Abstract
Previous studies have revealed that HIV infected individuals possess circulating CD4+CD8+ (DP) T-cells specific for HIV antigens. In the present study, we analyzed the proliferation and functional profile of circulating DP T-cells from 30 acutely HIV infected individuals and 10 chronically HIV infected viral controllers. The acutely infected group had DP T-cells which showed more proliferative capability and multifunctionality than both their CD4+ and CD8+ T-cells. DP T-cells were found to exhibit greater proliferation and higher multifunctionality compared to CD4 T-cells in the viral controller group. The DP T-cell response represented 16% of the total anti-HIV proliferative response and greater than 70% of the anti-HIV multifunctional response in the acutely infected subjects. Proliferating DP T-cells of the acutely infected subjects responded to all HIV antigen pools with equal magnitude. Conversely, the multifunctional response was focused on the pool representing Nef, Rev, Tat, VPR and VPU. Meanwhile, the controllers’ DP T-cells focused on Gag and the Nef, Rev, Tat, VPR and VPU pool for both their proliferative and multifunctional responses. Finally, we show that the presence of proliferating DP T-cells following all HIV antigen stimulations is well correlated with proliferating CD4 T-cells while multifunctionality appears to be largely independent of multifunctionality in other T-cell compartments. Therefore, DP T-cells represent a highly reactive cell population during acute HIV infection, which responds independently from the traditional T-cell compartments.
Introduction
Immature T cells express both CD4 and CD8 while undergoing thymic development(1). Traditionally it was believed that expression of either CD4 or CD8 was permanently lost once T-cells transitioned to naïve T-cell status and exited the thymus. Despite this, it has been observed that both healthy and diseased humans, chickens, monkeys, mice, rats and pigs exhibit a circulating pool of CD4+CD8+ double positive (DP) T-cells(2–7). The DP population generally represents around 3% of circulating T-cells but shows considerable variability across individuals(3). In patients suffering from various neoplastic and infectious diseases, as well as in some ostensibly healthy individuals, circulating DPs can represent up to 43% of circulating T-cells(8–9).
Circulating DPs have often been thought to arise from thymic leakage of immature T-cells(2, 10). Nonetheless, sporadic studies have examined the origins and functional abilities of extra-thymic DPs. DPs have been shown to localize to the sites of inflammatory processes in a variety of autoimmune disorders as well as in infectious diseases, such as HCV(9, 11–12). It has been reported that DPs with a highly activated memory phenotype and expressing both HIV co-receptors CCR5 and CXCR4 are located in the intestine, where HIV replication preferentially occurs during acute infection(13–14). Kitchen et. al. and others have also shown that HIV is capable of infecting DP cells both in vitro and in vivo(15–19). Since DP cells are located at the sites of active HIV infection, express the appropriate co-receptors and are capable of being infected, DP cells may be important targets of acute HIV infection.
The ability of the immune system to quickly respond during acute HIV infection and thereby decrease viral loads to low levels is believed to be an important determinant of long term prognosis(20). Interestingly, Howe and collaborators have shown that HIV infected patients possess circulating HIV-specific DP T-cells during the acute phase(21). These HIV-specific DP cells expressed interferon-γ and were either co-expressing IL-2 or a marker for degranulation (CD107a), and patients were more likely to have bifunctional DP cells than either bifunctional CD4 or CD8 T-cells. Simultaneous expression of 3 or more functions at a time within HIV and SIV-specific CD4 and CD8 T-cells has previously been associated with improved disease outcomes(22–26). CD4 and CD8 T-cell proliferation in response to HIV antigens has also been associated with better HIV disease status(27–28). Therefore, in addition to the possibility of being a target of acute HIV infection, DP cells may represent an important component of the HIV-specific cellular immune response. At present, little is known about the breadth and functional profile of the DP response to HIV. As a result, we sought to determine the ability of circulating DP cells to respond to HIV antigens with a wide range of functionalities during the acute phase of HIV infection. Finally, we went on to compare these acute responses with those observed in a group of HIV-1 controllers.
Materials and Methods
Subjects
Persons with acute HIV infection were identified based on clinical presentation or by screening conducted by the state of North Carolina’s Screening and Tracing Active Transmission (STAT) Program that has identified individuals with acute HIV infections (AHI) since 2002. Subjects are identified as being acutely infected through a combination of reported symptoms and serology(29). Acutely infected patients were then referred for further evaluation at either Duke University or the University of North Carolina-Chapel Hill. Following the provision of written informed consent, the referred patients were enrolled in either studies of antiretroviral treatment or an untreated longitudinal study (depending on patient choice) if they were 1) EIA Negative and nucleic acid amplification test (NAT) positive 2) EIA positive, NAT positive, Western Blot negative/indeterminate or 3) EIA positive, NAT positive, Western Blot positive and documented EIA negative within 45 days. Thirty patients who had been infected a median of 43 (range 22–105) days before entry were studied (Table 1)(29). Due to the study location, all 30 patients were presumed to have been infected with HIV-1 clade B viruses. At study entry, blood samples were acquired from these patients and peripheral blood mononuclear cells (PBMCs) were isolated using standard density gradient centrifugation. Isolated PBMCs were cryopreserved in fetal calf serum (FCS) supplemented with 10% DMSO and stored in vapor phase liquid nitrogen within 8 hours from collection. Viral load and CD4 counts were also obtained at study entry. This acute patient cohort had a median viral load of 259789.5 (range 688–1,1503,872) copies/mL and a median CD4 count of 476.5 (range 6–1175) cells/mm3.
Table 1.
Acute Patient Data
| PID | Days Post Infection |
Viral Load (copies/mL) |
CD4 Count (cells/mm3) |
DP Cells (% of CD3) |
FIEBIG |
|---|---|---|---|---|---|
| PHI02-70 | 54 | 6281 | 1175 | 4.97 | 5/6 |
| Z023 | 24 | 255507 | 392 | 2.04 | 3 |
| Z026 | 43 | 1338235 | 558 | 4.62 | 4 |
| Z029 | 36 | 2027400 | 316 | 10.05 | 3 |
| Z030 | 34 | 1408114 | 703 | 11.48 | 1/2 |
| Z032 | 57 | 559145 | N/A | 4.33 | 5 |
| Z035 | 26 | 2251415 | 291 | 8.21 | 1/2 |
| Z042 | 38 | 572904 | N/A | 6.48 | 5/6 |
| Z055 | 56 | 21488 | 311 | 3.62 | 5/6 |
| Z059 | 45 | 565421 | 538 | 5.55 | 5/6 |
| Z064 | 84 | 11503872 | 6 | 28 | 5/6 |
| Z065 | 49 | 3071359 | 342 | 4.87 | 5/6 |
| Z067 | 22 | 562000 | 521 | 5.08 | 1/2 |
| Z068 | 40 | 750001 | 180 | 5.55 | 3 |
| Z069 | 33 | 100001 | 250 | 4.37 | 3 |
| Z072 | 46 | 25788 | 558 | 2.44 | 5/6 |
| Z073 | 33 | 6732441 | 673 | 3.29 | 1/2 |
| Z075 | 38 | 170826 | 241 | 4.21 | 5/6 |
| Z078 | 58 | 750001 | 760 | 9.99 | 5/6 |
| Z080 | 58 | 264072 | 508 | 5.26 | 5/6 |
| Z085 | 67 | 189036 | 422 | 5.48 | 5/6 |
| Z091 | 43 | 37706 | 541 | 4.98 | 5/6 |
| Z095 | 26 | 37706 | 808 | 7.52 | 5/6 |
| Z098 | 43 | 59603 | 1106 | 6.73 | 5/6 |
| Z099 | 26 | 394649 | 377 | 5.13 | 1/2 |
| Z100 | 31 | 33959 | 806 | 5.44 | 5/6 |
| Z106 | 105 | 688 | 574 | 6.84 | 5/6 |
| Z109 | 74 | 46470 | 438 | 7.23 | 5/6 |
| Z112 | 51 | 189930 | 445 | 4.11 | 5/6 |
| Z117 | 48 | 193306 | 200 | 8.43 | 3 |
Additionally, 9 virus controllers were separately recruited from the Duke Adult Infectious Diseases Clinic with informed consent under Duke University Medical Center IRB approval. Virus controllers were required to have been diagnosed as HIV positive for greater than 1 year, be antiretroviral therapy naïve, have CD4 counts >600 cells/mm3 blood and have been controlling virus replication to less than 2,700 viral RNA copies/mL blood (Table 2)(30–32).
Table 2.
Controller Patient Data
| PID | Viral Load (copies/mL) |
CD4 Count (cells/mm3) |
DP Cells (% of CD3) |
|---|---|---|---|
| VC10 | 2670 | 902 | 3.72 |
| VC11 | 2020 | 924 | 4.00 |
| VC16 | 519 | 628 | 2.06 |
| VC18 | <48 | 1514 | 1.98 |
| VC20 | 601 | 627 | 2.65 |
| VC23 | 424 | 806 | 4.36 |
| VC24 | 894 | 643 | 2.24 |
| VC26 | 118 | 668 | 2.43 |
| VC27 | 2690 | 816 | 3.33 |
Peptides
Fifteen amino acid peptides overlapping by 11 amino acid residues representing the HIV-1 Clade B consensus sequences of ENV (#9480), Gag (#8117), Nef (#5189), Pol (#6208), Rev (#6445), Tat (#5138), VPR (#6447) and VPU (#6444) were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Individual peptides were resuspended in DMSO and pooled, at a final concentration of 500µg/mL, into a total of six peptide pools representing gp120, gp41, Gag, Pol peptides #5461–5585, Pol peptides #5586–5709, and a combination of Nef, Rev, Tat, VPR and VPU (VVNRT).
Proliferation Assays
Cryopreserved PBMC were thawed and washed twice with RPMI containing 10% FCS and 1% penicillin/streptomicin (R10) and enumerated using a Guava Count system (Millipore). Following cell counting, the PBMCs were washed twice with PBS and subsequently resuspended at 20×106 cells/ml. The cells were stained with Carboxyfluorescein succinimidyl ester (CFSE) for 8 minutes mixing once at 4 minutes(33). CFSE staining was quenched with 100% human serum for 2 minutes, and after washing with PBS, the cells were resuspended in R10 containing human serum. CFSE stained cells were then plated at 1×106 cells/mL and stimulated with peptide pools representing HIV GP120, GP41, Gag, Pol pool 1, Pol pool 2, VVNRT(1 ug/mL) or anti-CD3 (eBioscience) and -CD28 (BD Biosciences) antibodies. Stimulated cells were then incubated at 36°C with 5% CO2 for 6 days. Following 6 days of stimulation, the cultures were washed twice with PBS and stained with a violet vital dye (Invitrogen) for 20 minutes at room temperature in the dark. Cells were again washed twice with PBS and then stained with anti-CD3 APC-Cy7 (BD Biosciences; clone SK7), -CD4 PE-Cy5.5 (eBioscience; OKT4) and -CD8 Qdot605 (Invitrogen; 3B5) antibodies for 20 minutes at room temperature in the dark. Following antibody staining, the cells were washed thrice with PBS and fixed with 1% paraformaldehyde. Stained and fixed cells were then refrigerated in the dark until acquisition.
Intracellular Cytokine Staining Assays
Cryopreserved PBMC were thawed, resuspended at 2x106 cells/mL in R10 and rested overnight at 36°C with 5% CO2. After the overnight rest, the PBMCS were counted and resuspended at 1×106 cells/mL in R10. Cells were then stimulated for 6 hours at 36°C with 5% CO2with peptide pools representing CMV pp65, HIV GP120, GP41, Gag, Pol pool 1, Pol pool 2 or VVNRT (1 ug/mL). Stimulations were performed in the presence of 1ug/mL each of anti- CD28 (BD Biosciences; L293) and CD49d (BD Biosciences; L25) antibodies, anti-CD107a PE-Cy5(eBioscience; H4A3), 5ug/mL Brefeldin A (Sigma-Aldrich) and 1 ug/mL Monensin (BD Biosciences). Following the stimulation, the cells were washed with PBS containing 1% FCS and surface stained with Aqua Blue vital dye (Invitrogen), anti-CD4 PE Cy5.5 (eBioscience; OKT4), -CD8 Qdot605 (Invitrogen; 3B5), -CD27 PE-Cy7 (BD Biosciences; M-T271), -CD57 Qdot565 (AbD Serotec; TB01) and –CD45RO PE-TR (Beckman Coulter; UCHL1) for 20 minutes at room temperature in the dark. Following surface staining, the cells were washed again with PBS containing 1% FCS, and then subsequently fixed and permeabilized with cytofix/cytoperm and perm/wash buffer (BD Biosciences) for 20 minutes and washed twice. Following the fix/perm step, cells were stained intracellularly with anti-CD3 Qdot655 (Invitrogen; S4.1), -IFN-γ Alexa700 (BD Biosciences; B27), -IL-2 APC (BD Biosciences; MQ1-17H12) -MIP-1β FITC (R&D Systems; 24006), and –perforin PE (Cell Sciences;B-D48) for 20 minutes at room temperature in the dark. Subsequent to intracellular staining, cells were washed with perm/wash buffer and then fixed with 1% paraformaldehyde. Following fixation, cells were refrigerated in the dark until acquisition.
Flow Cytometry Acquisition and Analysis
Within 18 hours of staining, fully stained cells from the proliferation and intracellular cytokine staining (ICS) assays were acquired on a custom LSRII (BD Biosciences) using FACSDiva. Following acquisition, flow data was analyzed using FlowJo software v.9.3.2 (TreeStar). For all assays, gates were set to include singlet events, live CD3+ cells, lymphocytes and CD4+/CD8+/CD4+CD8+ subsets. For the proliferation assays, CFSElow populations were then identified from each lymphocyte subset (Supplemental Figure 1A). For the ICS assays, the naïve population (CD27+CD45RO−) was identified and excluded from each lymphocyte subset. Within the memory population, cellular function positive populations were identified individually for all cellular functions except perforin, which was only defined as positive if both perforin+ and IFN-γ+ (Supplemental Figure 1B). Using a boolean gating strategy, the 32 combinations of the 5 cellular functions were identified. Based on these frequencies, we also calculated the total frequency of families of subsets expressing the same number of functions.
Statistical Analysis
For the proliferation assays, relative proliferation values were obtained by subtracting the average CFSElow population frequency of a patient samples’ two unstimulated wells from the CFSElow population frequency following stimulation, and then dividing the resulting value by the average CFSElow population frequency of a patient samples’ two unstimulated wells. For the intracellular cytokine staining assays, Pestle was used for background subtractions and Prism (Graphpad) and SPICE were used for frequency analysis. PESTLE and SPICE were kindly provided by Dr. M. Roederer Vaccine Research Center, NIH, Bethesda, MD.
DP response ratios were calculated by first multiplying each patients’ frequency of HIV-specific DP, CD4 and CD8 T-cells by the mean cell count in the DP, CD4 and CD8 compartments for each stimulation condition to obtain a normalized HIV-specific DP, CD4 and CD8 cell count. The normalized HIV-specific DP cell count was then divided by the sum of the normalized HIV-specific DP, CD4 and CD8 cell counts to give the DP response ratio.
Comparisons of responses within patient groups were performed using a Wilcoxon Matched Pairs test (Prism). Comparison across patient groups used a Mann-Whitney U test (Prism). Regression analyses were performed using a linear regression (Prism). No adjustments for multiple comparisons were performed and p values should be interpreted with this in mind.
Results
HIV-specific Proliferation of DP cells
Previous studies reported a correlation between the magnitude of HIV-specific proliferation of T-cells and improved disease outcomes. Nonetheless, HIV-specific proliferation of DP cells has not been previously examined. Therefore, PBMCs from HIV+ patients were analyzed for their proliferative ability in response to stimulation with peptides representing the HIV clade B consensus peptide sequence. Following 6 days of stimulation, CD4+CD8− (CD4), CD4+CD8+ (DP) and CD4−CD8+ (CD8) populations were identified by flow cytometry (Supplemental Figure 1A). The loss of CFSE staining was used to indicate cells within these populations which had undergone proliferation during the stimulation(33). As expected, we observed proliferative responses to the HIV proteome within both the CD4 and CD8 compartments of the acutely infected subjects. The relative proliferation within the CD8 compartment was greater than that seen in the CD4 compartment for both the acute (median, range 1.78, −4.93–25.5 vs. 0.93,−3.86–31.22) and controller patients (3.28, −0.19–29.69 vs. 0.55, −0.32–20.89), although this difference was only significant in the controller cohort (Figure 1A). In both acutely infected (3.78, −3.42–36.36) and controller patients (6.61, −0.13–98.13), we observed significantly greater relative proliferation within the DP compartment following HIV antigen stimulation than the CD4 compartment. DP proliferative responses were also higher than those observed in the CD8 compartments of both cohorts, but reach statistical difference only in the acute cohort (p= 0.01). Next, we determined the portion of the total T-cell proliferative response to HIV which was attributable to the DP compartment in the acute and controller cohorts, a calculation we call the DP response ratio. In both cohorts, the median proliferative DP response ratio was approximately 16% (range 0–78; 0–39) (Figure 1B). In 5 of 30 acute patients the DP response ratio was greater than 40%. We also sought to determine if there was a relationship between the time since infection and the magnitude of the proliferative response. There was no correlation between the CD4 proliferative response and time following infection (Figure 1C). However, the HIV specific proliferative response within the DP and CD8 compartments increases with time since infection (p = 0.0198, 0.0406), suggesting that both the DP and CD8 proliferative responses gain strength as the immune system partially controls viral replication (Figure 1D and E). Meanwhile, the magnitude of the HIV-specific proliferative response within the controller cohort did not correlate with viral loads within these patients (p = 0.6312).
Figure 1. Total HIV-Specific Proliferation.
A, Cells were stained with CFSE and stimulated for 6 days with peptide pools representing the HIV proteome. The HIV-specific relative proliferation was calculated for each T-cell subtype and plotted for each patient in the acute and chronic cohorts. B, The percentage of the total anti-HIV proliferative response coming from the DP compartment was calculated for each patient in the acute and chronic cohorts. C, The days post infection at which an acute blood sample was drawn was plotted against the HIV-specific relative proliferation in the CD4 compartment D, DP compartment E, CD8 compartment
Polyfunctionality of HIV-specific DP cells
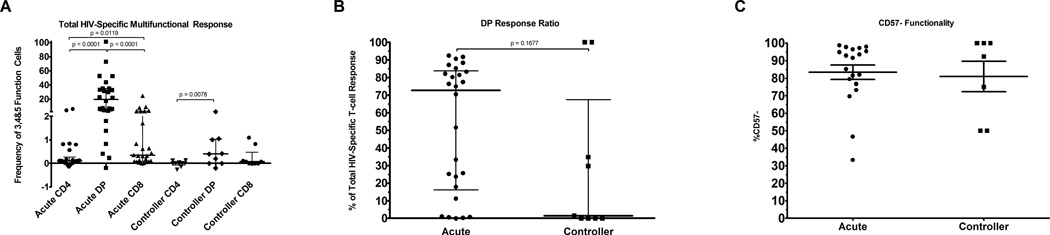
The polyfunctionality of HIV-specific cells was analyzed by staining for the expression of CD107a, IFN-γ, IL-2, MIP-1β and perforin after a six hour stimulation. HIV-specific functionality was determined according to the gating strategy displayed in supplemental figure 1B. Following the identification of each individual functional population, Boolean gating was applied to identify cells expressing all possible combinations of the 5 functions. We observed a significantly higher frequency of CD8 T-cells expressing 3 or more functions in the acutely infected group than we observed within the CD4 compartment (Figure 2A). HIV-specific multifunctional cells within the DP compartment (20, −0.19–100) were significantly more frequent than in the CD8 compartment (0.34, 0.01–24.84) in the acute infection group (p = 0.0002). Of interest, 100% of patient Z68’s DP cells were specific for HIV and multifunctional at study entry.
Figure 2. Total HIV-Specific Multifunctionality.
A, Cells were stimulated for 6 hours with peptide pools representing the HIV proteome and then expression of CD107a, IFN-γ, IL-2, MIP-1β and perforin. Using Boolean gating expression of all possible combinations of these functions was determined. The HIV-specific frequency of cells expressing 3, 4 or 5 of these functions within each T-cell subtype was plotted for each patient. B, The percentage of the total 3, 4 or 5 function response coming from the DP compartment was calculated for each patient in the acute and chronic cohorts. C, The percentage of multifunctional DP cells lacking expression of CD57.
Using the multifunctional response, we again compared the DP response ratios across cohorts to determine the fraction of all HIV-specific multifunctional T-cells which reside within the DP compartment (Figure 2B). The acute cohort had a median multifunctional DP response ratio of 73% (0–92%) while the controllers exhibited a median of 2% (0–100%). It is notable that the controllers exhibited an extremely variable DP response ratio with the majority well below the acute cohorts’ median and two patients presenting a DP ratio of 100%. Due to this high variability, the response ratios of the two groups only trended towards a significant difference (p = 0.1677).
In order to understand the differentiation stage of the whole and HIV-1-specific multifunctional DP cell subsets, we also examined their CD27, CD45RO, and CD57 expression. Within the general memory population of the CD4, DP and CD8 memory compartments (Supplemental Figure 2A-F), we observed that the CD4 memory compartment was predominantly CD27+CD45RO+CD57−; the CD8 memory compartment was evenly distributed between CD27 and CD45RO expression status with the frequency of cells lacking CD57 expression being approximately two-fold higher than those displaying CD57 expression. The DP compartment was often intermediate to the frequencies displayed in either the CD4 or CD8 compartments but more closely resembled the CD8 compartment. When the HIV-1-specific subsets were analyzed, we found that in both the acute and controller cohorts, a median of 92% (33–99%; 50–100%) of multifunctional DP cells lacked CD57 expression and therefore were not terminally differentiated (Figure 2C). Within the time span covered by our acute cohort, there were no significant correlations between the frequencies of CD4, DP or CD8 multifunctional cells and time post-infection (Supplemental Figure 3A, B and C). Conversely, the frequency of HIV-specific multifunctional DP cells within the controller cohort correlated with the viral loads within these patients (p = 0.0282).
Antigen-Specific Contribution to HIV-specific responses
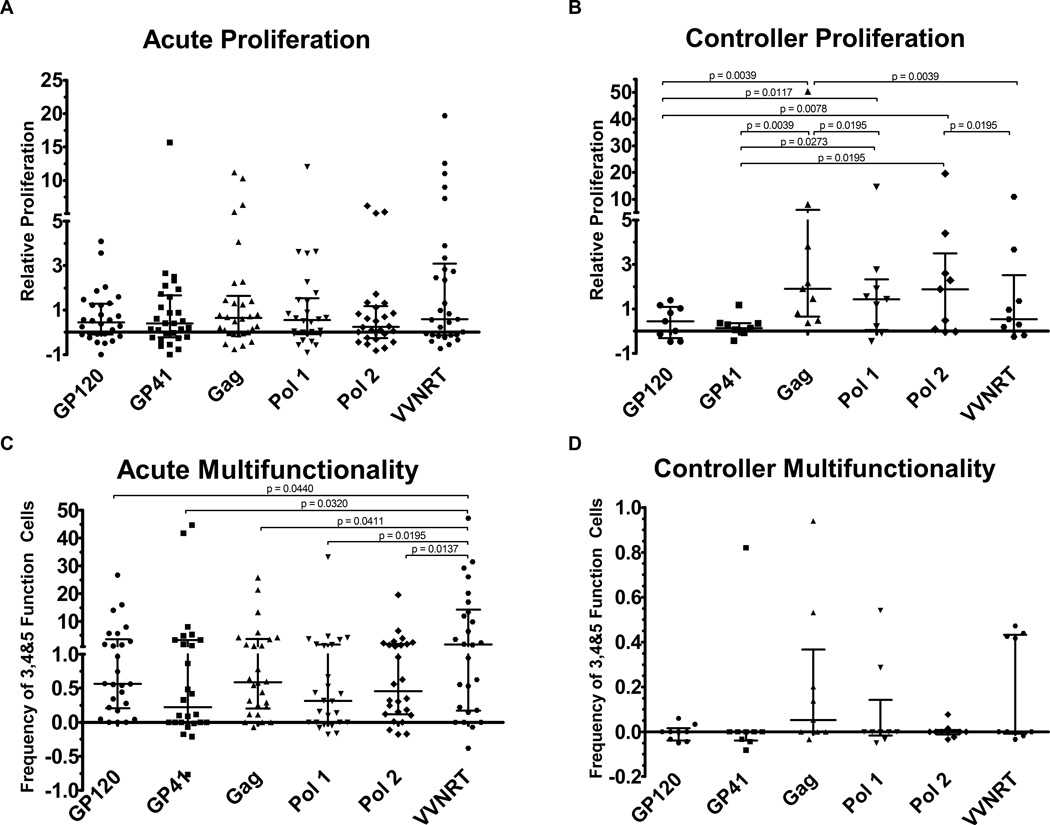
Next, we sought to identify the antigenic regions responsible for evoking the proliferation and multifunctionality observed within the DP compartment. Within the acute HIV infection cohort, we observed no significant antigen-specific differences in proliferative capacity of the DP cells (Figure 3A). On the other hand, the controllers’ DP cells focused on all non-Env peptide pools (Figure 3B). In fact, the DP proliferative response to Gag was significantly higher within the controller cohort than within the acute cohort (p = 0.0437). All other antigen-specific responses were not significantly different between the patient cohorts (p-values summarized in Supplemental Table 1).
Figure 3. HIV Antigen Specific Responses.
A, The relative proliferation of the DP compartment against each HIV antigen pool for patients in the acute cohort. B, within the controller cohort. C, The frequency of antigen specific 3, 4 or 5 function memory T cells responding to each HIV antigen pool in the acute cohort. D, within the controller cohort.
In contrast to their proliferative response, the acute patients’ multifunctional response was most pronounced to VVNRT, though responses were robust to most of the peptide pools (Figure 3C). Meanwhile, the controllers’ multifunctional response did not focus on an individual HIV antigen pool (Figure 3D). We compared the multifunctional responses to each antigen between patient cohorts and observed that they were significantly higher among the acute patients than the controllers for all peptide pools except the Pol pool 1, which displayed a trend towards significance (p-values summarized in Supplemental Table 1).
We also compared the proliferative and functional responses across assays for the two peptide pools (Gag and VVNRT) that generated the strongest functional and proliferative responses. This analysis revealed no correlation between the level of proliferating DP cells and the frequency of multifunctional DP cells (Figure 4A and B). This was observed in both the acute and controller cohorts.
Figure 4. Relationship between Multifunctionality and Proliferation.
A, The frequency of antigen specific 3, 4 or 5 function DP cells is plotted against the relative proliferation of DP cells in response to gag stimulation. B, in response to VVNRT stimulation.
Relationship between responses within the DP subset and the CD4+ and CD8+ T-cell subsets
It is not clear if DP cells leave the thymus as DP cells or if they are derived in the periphery from the CD4 and/or CD8 compartments. In order to gain some insight into the origins of the DP response, we also determined the degree to which the magnitude of HIV-1-specific DP responses resembled that found within either the CD4 or CD8 compartments. Within the acute cohort, the level of proliferated DP cells was directly related to the proliferation observed within the CD4 compartment following stimulation with each HIV antigen (Figure 5A and not shown). Furthermore, the magnitude of DP proliferation following stimulations with Gag, both Pol pools and VVNRT also correlated with the proliferation observed in the CD8 compartment (Figure 5A and not shown). Similarly, the controller DP proliferative response correlated with the CD4 response following stimulations with Gag and both Pol pools (Figure 5B and not shown). While the DP anti-Gag and VVNRT proliferative response correlated with the observed CD8 proliferation. Conversely, there were no significant correlations between the acute DP multifunctional response and the CD4 response. The magnitude of the acute anti-GP120 multifunctional DP response did correlate with the multifunctional frequencies observed in the CD8 compartment (Figure 5C and not shown). Meanwhile, the controller DP multifunctional responses did not correlate with any CD4 or CD8 multifunctional responses (Figure 5D and not shown). The p-values obtained from each of these comparisons are summarized in Supplemental Table 1.
Figure 5. CD4 or CD8 Responses vs. DP Responses.

A, The antigen specific relative proliferation in the CD4 or CD8 compartment for each patient is plotted against that patient’s antigen specific relative proliferation in the DP compartment for patients in the acute cohort. B, for patients in the controller cohort. C, The antigen specific frequency of 3, 4 or 5 function CD4 or CD8 cells is plotted against that patient’s antigen specific frequency of 3, 4 or 5 function DP cells for patients in the acute cohort. D, for patients in the controller cohort.
Discussion
Relative to the extensive analysis of the CD4+ and CD8+ T-cell compartments, the role of circulating DP cells in immune responses to pathogens and cancer has rarely been studied. As a result, their immune function is poorly understood. In this study, we present the first ever evidence of antigen-specific DP cells simultaneously producing 3 or more functions.
Previously, a cohort of patients recently infected with HIV-1 was shown to contain DP cells which produced IFN-γ alone or in combination with either IL-2 or expression of CD107a in response to HIV(21). This work was performed at the level of the entire HIV proteome, leaving the antigen-specific breadth of DP cells unknown. Additionally, we present the first ever examination of HIV-specific DP cells within patients who naturally control HIV-1 infection. In this study, we sought to determine the antigen specificity of DP cells and if DP cells were capable of mounting a wide variety of response modalities (CD107a, IFN-γ, IL-2, MIP-1β, and/or perforin as well as proliferation) or were restricted to a narrow range of response types. This was done using peptide pools representing the majority of the HIV genome to stimulate cells isolated from acutely infected HIV patients. This study has shown that within these patients, DP cells represent an immune subset which is capable of proliferation and mounting a multifunctional response. We show for the first time that antigen-specific DP cells are capable of expressing 3 or more functions at a time. In fact, within the acute cohort studied here, anti-HIV T-cells expressing all 5 functions were almost exclusively DP cells (data not shown). Moreover, the multifunctional DP cells from the acute cohort were primarily focused on the VVNRT peptide pool, while the controller multifunctional DP cells were less focused in their antigen specificity.
The presence of multifunctional cells within the CD8 compartment during chronic infection has previously been shown to correlate with improved disease outcomes(22). Therefore, it will be important to track the DP response in treatment naïve patients to determine if multifunctional DP cells are as protective as multifunctional CD8 cells. Interestingly, the presence of multifunctionality in the DP population did not correlate with multifunctionality in the CD8 compartment. Our previous findings have shown that it may take as long as 55 weeks to develop significant multifunctional CD8 responses(34). As a result, it is unclear if there is truly a lack of a relationship between DP and CD8 multifunctionality or if multifunctionality within the DP compartment may precede development of CD8 multifunctionality. Nonetheless, within our limited cohort of controllers there was a correlation between the frequency of multifunctional DP cells and lower viral loads at the time of sample acquisition. This suggests that multifunctional DP cells may have an impact on the successful control of virus replication.
We have shown that HIV-specific DP cells display both proliferative and multifunctional capabilities. These two qualitative aspects of the anti-HIV immune response have previously been correlated with improved disease outcomes when examined within other T-cell compartments. It is important to note that the samples in this study were obtained while the patients’ immune response would be expected to establish a partial control of viral replication toward the viral set point. Therefore, the DP responses described here are temporally associated with an expected decline in viral load and consequently may be important mediators of the temporary viral control experienced late in acute infection. For that reason, it will be important to isolate HIV-specific DP cells in order to test their ability to inhibit HIV in vitro either through direct inhibition of viral replication, by cytotoxic killing of HIV infected cells and/or stimulation of antibody dependent cellular cytotoxicity. Additionally, recent improvements in humanized mouse models may allow the selective addition and subtraction of DP cells in order to determine the role DP cells may play in the initial decline in viral loads following acute infection(35–38). If DP cells are able to not just respond to HIV antigens but in fact inhibit HIV, then it is important to note that a therapeutic vaccine trial has shown the ability to significantly increase HIV-specific IFN-γ production by DP cells in chronically infected patients with a single intramuscular injection(39). This vaccine trial was able to demonstrate that not only are HIV-specific DP cells present in chronic infection, but that memory T-cell responses result in the expansion of HIV-specific DP cells in addition to traditional CD4 and CD8 expansion. Therefore, DP cells may need to be considered in the development of therapeutic HIV vaccines. Additionally, Pahar and colleagues have shown that DP cells are highly enriched in the gut, which is an important target site for protective HIV vaccine development(13). Our study revealed that the circulating HIV-specific multifunctional DP population generally lacks expression of CD57 and is therefore not terminally differentiated. Thus, it may be possible to generate a long lasting protective DP memory pool. Unfortunately, Brenchley et.al. have shown that CD57− memory T-cells preferentially harbor HIV(18). Therefore, the DP cells we may want to elicit for protective immunity may also be the subset of DP cells HIV prefers to infect. As a result, extensive examination of dynamics between DP mediated anti-HIV responses and infectability may be necessary.
Given previous work showing that DP cells are generally highly differentiated, our observation that they show evidence of proliferation following 6 days raises important questions about DP cell origins, at least in the HIV-1 infection model. Since a portion of DP cells resemble central memory T-cells or even naïve T-cells, it is possible that the DP population is a self-sustaining population(21). Alternatively, a portion of single positive CD4 and/or CD8 T-cells could transition to DP status following antigenic activation and differentiation. In fact, previous work has shown that heavy stimulation (CD3/28, SEB, etc.) of CD8 T-cells sustained over multiple days causes dim expression of CD4 (16, 40–41). We observed CD4bright in addition to CD4dim DP cells within both stimulated and unstimulated conditions, therefore sustained strong stimulation of CD8 cells is unlikely to explain the origin of the DP cells described in this study. Additionally, DP cells were also present and highly active in the ICS assay for which stimulations only lasted 6 hours rather than multiple days as in the above studies. If the HIV-specific DP cells we observe are originating from a single positive population, then the strong correlation between proliferated CD4 and DP cells means that it is likely that the CD4 compartment would be the primary source. Similarly, Colombatti and colleagues’ work showing that DP cells show greater clonal similarity with CD4 cells than CD8 cells further supports this hypothesis(42).
In summary, we have demonstrated that DP cells are capable of mounting a large and highly diversified response to HIV antigens. Additionally, the DP response is maintained in viral controllers and similar responses within the CD4 and CD8 compartments have previously been correlated with improved disease outcomes. Therefore, it is important that this study form the basis of further work delineating the effect this response has on viral replication and long term disease outcomes. Furthermore, these DP cells may be an important vaccine target, which means we must work to more fully understand DP cell origins and development. In total, we have established DP cells as a major responding population to acute HIV infection and established a basis for extensive exploration of their origins and role in successfully combating HIV and other pathogens.
Supplementary Material
Acknowledgements
We thank Susan Stager, Drs. Sunita Patel, Stephanie Freel and Coleen Cunningham, the staff and patients of the Duke and UNC Infectious Diseases Clinics.
Grant Support:
This work was supported by NIH grants R01AI050483 and R01AI052279. We would like to acknowledge the internal support of the Molecular Virology and Clinical Cores of the Duke University Center for AIDS Research (CFAR: NIH funded program 2P30 AI064518).
Abbreviations
- DP
CD4+CD8+ T cells
References
- 1.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11(8):666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez E, et al. Rat Peripheral CD4+CD8+ T Lymphocytes Are Partially Immunocompetent Thymus-Derived Cells That Undergo Post-Thymic Maturation to Become Functionally Mature CD4+ T Lymphocytes. The Journal of Immunology. 2002;168(10):5005–5013. doi: 10.4049/jimmunol.168.10.5005. [DOI] [PubMed] [Google Scholar]
- 3.Blue M, et al. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. The Journal of Immunology. 1985;134(4):2281–2286. [PubMed] [Google Scholar]
- 4.Hernández J, et al. Comparative evaluation of the CD4+CD8+ and CD4+CD8− lymphocytes in the immune response to porcine rubulavirus. Veterinary Immunology and Immunopathology. 2001;79(3–4):249–259. doi: 10.1016/s0165-2427(01)00259-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhargava Periwal S, Cebra JJ. Respiratory Mucosal Immunization with Reovirus Serotype 1/L Stimulates Virus-Specific Humoral and Cellular Immune Responses, Including Double-Positive (CD4+/CD8+) T Cells. J. Virol. 1999;73(9):7633–7640. doi: 10.1128/jvi.73.9.7633-7640.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ober BT, et al. Vaccine-Induced, Pseudorabies Virus-Specific, Extrathymic CD4+CD8+ Memory T-Helper Cells in Swine. J. Virol. 1998;72(6):4866–4873. doi: 10.1128/jvi.72.6.4866-4873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam K-H, et al. Peripheral blood extrathymic CD4+CD8+ T cells with high cytotoxic activity are from the same lineage as CD4+CD8− T cells in cynomolgus monkeys. International Immunology. 2000;12(7):1095–1103. doi: 10.1093/intimm/12.7.1095. [DOI] [PubMed] [Google Scholar]
- 8.Sala P, et al. Persistent expansions of CD4+ CD8+ peripheral blood T cells. Blood. 1993;82(5):1546–1552. [PubMed] [Google Scholar]
- 9.Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmunity Reviews. 2004;3(3):215–220. doi: 10.1016/j.autrev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Bonomo A, Kehn P, Shevach E. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunity. The Journal of Immunology. 1994;152(4):1509–1514. [PubMed] [Google Scholar]
- 11.Nascimbeni M, Pol S, Saunier B. Distinct CD4<sup>+</sup>CD8<sup>+</sup> Double-Positive T Cells in the Blood and Liver of Patients during Chronic Hepatitis B and C. PLoS ONE. 2011;6(5):e20145. doi: 10.1371/journal.pone.0020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwatani Y, et al. Intrathyroidal lymphocyte subsets, including unusual CD4+ CD8+ cells and CD3loTCRαβ1o/-CD4−CD8−cells, in autoimmune thyroid disease. Clinical & Experimental Immunology. 1993;93(3):430–436. doi: 10.1111/j.1365-2249.1993.tb08196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. European Journal of Immunology. 2006;36(3):583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 14.Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Current HIV/AIDS Reports. 2007;4(1):10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- 15.Hughes GJ, et al. HIV-1-infected CD8+CD4+ T cells decay in vivo at a similar rate to infected CD4 T cells during HAART. AIDS. 2008;22(1):57–65. doi: 10.1097/QAD.0b013e3282f151b9. 10.1097/QAD.0b013e3282f151b9. [DOI] [PubMed] [Google Scholar]
- 16.Kitchen SG, et al. Costimulation of Naive CD8+ Lymphocytes Induces CD4 Expression and Allows Human Immunodeficiency Virus Type 1 Infection. J. Virol. 1998;72(11):9054–9060. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochrane A, et al. High Levels of Human Immunodeficiency Virus Infection of CD8 Lymphocytes Expressing CD4 In Vivo. J. Virol. 2004;78(18):9862–9871. doi: 10.1128/JVI.78.18.9862-9871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley JM, et al. T-Cell Subsets That Harbor Human Immunodeficiency Virus (HIV) In Vivo: Implications for HIV Pathogenesis. J. Virol. 2004;78(3):1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imlach S, et al. Activated Peripheral CD8 Lymphocytes Express CD4 In Vivo and Are Targets for Infection by Human Immunodeficiency Virus Type 1. J. Virol. 2001;75(23):11555–11564. doi: 10.1128/JVI.75.23.11555-11564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho DD. Viral Counts Count in HIV Infection. Science. 1996;272(5265):1124–1125. doi: 10.1126/science.272.5265.1124. [DOI] [PubMed] [Google Scholar]
- 21.Howe R, et al. Phenotypic and Functional Characterization of HIV-1-Specific CD4+CD8+ Double-Positive T Cells in Early and Chronic HIV-1 Infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;50(5):444–456. doi: 10.1097/qai.0b013e31819aa8c4. 10.1097/QAI.0b013e31819aa8c4. [DOI] [PubMed] [Google Scholar]
- 22.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson NA, et al. Vaccine-Induced Cellular Responses Control Simian Immunodeficiency Virus Replication after Heterologous Challenge. J. Virol. 2009;83(13):6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen SG, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15(3):293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannanganat S, et al. Human Immunodeficiency Virus Type 1 Controllers but Not Noncontrollers Maintain CD4 T Cells Coexpressing Three Cytokines. J. Virol. 2007;81(21):12071–12076. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferre AL, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113(17):3978–3989. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg ES, et al. Vigorous HIV-1-Specific CD4+ T Cell Responses Associated with Control of Viremia. Science. 1997;278(5342):1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 28.Lichterfeld M, et al. Loss of HIV-1-specific CD8+ T Cell Proliferation after Acute HIV-1 Infection and Restoration by Vaccine-induced HIV-1-specific CD4+ T Cells. The Journal of Experimental Medicine. 2004;200(6):701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gay C, et al. Cross-Sectional Detection of Acute HIV Infection: Timing of Transmission, Inflammation and Antiretroviral Therapy. PLoS ONE. 2011;6(5):e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freel SA, et al. Phenotypic and Functional Profile of HIV-Inhibitory CD8 T Cells Elicited by Natural Infection and Heterologous Prime/Boost Vaccination. J. Virol. 2010;84(10):4998–5006. doi: 10.1128/JVI.00138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders KO, et al. Epigenetic regulation of CD8+ T-lymphocyte mediated suppression of HIV-1 replication. Virology. 2010;405(1):234–242. doi: 10.1016/j.virol.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders KO, et al. Secretion of MIP-1(beta) and MIP-1(alpha) by CD8+ T-lymphocytes correlates with HIV-1 inhibition independent of coreceptor usage. Cellular Immunology. 2011;266(2):154–164. doi: 10.1016/j.cellimm.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. Journal of Immunological Methods. 1994;171(1):131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari G, et al. Relationship between Functional Profile of HIV-1 Specific CD8 T Cells and Epitope Variability with the Selection of Escape Mutants in Acute HIV-1 Infection. PLoS Pathog. 2011;7(2):e1001273. doi: 10.1371/journal.ppat.1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shacklett BL. Can the New Humanized Mouse Model Give HIV Research a Boost? PLoS Med. 2008;5(1):e13. doi: 10.1371/journal.pmed.0050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denton PW, et al. Antiretroviral Pre-exposure Prophylaxis Prevents Vaginal Transmission of HIV-1 in Humanized BLT Mice. PLoS Med. 2008;5(1):e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melkus MW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. The Journal of Experimental Medicine. 2007;204(4):705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suni MA, et al. CD4+CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. European Journal of Immunology. 2001;31(8):2512–2520. doi: 10.1002/1521-4141(200108)31:8<2512::aid-immu2512>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan YB, et al. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology. 2001;103(3):270–280. doi: 10.1046/j.1365-2567.2001.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flamand L, et al. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: Implications for HIV pathogenesis. Proceedings of the National Academy of Sciences. 1998;95(6):3111–3116. doi: 10.1073/pnas.95.6.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombatti A, et al. Age-Related Persistent Clonal Expansions of CD28-Cells: Phenotypic and Molecular TCR Analysis Reveals both CD4+and CD4+CD8+Cells with Identical CDR3 Sequences. Clinical Immunology and Immunopathology. 1998;89(1):61–70. doi: 10.1006/clin.1998.4580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.