs) | 0.7471 (0.3769) |
0.0489 | 0.5038 (0.3451) |
0.1459 | 0.1504 (0.1242) |
0.2277 | 0.0026 (0.0019) |
0.1699 | −0.0036 (0.0018) |
0.0436 | 0.0092 (0.0026) |
0.0006 |
| IAAT | 0.0772 (0.0634) |
0.2253 | 0.0676 (0.0549) |
0.2201 | −0.0378 (0.0228) |
0.0984 | 0.0011 (0.0004) |
0.0031 | 0.0011 (0.0003) |
0.0034 | −0.0024 (0.0005) |
<0.0001 |
| Baseline | ||||||||||||
| IL6 (pg/mL) |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| ≤1.16 1.17–1.7 |
0.0884 (8.0199) |
0.9153 | 1.0305 (6.9239) |
0.4528 | −3.9776 (2.9889) |
0.4141 | 0.04407 (0.0561) |
0.1292 | 0.0311 (0.0593) |
0.6789 | −0.1850 (0.0902) |
0.0647 |
| >1.7 | 3.0309 (8.0648) |
8.1871 (6.9768) |
−2.0212 (2.9984) |
−0.0723 | 0.0523 (0.0598) |
−0.829 (0.0902) |
||||||
| Race | ||||||||||||
| (White vs.Black) |
5.5457 (4.6050) |
0.2299 | 3.7329 (4.2044) |
0.3757 | −3.9937 (1.5249) |
0.0095 | 0.1444 (0.0230) |
<0.0001 | −0.0456 (0.0221) |
0.0362 | 0.2201 (0.0326) |
<0.0001 |
| Visit | −2.7972 (4.1007) |
0.0511 | −3.5108 (3.4383) |
0.0280 | 4.2739 (1.5831) |
0.0015 | −0.0976 (0.0302) |
<0.0001 | −0.0961 (0.3236) |
<0.0001 | 0.0674 (0.0491) |
<0.0001 |
| Baseline | ||||||||||||
| IL6 (pg/mL) *visit |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| ≤ 1.16 1.17–1.7 |
−5.0884 (5.0458) |
0.5689 | −2.1198 (4.1542) |
0.6353 | −0.4114 (2.0151) |
0.6996 | −0.0365 (0.0403) |
0.1321 | 0.0021 (0.0435) |
0.6550 | 0.1051 (0.0662) |
0.1919 |
| >1.7 | −3.8249 (4.9417) |
−3.8658 (4.0595) |
−1.6321 (1.9830) |
0.0475 (0.0400) |
−0.0340 (0.0435) |
0.1032 (0.0655) |
||||||
Abbreviations: IAAT, intraabdominal adipose tissue; IL-6, interleukin-6.
Table 4.
Relationship between change in plasma lipids and baseline TNF-α tertiles during weight loss1
| Factor | Total Cholesterol | LDL-C | HDL-C | Triglycerides (log10) | Insulin (logj10) | Si (log10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | β(se) | p | |
| Age (yrs) | 0.8577 (0.3697) |
0.0214 | 0.6108 (0.3404) |
0.0743 | 0.1372 (0.1246) |
0.2722 | 0.00028 (0.0018) |
0.1242 | −0.0036 (0.0019) |
0.0423 | 0.0086 (0.0027) |
0.0017 |
| Visceral fat |
0.0497 (0.0622) |
0.4249 | 0.0604 (0.0539) |
0.2644 | −0.0528 (0.0227) |
0.0208 | 0.0011 (0.0003) |
0.0054 | 0.0012 (0.0003) |
0.0017 | −0.0026 (0.0005) |
<0.0001 |
| Baseline | ||||||||||||
| TNF-α (pg/mL) ≤ 0.5 |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| 0.51–0.85 | 4.3115 (7.8128) |
0.4510 | 8.4714 (6.7653) |
0.3221 | −2.2228 (2.9368) |
0.4171 | −0.0206 (0.0558) |
0.6822 | 0.0494 (0.0575) |
0.2320 | −0.0625 (0.0898) |
0.6344 |
| >0.85 | 10.3812 (8.2160) |
9.4952 (7.0947) |
1.8654 (3.0975) |
0.0309 (0.0587) |
0.1040 (0.0606) |
0.0227 (0.0929) |
||||||
| Race | ||||||||||||
| (White vs.Black) |
4.7067 (4.5967) |
0.3071 | 2.5092 (4.2242) |
0.5532 | −3.4580 (1.5534) |
0.0271 | 0.1383 (0.0233) |
<0.0001 | −0.0449 (0.0223) |
0.0459 | 0.2262 (0.0332) |
<0.0001 |
| Visit | −6.2729 (4.0310) |
0.0375 | −4.8691 (3.3617) |
0.0320 | 3.7324 (1.5774) |
0.0056 | −0.1079 (0.0308) |
<0.0001 | −0.0578 (0.0316) |
<0.0001 | 0.2262 (0.0333) |
0.0002 |
| Baseline | ||||||||||||
| TNF-α (pg/mL) *visit ≤ 0.5 |
0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| 0.51–0.85 | −2.8457 (4.7778) |
0.5034 | −3.6444 (3.9046) |
0.3698 | −1.0745 (1.9301) |
0.8537 | 0.0335 (0.0397) |
0.6415 | −0.0630 (0.0415) |
0.1216 | 0.0354 (0.0648) |
0.5711 |
| >0.85 | 3.2680 (5.1836) |
2.2320 (4.2416) |
−0.6890 (2.0877) |
0.0005 (0.0425) |
−0.0865 (0.0445) |
−0.0378 (0.0685) |
||||||
Abbreviations: IAAT, intraabdominal adipose tissue; TNF-α, tumor necrosis factor-
Figure 1.
Changes in plasma triglycerides (A) and Si (B) induced by weight loss stratified by baseline CRP levels (squares = CRP < 1; circles = CRP 1–3; triangles = CRP > 3 mg/L). Values are predicted means controlling for changes in visceral fat, race, age and visit. (A) P < 0.05 for CRP < 1 vs. 1–3 mg/L. (B) P < 0.05 for CRP < 1 and 1–3 vs. > 3 mg/L.
Similarly for IL-6 (Table 3), the baseline IL-6-by-time interaction was not significant for any dependent variable. Finally, the baseline TNF-α by time interaction was not significant for any dependent variable (Table 4).
DISCUSSION
Results of this study showed that the changes in serum lipid concentrations and insulin sensitivity with weight loss did not vary depending on the subject’s baseline inflammatory status. Results also showed that inflammatory status was associated with traditional cardiovascular and diabetes risk factors. Individuals with higher markers of inflammation also had a less desirable lipid profile and lower Si. In fact, for all 3 inflammatory markers studied, CRP, IL-6, and TNF-α, the metabolic risk factors were more adverse in individuals with higher inflammatory status than in those in the lowest tertile of inflammation. Insulin sensitivity was lower in individuals in the highest tertile of CRP and IL-6 whereas TG was higher in women in the 2 higher tertiles of CRP.
Heliovaara et al. (18) have previously shown that, in normal weight men and women, Si is inversely associated with IL-6 but did not find the association, as we have, with CRP. Piche et al. (19) also found that post-menopausal women in the highest CRP tertile (≥ 3.0 mg/L) had higher TG and lower Si than women in the lowest tertile (< 1.0 mg/L). However, unlike our results, they found that differences in TG between CRP tertiles disappeared after adjustment for IAAT. Differences in Si were maintained. Differences between those results and ours with regards to differences in TG with increasing CRP levels may be due to the larger variability in IAAT in their cohort and higher IAAT levels in their postmenopausal women relative to our pre-menopausal women. IAAT may be a more important modulator of lipoproteins at higher levels of IAAT than at lower levels. Piche et al. (19) concluded that CRP levels had no independent effect on plasma lipoproteins. Based on our data, it may be necessary to specify that this may be the case only in post-menopausal women or in women with high IAAT. This is supported by findings from another group who found, as we did, a negative association between IL-6, CRP and Si (20). This group also reported that, in their population of overweight men and women, Si was the strongest predictor of CRP and IL-6, above and beyond the role percent body fat plays on Si.
No previous study has examined the role of pre-weight loss inflammatory status on the lipid response to weight loss. Several studies have looked at the association between baseline inflammatory status and the response to diet composition (8–11). Zhao et al. (10) reported a 29% lower cholesterol-lowering response to high polyunsaturated fat diets in subjects with elevated CRP concentrations relative to those with lower CRP levels. These data differ from those of Desroches et al. (8) who found greater reductions in TC and LDL-C with consumption of a high monounsaturated fat diet by subjects with high baseline CRP concentrations than those with lower CRP concentrations at baseline. However, TG reductions were greater in subjects with low baseline CRP relative to high CRP subjects. Further, those with higher baseline CRP had increases in TG when placed on a low-fat diet, whereas those with low CRP at baseline had a reduction in TG. These results are in accordance with those of Hilpert et al. (9) who also found that, subjects with high CRP levels had increases in atherogenic risk factors when placed on a low-fat diet, whereas those with low CRP had reductions in risk. Improvements in TC and LDL-C were also found to be greater in subjects with low relative to high baseline CRP when placed on the Dietary Approaches to Stop Hypertension (DASH) diet (11).
In light of the studies described above, we had hypothesized that lipid responses to weight loss would differ based on the subject’s baseline inflammatory status. We did not find such interaction of inflammatory status by weight loss on the metabolic risk variables studied. However, we may have needed a larger sample size to detect the impact of baseline CRP levels on TG and Si, which showed a trend for an effect in our study. In our study, few women had elevated CRP levels at baseline with only 23.1% having CRP >3 mg/dL. Perhaps an effect of baseline CRP on changes in TG and Si with weight loss would have been detected if our sample had more heterogeneous baseline CRP levels.
There are some unique and important aspects to this study that deserve mention. First, a similar number of Black and White women participated in this study, allowing us to examine potential racial differences in outcomes of interest. Second, women were in energy balance during both measurement periods and the foods they consumed during those periods were identical. Thus, our results were not affected by the subjects’ food choices and energy status.
This study also has several limitations. First, only pre-menopausal women were included. It is therefore unknown whether similar results would apply to post-menopausal women and men. Race and gender differences have been found in CRP (21). A report by our group showed that nitrate and nitrite levels are different between Black and White women at similar body weight and that myeloperoxidase levels change in opposite directions in response to weight loss in Black and White women (22). One study examining gender differences in correlations between different cardiovascular disease risk factors reported more and stronger correlations in female subjects than males (23).
Second, only healthy women were included in this study and all were within a narrow BMI range of 27–30. It is possible that different results would be observed if our sample had been more heterogeneous in health and BMI status. The men in the study by Desroches et al. (8), for example, had a wider range of BMI than women in our study and they found that baseline CRP concentrations influenced the lipid responses to diets. Similarly, subjects in the study by Hilpert et al. (9) and by Zhao et al. (10) had a wider range of BMI and higher plasma lipid concentrations than the women in this study.
In conclusion, this study did not find that inflammatory status was related to the lipid and insulin response to a weight loss program in otherwise healthy, overweight women. More research is necessary to determine whether similar results would be obtained in subjects at risk of cardiovascular disease and diabetes. However, our data confirm previous findings that individuals with elevated markers of inflammation also have other elevated risk factors for cardiovascular disease and diabetes, even in moderately overweight, otherwise healthy young women.
ACKNOWLEDGEMENTS
Funded by R01DK49779, GCRC grant M01-RR-00032, and CNRU grant P30-DK56336.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of Creactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A–I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. Jama. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 4.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 19:1999. 972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 5.Festa A, D'Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Hanley AJ, Festa A, D'Agostino RB, Jr., et al. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 2004;53:1773–1781. doi: 10.2337/diabetes.53.7.1773. [DOI] [PubMed] [Google Scholar]
- 7.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001:285, 2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Desroches S, Archer WR, Paradis ME, et al. Baseline plasma C-reactive protein concentrations influence lipid and lipoprotein responses to low-fat and high monounsaturated fatty acid diets in healthy men. J Nutr. 2006;136:1005–1011. doi: 10.1093/jn/136.4.1005. [DOI] [PubMed] [Google Scholar]
- 9.Hilpert KF, Kris-Etherton PM, West SG. Lipid response to a low-fat diet with or without soy is modified by C-reactive protein status in moderately hypercholesterolemic adults. J Nutr. 2005;135:1075–1079. doi: 10.1093/jn/135.5.1075. [DOI] [PubMed] [Google Scholar]
- 10.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 11.Erlinger TP, Miller ER, 3rd, Charleston J, Appel LJ. Inflammation modifies the effects of a reduced-fat low-cholesterol diet on lipids: results from the DASH-sodium trial. Circulation. 2003;108:150–154. doi: 10.1161/01.CIR.0000080288.30567.86. [DOI] [PubMed] [Google Scholar]
- 12.Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 13.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man. Measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J.Clin.Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacini G, Bergman RN. MINROD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose toerance test. Comp Meth Prog Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 15.Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am.J.Physiol. 1987;253:E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 15.Heliovaara MK, Teppo AM, Karonen SL, Tuominen JA, Ebeling P. Plasma IL-6 concentration is inversely related to insulin sensitivity, and acutephase proteins associate with glucose and lipid metabolism in healthy subjects. Diabetes Obes Metab. 2005;7:729–736. doi: 10.1111/j.1463-1326.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 16.Piche ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Bluher M, Fasshauer M, Tonjes A, Kratzsch J, Schon MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113:534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 18.Khera A, McGuire DK, Murphy SA, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 19.Fenster CP, Darley-Usmar VM, Landar AL, et al. Weight loss and race modulate nitric oxide metabolism in overweight women. Free Radic Biol Med. 2004;37:695–702. doi: 10.1016/j.freeradbiomed.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Oda E, Abe M, Kato K, Watanabe K, Veeraveedu PT, Aizawa Y. Gender differences in correlations among cardiovascular risk factors. Gend Med. 2006;3:196–205. doi: 10.1016/s1550-8579(06)80208-1. [DOI] [PubMed] [Google Scholar]