Abstract
Background and Purpose
We recently reported that delayed lithium therapy can improve stroke recovery in rats by augmenting neurovascular remodeling. We tested the hypothesis that lithium can promote the expression of growth factors in brain endothelial cells and astrocytes.
Methods
Human brain microvascular endothelial cells and primary rat cortical astrocytes were exposed to lithium chloride in serum-free medium. We examined 2 representative growth factors: brain-derived neurotrophic factor and vascular endothelial growth factor (VEGF). Cell lysates were collected for Western blot analysis. Conditioned media was analyzed with enzyme-linked immunosorbent assay. SB-216763 and LY294002 were used to assess the roles of the glycogen synthase kinase-3β (GSK-3β) and PI3-K signaling in the lithium-induced responses.
Results
No consistent responses were observed for brain-derived neurotrophic factor. However, lithium (0.2 to 20 mmol/L) increased the phosphorylation of GSK-3β and promoted VEGF secretion in a concentration-dependent manner in both endothelial and astrocyte cells. For endothelial cells, the potent GSK-3β inhibitor SB-216763 upregulated VEGF, whereas inhibition of PI3-K with LY294002 suppressed lithium-induced responses in both phospho-GSK-3β and VEGF. In contrast, neither inhibition of GSK-3β nor inhibition of PI3-K had any detectable effects on VEGF levels in astrocytes.
Conclusions
Lithium promotes VEGF expression through PI3-K/GSK-3β-dependent and -independent pathways in brain endothelium and astrocytes, respectively. This growth factor signaling mechanism may contribute to lithium's reported ability to promote neurovascular remodeling after stroke.
Keywords: growth factor, neuroprotection, neurovascular unit, stroke recovery
The mood stabilizer lithium has been reported as a potential neuroprotectant against many central nervous system disorders, including stroke and Alzheimer disease.1–3 Although the neuroprotective mechanisms of lithium are still not clearly defined, known molecular targets for lithium include inositol monophosphatase, proteasome, and glycogen synthase kinase-3 (GSK-3).1,3–5
We recently showed that delayed treatment with lithium improved functional MRI outcomes in a rat model of stroke recovery.6 Within peri-infarct cortex, lithium-treated rats demonstrated increased brain activation after forepaw stimulation, and these areas corresponded with changes in vascular density. Others have showed that brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) contribute to neurovascular remodeling after stroke, and these responses involve both recovering endothelium and reactive astrocytes.7 Therefore, we now ask whether lithium can upregulate BDNF and VEGF in brain endothelial and astrocyte cells.
Materials and Methods
A previously characterized human brain microvascular endothelial cell line8 was seeded in fibronectin-coated plates and exposed to lithium chloride (LiCl; Sigma) in serum-free medium after 6-hour serum starvation; NaCl (Sigma) was used as a control. Primary cultures of rat cortical astrocytes were prepared following standard techniques with cells from newborn (<2 days) Sprague-Dawley rats seeded in collagen I-coated plates for serum starvation and exposure to LiCl. After 30 minutes incubation, endothelial or astrocyte lysates were collected for Western blot with antibodies against phospho-GSK-3β (Ser9) or total GSK-3β (Cell Signaling). After 20 hours, enzyme-linked immunosorbent assays were used to measure BDNF (Promega) and VEGF (R&D Systems) in endothelial- or astrocyte-conditioned media. SB-216763 (Sigma) and LY294002 (Sigma) were used to inhibit GSK-3β and PI3-K, respectively. Standard lactate dehydrogenase assays confirmed that the treatments were not cytotoxic. Data were analyzed with analysis of variance followed by Tukey-Kramer tests.
Results
Levels of 2 representative growth factors, BDNF and VEGF, were assessed in conditioned media. Treatment with LiCl (0.2 to 20 mmol/L) for 20 hours did not produce a consistent change in BDNF levels in either endothelial cells or astrocytes (data not shown). Levels of VEGF were easily measured in conditioned media from brain endothelial cells (487.6±33.2 pg/mL) and in astrocytes (46.8±5.3 pg/mL). Exposure to LiCl for 20 hours increased VEGF in a concentration-dependent manner by 2- to 4-fold in both endothelial cells (Figure 1A) and astrocytes (Figure 1B). Treatment with NaCl had no detectable effects.
Figure 1.
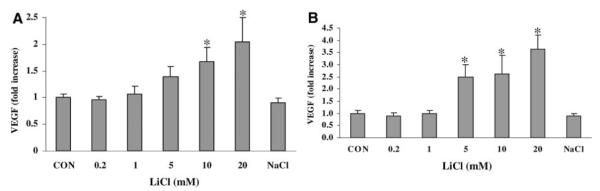
A, LiCl increased VEGF production by brain endothelial cells. B, LiCl increased VEGF production by brain astrocyte cells. *P<0.05 versus CON. N=3 independent experiments performed in triplicate.
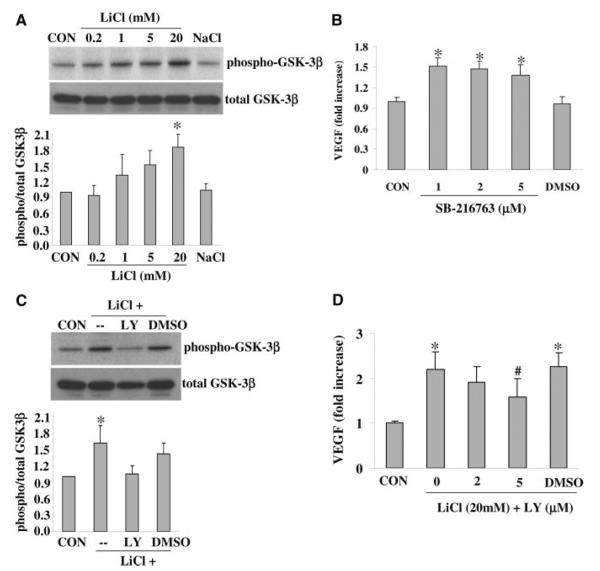
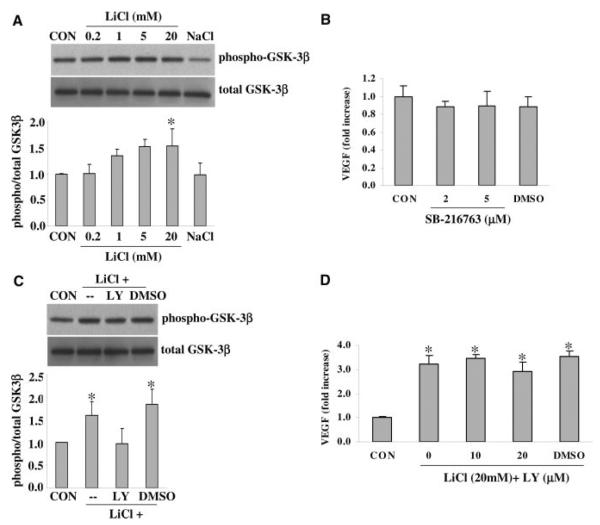
Western blot of cell lysates demonstrated that Ser-9 phosphorylation of GSK-3β was increased by LiCl in a concentration-dependent manner in endothelial cells (Figure 2A). GSK-3β activity is decreased by Ser-9 phosphorylation. Consistent with this phenomenon, the GSK-3 inhibitor SB-216763 similarly elevated VEGF levels in this brain endothelial cell model (Figure 2B). Next, we examined the closely related PI-3K pathway. The potent PI3-K inhibitor LY294002 prevented the phosphorylation of GSK-3β by LiCl (Figure 2C). Concomitantly, inhibition of PI3-K also prevented the lithium-induced upregulation of VEGF (Figure 2D).
Figure 2.
A, Exposure to LiCl triggered GSK-3β signaling as indicated by elevated levels of phospho-GSK-3β in cell lysates. B, The GSK-3 inhibitor SB-216763 also significantly upregulated VEGF production. C, LiCl-induced GSK-3β signaling requires PI3-K, because the PI3-K inhibitor LY294002 (LY) suppressed phospho-GSK-3β levels. A total of 20 mmol/L LiCl and 5 μmol/L LY294002 was used. D, LY294002 prevented the LiCl-induced upregulation of VEGF in brain endothelial cells. *P<0.05 between controls (CON) versus LiCl-treated cells. #P<0.05 between LiCl alone versus LiCl plus LY294002. N=3 independent experiments performed in triplicate. DMSO was used as a vehicle control.
In astrocyte cells, the phosphorylation of GSK-3β was also increased by LiCl (Figure 3A). However, surprisingly, inhibition with SB-216763 did not elevate VEGF levels (Figure 3B). The PI3-K inhibitor LY294002 prevented the lithium-induced phosphorylation of GSK-3β (Figure 3C), but it had no effect on lithium-induced VEGF levels (Figure 3D).
Figure 3.
A, Exposure to lithium triggered GSK-3β signaling in astrocytes as indicated by elevated levels of phospho-GSK-3β in cell lysates. B, The GSK-3 inhibitor SB-216763 did not change the VEGF production. C, Lithium-induced GSK-3β signaling requires PI3-K, because the PI3-K inhibitor LY294002 (LY) suppressed phospho-GSK-3β levels. A total of 20 mmol/L LiCl and 20 μmol/L LY294002 was used. D, LY294002 had no effect on lithium-induced upregulation of VEGF in astrocytes. *P<0.05 between controls (CON) versus lithium-treated cells. N=3 independent experiments performed in duplicate or triplicate. DMSO was used as a vehicle control.
Discussion
Recently, we showed that delayed treatment with lithium may augment neurovascular remodeling and improve stroke recovery.6 The present study suggests that some of lithium's effects might involve its ability to upregulate VEGF in brain cells.
Although lithium can trigger a wide range of biological actions in cells,3 our data here suggest that the GSK-3β and PI3-K signaling pathways may play a central role. GSK-3β can modulate VEGF expression and help regulate angiogenesis.9 It has been shown that lithium increases VEGF mRNA in myocardium after cardiac ischemia.10 Here, the ability of lithium to upregulate VEGF in brain endothelial cells also appeared to function through the GSK-3β and PI3-K pathway. It has also been reported that lithium can induce GSK-3-independent mechanisms in neural cells.11 Surprisingly, however, lithium-induced VEGF in astrocytes appears to be independent of GSK-3β and PI3-K signaling, at least in our system. Besides the glial and vascular compartment, neurons may also be beneficially affected. Lithium prevents VEGF reduction in immature neurons of hippocampus after chronic mild stress in rats.12 Hence, the ability of lithium to upregulate VEGF in brain endothelium and astrocytes might allow both autocrine and paracrine protection to the entire neurovascular unit after stroke.
Nevertheless, several questions remain. Lithium increases BDNF secretion in cultured rat cortical neurons and protects them from glutamate toxicity.13 Recently, lithium was found to selectively promote the BDNF promoter IV activity, causing an increase in exon IV-containing BDNF mRNA and total BDNF protein levels in rat neurons.14 However, in our brain endothelial cell and astrocyte models, BDNF was not affected. Why? Besides VEGF and BDNF, what other growth factors might be altered by lithium signaling? Besides GSK-3β and PI3-K signals, what other molecular pathways may mediate VEGF, especially in astrocytes?
Finally, besides stroke, might neurovascular actions of lithium also apply in other central nervous system disorders? A small clinical trial recently suggested that lithium may improve survival in ALS patients.15 Others have proposed that VEGF may protect neurons in experimental models of ALS,16 whereas early neurovascular alterations may contribute to ALS pathophysiology.17,18 Hence it is possible that the ability of lithium to upregulate endothelial or astrocytic VEGF and other growth factors may contribute to its beneficial effects in ALS and other neurodegenerative diseases. These broader neurovascular signaling actions of lithium warrant further investigation.
In conclusion, lithium or other GSK-3β-modulating drugs may provide a therapeutic approach for augmenting VEGF and perhaps promoting neurovascular remodeling during stroke recovery. The initial cell data presented here require further confirmation and analysis with in vivo model systems.
Acknowledgments
Sources of Funding
Supported in part by an American Heart Association Bugher award and National Institutes of Health grants R01-NS48422, R01-NS53560, P50-NS10828, and P01-NS55104.
Footnotes
Disclosures
None.
References
- 1.Aghdam SY, Barger SW. Glycogen synthase kinase-3 in neurodegeneration and neuroprotection: lessons from lithium. Curr Alzheimer Res. 2007;4:21–31. doi: 10.2174/156720507779939832. [DOI] [PubMed] [Google Scholar]
- 2.Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disord. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- 4.Chin PC, Majdzadeh N, D'Mello SR. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 6.Kim YR, van Meer MP, Tejima E, Murata Y, Mandeville JB, Dai G, Chuang DM, Rosen BR, Lo EH. Functional MRI of delayed chronic lithium treatment in rat focal cerebral ischemia. Stroke. 2008;39:439–447. doi: 10.1161/STROKEAHA.107.492215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan MK, Williams KA, Kivisakk P, Pearce D, Stins MF, Ransohoff RM. CXCR3 marks CD4+ memory T lymphocytes that are competent to migrate across a human brain microvascular endothelial cell layer. J Neuroimmunol. 2004;153:150–157. doi: 10.1016/j.jneuroim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Skurk C, Maatz H, Rocnik E, Bialik A, Force T, Walsh K. Glycogensynthase kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ Res. 2005;96:308–318. doi: 10.1161/01.RES.0000156273.30274.f7. [DOI] [PubMed] [Google Scholar]
- 10.Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40:138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E. Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J Neurochem. 2003;87:417–426. doi: 10.1046/j.1471-4159.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva R, Martins L, Longatto-Filho A, Almeida OF, Sousa N. Lithium prevents stress-induced reduction of vascular endothelium growth factor levels. Neurosci Lett. 2007;429:33–38. doi: 10.1016/j.neulet.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2007 Oct 9; doi: 10.1038/sj.mp.4002099. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Fornai F, Longone P, Carafo L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazerri G, Spalloni A, Bellio N, Lenzi P, Modugno N, Siciliano G, Isidoro C, Murri L, Ruggieri S, Paparelli A. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 17.Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS ONE. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalvo Y, O'Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]