Abstract
Background
Injury to peritubular capillaries and capillary basement membrane multilamination (PTCL) is a hallmark of antibody-mediated chronic renal allograft rejection. However, the predictive diagnostic value of PTCL is incompletely studied.
Methods
We analyzed the diagnostic significance of PTCL and propose diagnostic strategies. We evaluated 360 diagnostic native and 187 transplant kidney specimens by electron microscopy (terminology: PTCL-C, severe; PTCL subgroup C3, very severe multilamination; see Materials and Methods for definitions).
Results
PTCL was not pathognomonic for any specific disease. PTCL- C/C3 was rare in native kidneys (C, 6%; C3, 1 %), associated mainly with late thrombotic microangiopathy (C: 78%; C3: 11% of cases). In allografts, PTCL-C/C3 was significantly more common, especially in specimens more than 24 months after transplantation (C, 47%; C3, 31%). PTCL-C/C3 was found in acute (C, 20%; C3, 7%) and chronic T-cell rejection (C, 67%; C3,29%), calcineurin inhibitor toxicity (C, 36%; C3, 18%), or C4d+ specimens (C, 61%; C3, 50%) with odds ratios between 4 and 36. PTCL-C3 was more predominant in cases with antibody-mediated injury. Highest odds ratios (81–117) for PTCL-C/C3 were noted in combined injuries, that is, mixed chronic T-cell and concurrent chronic antibody–mediated rejection. Positive predictive values of PTCL-C and C3 are the following: all rejection types, 89% and 93%; all Banff chronic rejection types, 69% and 71%; and chronic presumptive antibody rejection, 37% and 49%, respectively. Corresponding negative predictive values of C and C3 for different Banff rejection categories are between 50% and 94%.
Conclusions
The presence of PTCL-C3 is a helpful adjunct finding to diagnose rejection-induced tissue injury but cannot precisely predict the Banff rejection category. Conversely, the absence of PTCL-C3 is helpful in excluding chronic, Banff category II antibody-mediated rejection.
Keywords: Basement membrane, Electron microscopy, Peritubular capillary, Rejection, Kidney transplantation
The diagnosis and classification of kidney allograft rejection depends heavily on changes seen by light microscopy. Ultrastructural lesions are not specifically detailed in the “Banff classification scheme,” and electron microscopy (EM) is only infrequently performed during the systematic evaluation of graft biopsies. EM can, however, show intriguing abnormalities, including glomerular capillary wall remodeling and peritubular capillary basement membrane multilaminations (PTCL) that are interpreted by some as signs of chronic antibody-mediated injury (1, 2).
Following the seminal descriptions by Monga et al. (3,4), subsequently Drachenberg et al. (5), Ivanyi et al. (6), and Gough et al. (1) further reported on PTCL. Unfortunately, nearly all studies on PTCL are limited by selected case series, lack of comparable scoring criteria or comparative data from native kidneys, and occasionally even lack of EM as an investigative tool (3, 7–12). In renal allografts, studies primarily focused on targeted correlations between PTCL and specific morphologic changes, such as transplant glomerulopathy (TGL) (1, 3–5, 7, 11, 13, 14) or C4d staining (8,9,11).
Our current EM-based study in for-cause renal specimens aims to define the diagnostic significance of PTCL in renal allografts by evaluating a large cohort of native and kidney transplant specimens. In a unique approach, we analyzed the relationship of PTCL to histologic changes, disease categories, selected demographic and laboratory parameters, and calculated odds ratios (ORs). We determined positive and negative predictive values of the most severe forms of PTCL for “Banff rejection categories.” We suggest guidelines for the incorporation of PTCL into the diagnostic evaluation of kidney transplant biopsies.
RESULTS
Forty-eight percent (265) of 547 specimens showed only circumferential multilayering in the three capillaries scored, 5% (26) revealed only segmental multilayering, and 47% (256) demonstrated a combination of segmental and circumferential laminations. Seventeen percent (90) of 547 specimens showed intracase variations of three or more basement membrane layers when comparing the most affected to the least affected capillary.
Interobserver Reproducibility
Two observers re-evaluated 150 capillaries to determine the reproducibility of PTCL counts. Unweighted κ Cohen statistics showed good agreement levels of 0.66 for observer 1 (95% confidence interval, 0.48 and 0.84) and 0.73 for observer 2 (95% confidence interval, 0.57 and 0.89) between original and PTCL recounts.
Native Kidneys
PTCL-A was found in 274 (76%) of 360 cases; PTCL-B, 63 (18%) of 360; PTCL-C, 23 (6%) of 360 (C1, 6 of 23 [26%]; C2, 13 of 23 [57%]; C3, 4 of 23 [17%]).
PTCL and patient demographics. PTCL group distributions were not significantly associated with age, race, or sex.
PTCL and disease categories (diabetes mellitus, arterionephrosclerosis, focal and segmental glomerulosclerosis, various immune complex-mediated glomerulonephritides, thrombotic microangiopathies [TMAs], ANCA-associated vascu-litides, acute tubular injury, pyelonephritis, and end-stage renal diseases). In native kidney specimens, PTCL-C was a rare finding (overall, 6%) with the exception of TMAs, in particular the late disease stages, which demonstrated a significantly higher prevalence of PTCL-C (14% in early and 78% in late TMA phases). PTCL-C3 was highly uncommon in all native disease categories (1%).
PTCL and selected morphologic changes. Advanced sclerotic changes grade II (global glomerulosclerosis, interstitial fibrosis/tubular atrophy, and arteriosclerosis and arteriolosclerosis) correlated significantly with PTCL-C (prevalence of PTCL-C in chronic changes grade I vs. grade II, 1%–4% vs. 10%–14%, respectively; all differences P<0.05) but not with subgroup PTCL-C3. No significant correlations were found between inflammatory changes, capillaritis, or endothelial cell activation and PTCL-C/C3.
Kidney Allografts
PTCL-A was found in 72 (38%) of 187 cases; PTCL-B, 48 (26%) of 187; PTCL-C, 67 (36%) of 187 (C1, 14 [21%] of 67; C2, 10 [15%] of 67; C3, 43 [64%] of 67).
In comparison with native specimens, transplants showed a right shift with significantly fewer cases in PTCL-A (P<0.001) and more in PTCL-B, C, and subgroup C3 (P<0.05, P<0.001, and P<0.01, respectively). The overall prevalence of PTCL-C of 36% in transplants was only paralleled in one native disease category: TMA (39% with PTCL-C, difference from transplant group not significant). Compared with native kidneys, PTCL-C and subgroup C3 were most typical for changes in allografts; therefore, these groups were used for subsequent statistical analyses.
Time of Biopsy After Transplantation
The degree of severe PTCL-C increased after transplantation: 5% (C3, 0%) at year 1, 29% (C3, 18%) at year 2, 36% (C3, 14%) at year 3, and 61% (C3, 48%) at more than 10 years.
Histologic Changes
Marked PTCL was significantly more common in cases with peritubular capillaritis grade II versus grade I (PTCL-C, 57% vs. 14%; PTCL-C3, 38% vs. 6%; P<0.0001). Ultrastructural capillaritis and endothelial cell activation correlated with PTCL-C (P<0.05) but not PTCL-C3. Grade II versus grade I sclerotic changes (global glomerulosclerosis and interstitial fibrosis/tubular atrophy) correlated significantly with PTCL-C and C3 (all P<0.05).
In comparison with early biopsies obtained during the first 12 months, PTCL-C/C3 increased significantly in specimens obtained after year 1 (P=0.001). Severe PTCL was most prevalent in late biopsies with transplant glomerulopathy glomerulitis, C4d positivity, or transplant sclerosing vasculopathy. With the exception of recurrent diabetic nephropathy, marked PTCL was overall significantly less frequent in the comparative transplant control group (40/64 biopsies taken >1 year after grafting; PTCL-C, 5% ; and PTCL-C3, 2%) (Table 1).
TABLE 1.
Transplant biopsy specimens: PTCL groups C (1–3) and PTCL Subgroup C3 in histologic categoriesa
| Group C (1–3) |
Subgroup C3 |
|||
|---|---|---|---|---|
| Histologic Findings | n | C in total n, % | C3/C (1–3) | Subgroup C3 in group C (1–3), % |
| Tubulointerstitial rejectiona (n=70) | 38 | 54b | 24/38 | 63b |
| Transplant endarteritisa (n=8) | 3 | 38b | 2/3 | 67 |
| Transplant glomerulitisa (n=16) | 12 | 75b | 7/12 | 58b |
| All transplant glomerulopathya (n=50) | 32 | 64 | 24/32 | 75 |
| Global (n=38) | 29 | 76b | 22/29 | 76b |
| Segmental (n=12) | 3 | 25 | 2/3 | 67 |
| All transplant sclerosing vasculopathya (n=45) | 30 | 67b | 18/30 | 60b |
| Active with endarteritis (n=22) | 16 | 73b | 12/16 | 75b |
| Inactive without endarteritis (n=23) | 14 | 61b | 6/14 | 43b |
| Calcineurin inhibitor toxicity (n=11) | 4 | 36b | 2/4 | 50 (NS) |
| All comparative transplant control group (n=64) | 3 | 5 | 1/3 | 33 |
| Others (n=48) | 1 | 2 | 0/1 | 0 |
| Recurrence/de novo diseases (n=11) | 0 | 0 | 0/0 | 0 |
| Diabetes mellitus (n=5) | 2 | 40 | 1/2 | 50 |
| All C4d+a (n=56) | 34 | 61b | 28/34 | 82b |
| C4d+ diffuse (n=40) | 27 | 68b | 23/27 | 85b |
| C4d+ focal (n=16) | 7 | 44b | 5/7 | 71b |
| All C4d−a (n=127) | 31 | 24 | 13/31 | 41 |
| All biopsies ≤1 yra (n=38) | 2 | 5 | 0/2 | 0 |
| Tubulointerstitial rejection (n=8) | 0 | 0 | 0/0 | 0 |
| Transplant endarteritis without (n=2) | 0 | 0 | 0/0 | 0 |
| Transplant glomerulitis (n=l) | 0 | 0 | 0/0 | 0 |
| Transplant glomerulopathy (n=2) | 0 | 0 | 0/0 | 0 |
| Transplant sclerosing vasculopathy (n=3) | 1 | 33 | 0/1 | 0 |
| All C4d+ (n=9) | 0 | 0 | 0/0 | 0 |
| C4d+ diffuse (n=5) | 0 | 0 | 0/0 | 0 |
| C4d+ focal (n=4) | 0 | 0 | 0/0 | 0 |
| All C4d−(n=29) | 2 | 7 | 0/2 | 0 |
| All biopsies >1 yra (n=145) | 63 | 43 | 41/63 | 65 |
| Tubulointerstitial rejection (n=62) | 38 | 61 | 24/38 | 63 |
| Transplant endarteritis (n=6) | 3 | 50 | 2/3 | 67 |
| Transplant glomerulitis (n=15) | 12 | 80 | 7/12 | 58 |
| Transplant glomerulopathy (n=48) | 32 | 67 | 24/32 | 75 |
| Transplant sclerosing vasculopathy (n=42) | 29 | 69 | 18/29 | 62 |
| All C4d+ (n=47) | 34 | 72 | 28/34 | 82 |
| C4d+ diffuse (n=35) | 27 | 77 | 23/27 | 85 |
| C4d+ focal (n=12) | 7 | 58 | 5/7 | 71 |
| All C4d− (n=98) | 29 | 30 | 15/29 | 52 |
Cases can show more than one rejection-associated histologic change.
Comparison of PTCL-C1–3 and PTCL Subgroup C3 with transplant control group, P<0.05.
Specimens in the “comparative control group” and in the “calcineurin inhibitor toxic group” lack per definition any evidence of rejection or C4d positivity. No analysis performed in some groups because of either overlap with cases in control category or small case numbers.
PTCL, peritubular capillary basement membrane multilamination; NS, not significant.
Transplant Glomerulopathy
At time of initial biopsy diagnosis of TGL, 65% of biopsies showed PTCL-C; and 53%, C3. Marked PTCL was significantly more common in C4d+ (C, 81%; C3, 71%) compared with C4d− (C, 47%; C3, 32%; P<0.05) and in global (C, 77%; C3, 63%) compared with segmental TGL (C, 30%; C3, 20%; P<0.03).
TGL is a morphologic phenotype caused by glomerular capillary injury of different etiologies (15, 16). In a sub-analysis, we grouped TGL according to the presumptive underlying cause. Comparing rejection with nonrejection-induced TGL, all rejection cases, whether presumably cell or antibody mediated, were significantly more often global (84% vs. 44% in nonrejection TGL, P<0.03) than segmental. Rejection-associated TGL was more often associated with marked PTCL (C, 77%; C3, 65%) than nonrejection TGL (C, 22%; C3, 11%; P<0.01).
Diagnostic Categories
Biopsy-based approach. Table 2 groups all 183 biopsies with C4d data according to diagnostic categories (for diagnostic group I-IX definitions, see Table 2). Acute rejection defined as diagnostic groups I to III: PTCL-C in 20% to 44% of biopsies with either cellular or presumptive antibody-mediated rejection, PTCL-C3 in 7% to 33% (all differences for PTCL-C not significant). Chronic active rejection IV to VI: PTCL-C in 67% to 79% of biopsies with either cell or presumptive antibody–mediated chronic rejection (all differences not significant), PTCL-C3 in 29% (IV) to 67% (VI) (difference between IV and combined V VI, P<0.02). Chronic active IV to VI versus chronic inactive VII rejection: PTCL-C/C3 significantly more frequent in the combined chronic active (C, 73%; C3, 49%) than chronic inactive rejection groups (C, 38%; C3, 15%; P<0.04). Acute I to III versus corresponding chronic IV to VII rejection groups: less PTCL in combined acute groups (C, 33%; C3, 23%) than in combined chronic groups (C, 66%; C3, 43%; P=0.001 and P<0.04, respectively). Pronounced differences between group I (acute cellular) and IV (chronic active cellular rejection C, 20% vs. 67%, P=0.008; C3, 7% vs. 29%, not statistically analyzed because of small case numbers). All cellular I and IV versus all presumptive antibody–mediated II, III, V, and VI rejection groups: PTCL-C (49% vs. 61%, difference not significant). Significantly less PTCL-C3 in cellular (21%) versus presumptive antibody–mediated rejection (50%, P<0.01). Calcineurin inhibitor toxicity VIII versus acute I to III and chronic IV to VII rejection: PTCL-C and C3 in 36% and 18% of biopsies, respectively, with CNI toxicity, not significantly different from acute rejection groups I to III or chronic inactive rejection group VII. PTCL-C was significantly more frequent in the combined chronic active rejection groups IV to VI (73%, P<0.04; C3 not statistically analyzed because of small case numbers). Comparative control group IX: PTCL-C (5%) and PTCL-C3 (2%).
Patient-based approach: 144 patients with available data on the C4d staining profile were grouped into diagnostic categories according to histologic findings in their last available transplant biopsy, including historic C4d staining results from preceding biopsies in 48 of 144 patients. This approach accounted for possible transient historic antibody–mediated injury/C4d positivity that could have induced PTCL and may have been underestimated in the “biopsy-based approach” outlined previously. Fifty-one (35%) of 144 patients showed PTCL-C, and 35 (24%) of 144 patients showed PTCL-C3 in a distribution among diagnostic categories similar to that listed in Table 2. Seven of 51 patients with PTCL-C (C3, 3 of 35) showed no evidence/history of C4d positivity and no evidence/history of capillaritis, that is, no glomerulitis and no more than minimal peritubular capillaritis (grade I, data not shown).
TABLE 2.
Transplant biopsy specimens: PTCL groups C (1–3) and PTCL Subgroup C3 in diagnostic categories
| Group C (1–3) |
Subgroup C3 |
||||
|---|---|---|---|---|---|
| Diagnostic Categories in Biopsiesa | n=183 | n | C in total n, % | n | Subgroup C3 in group C (1–3), % |
| I. ACR C4d− | n=15 | 3 | 20 | 1/3 | 33 |
| II. Presumptive acute AMR C4d+ | n=7 | 2 | 29 | 2/2 | 100 |
| III. Mixed ACR and presumptive AMR C4d+ | n=18 | 8 | 44b | 6/8 | 75b |
| IV. Chronic active T-cell-mediated rejection, C4d− | n=24 | 16 | 67b | 7/16 | 44b |
| V. Chronic mixed active T-cell rejection and presumptive AMR, C4d+ |
n=19 | 15 | 79b | 12/15 | 80b |
| VI. Chronic active presumptive AMR, C4d+ | n=12 | 9 | 75b | 8/9 | 89b |
| VII. Chronic inactive rejection, C4d− | n=13 | 5 | 38b | 2/5 | 40 |
| VIII. Calcineurin inhibitor-induced toxicity | n=11 | 4 | 36b | 2/4 | 50 |
| IX. Comparative transplant control group | n=64 | 3 | 5 | 1/3 | 33 |
| X. Early rejection <1 yr after transplantation | n=14 | 1 | 7 | 0/1 | 0 |
| XI. Late rejection >1 yr after transplantation | n=94 | 57 | 61 | 38/59 | 64 |
Definitions are as follows:
I. ACR: tubulointerstitial rejection or endarteritis, C4d−, no chronic rejection (Banff category 4 types 1 and 2).
II. Presumptive acute AMR: C4d+ with tissue injury but without ACR and no chronic rejection (Banff category 2 types 1–3).
III. Mixed ACR and presumptive acute AMR, C4d+: changes of groups I and II combined.
IV. Chronic active T-cell–mediated rejection: ACR with transplant glomerulopathy with or without glomerulitis or transplant sclerosing vasculopathy, C4d− (Banff category 4, chronic rejection).
V. Chronic mixed active T-cell rejection and presumptive AMR: ACR with transplant glomerulopathy or transplant sclerosing vasculopathy, C4d+: changes of groups IV and VI combined.
VI. Chronic active presumptive AMR: presence of transplant glomerulopathy or transplant sclerosing vasculopathy, no ACR, C4d+ (Banff category 2, chronic rejection).
VII. Chronic inactive rejection: presence of transplant glomerulopathy or transplant sclerosing vasculopathy, no ACR, C4d− (no Banff designation).
VIII. Calcineurin inhibitor-induced toxicity: calcineurin inhibitor-induced toxicity, no evidence of acute or chronic rejection, C4d−.
IX. Comparative transplant control group: No acute or chronic rejection, C4d−, various histologic changes including glomerulonephritides and diabetes mellitus.
X. The rejection group during the first year after transplantation included the following: ACR, n=2; AMR, n=3; Mixed ACR and AMR, n=5; Chronic mixed active T-cell rejection and AMR, n=l; Chronic inactive rejection, n=3.
XI. Late rejection included cases from groups I–VII.
In comparison with control group IX, P<0.05.
ACR, acute cellular rejection; AMR, antibody–mediated rejection; PTCL, peritubular capillary basement membrane multilamination.
Donor-specific Antibodies
Donor-specific antibodies (DSAs) were checked in 42 of 144 patients at 54 of 183 biopsy time points (DSA positivity, 20/42 patients at 25/54 biopsy time points/samples). DSA positivity was associated in 48% (12/25 samples) with PTCL-C and 40% (10/25) with PTCL-C3. DSA negativity was associated in 28% (8/29) with PTCL-C and 17% (5/29) with PTCL-C3 (all differences not significant). Eighteen (72%) of 25 DSA+ biopsies were additionally C4d+ (PTCL-C, 9 [50%] of 18; PTCL-C3, 7 [39%] of 18). In comparison, 23 (80%) of 29 DSA− biopsies were also C4d− (PTCL-C, 5 [22%] of 23; C3, 2 [9%] of 23; difference between DSA+/ C4d+ and DSA /C4d groups: PTCL-C, not significant; PTCL-C3, P<0.03).
Logistic Regression
Both positivity for C4d staining and a biopsy more than 24 months after grafting significantly increased the OR of PTCL-C and PTCL-C3.
In a subanalysis of categorical diagnoses using the control group as a reference and adjusting for time, all diagnostic categories correlated significantly with PTCL-C and C3. The likelihood of PTCL-C/C3 was lowest in cases with acute rejection or calcineurin inhibitor–induced toxicity and highest in cases with mixed T-cell and presumptive antibody-mediated chronic rejection (Table 3).
TABLE 3.
Logistic regression for PTCL-C1–3 and PTCL Subgroup C3
| A. Odds Ratios Dependent on the Following Features Indicated | ||||||
|---|---|---|---|---|---|---|
| PTCL-C1–3 |
PTCL Subgroup C3 |
|||||
| Histologic Findings | OR | CI | p-value | OR | CI | p-value |
| C4d+ | 3.7 | 1.4–9.6 | 0.007 | 7.8 | 2.7–22.6 | <0.001 |
| Time after transplantation >24 mo | 3.8 | 1.5–9.6 | 0.004 | 3.7 | 1.2–11.5 | 0.03 |
| Transplant glomerulopathy | 2.4 | 1–5.9 | NS | 4.2 | 1.5–11.4 | 0.005 |
| Transplant sclerosing vasculopathy | 4.3 | 1.5–12.1 | 0.006 | 2.4 | 0.7–7.6 | NS |
| Control | 0.2 | 0.06–1.1 | NS | 0.3 | 0.03–2.4 | NS |
| B. ORs with control group as reference adjusted for time after transplantation | ||||||
| Biopsy diagnosis | ||||||
| Control group (reference value) | 1.00 | (ref) | (ref) | 1.00 | (ref) | (ref) |
| ACR | 6.2 | 1–37.5 | 0.05 | a | a | a |
| Presumptive acute AMR C4d+ | 9.9 | 1.1–89.1 | 0.04 | a | a | a |
| Mixed ACR and presumptive AMR C4d+ | 27 | 5.4–133.6 | <0.001 | 56 | 5.5–569.7 | 0.001 |
| Chronic active T-cell-mediated rejection C4d | 36 | 8–161 | <0.001 | 20.7 | 2.3–188.9 | 0.007 |
| Chronic mixed active T-cell rejection and presumptive AMR C4d+ | 81 | 15.2–431.2 | <0.001 | 117 | 12.3–1118.9 | <0.001 |
| Chronic active presumptive AMR C4d+ | 40.5 | 6.3–261.8 | <0.001 | 81.3 | 7.2–914.6 | <0.001 |
| Chronic inactive rejection C4d | 15.6 | 2.8–85.7 | 0.002 | a | a | a |
| Calcineurin inhibitor-induced toxicity | 8.4 | 1.4–50.5 | 0.02 | a | a | a |
Analyses were not performed because of the small case numbers.
ACR, acute cellular rejection; AMR, antibody–mediated rejection; CI, confidence interval; PTCL, peritubular capillary basement membrane multi-lamination; OR, odds ratio; NS, not significant.
PTCL and Predictive Diagnostic Values
When PTCL-C (or subgroup C3) was present, samples were obtained more than 1 year after transplantation in 97% of cases (C3, 100%), showed associated elevated DSAs in 60% (C3, 67%), showed C4d positivity in 52% (C3, 68%), and showed TGL in 49% (C3, 59%). Biopsies with PTCL-C or subgroup C3 showed chronic active rejection with C4d positivity in 37% (C3,49%), chronic active T-cell–mediated rejection in 25% (C3, 17%), acute rejection with C4d positivity in 15% (C3, 20%), chronic inactive rejection in 8% (C3, 5%), calcineurin inhibitor-induced toxicity in 6% (C3, 5%), acute T-cell–mediated rejection in 5% (C3, 2%), and other diagnoses (such as glomerular diseases or infections) in 4% (C3,2%). Table 4 summarizes predictive values of PTCL-C and C3 for different rejection categories.
TABLE 4.
Predictive value of PTCL
| All Rejection Typesa |
All Presumptive Antibody–mediated RejectionbC4d+ (acute/chronic) |
Chronic Rejectionc (all types) |
Chronic Active T-cell–mediated Rejectiond |
Chronic Active Presumptive Antibody-mediated Rejectione C4d+ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTCL | Present | Absent | Present | Absent | Present | Absent | Present | Absent | Present | Absent | ||||||
| PTCL-C1–3 present | 58 | 7 | 34 | 31 | 45 | 20 | 16 | 49 | 24 | 41 | ||||||
| PTCL-C1–3 absent | 50 | 68 | 22 | 96 | 23 | 95 | 8 | 110 | 7 | 111 | ||||||
| Sensitivity, % | 54 | 61 | 66 | 67 | 77 | |||||||||||
| Specificity, % | 91 | 76 | 83 | 69 | 73 | |||||||||||
| PPV, % | 89 | 52 | 69 | 25 | 37 | |||||||||||
| NPV, % | 58 | 81 | 81 | 93 | 94 | |||||||||||
| PTCL subgroup C3 present | 38 | 3 | 28 | 13 | 29 | 12 | 7 | 34 | 20 | 21 | ||||||
| PTCL subgroup C3 absent | 70 | 72 | 28 | 114 | 39 | 103 | 17 | 125 | 11 | 131 | ||||||
| Sensitivity, % | 35 | 50 | 43 | 29 | 65 | |||||||||||
| Specificity, % | 96 | 90 | 90 | 79 | 86 | |||||||||||
| PPV, % | 93 | 68 | 71 | 17 | 49 | |||||||||||
| NPV, % | 51 | 80 | 73 | 88 | 92 | |||||||||||
See definitions in Table 4 (combined groups I, II, III, IV, V, VI, VII).
See definitions in Table 4 (combined groups II, III, V, VI).
See definitions in Table 4 (combined groups IV, V, VI, VII).
See definitions in Table 4 (group IV).
See definitions in Table 4 (combined groups V, VI).
NPV, Negative predictive value; PPV, Positive predictive value; PTCL, peritubular capillary basement membrane multilamination.
DISCUSSION
Our study of native and renal transplant specimens is the largest most comprehensive analysis of the diagnostic significance of PTCL published to date. For the first time, PTCL categories are systematically studied in native and transplanted kidneys, results correlated with Banff rejection categories, ORs calculated, and positive and negative diagnostic predictive values defined. Our in-depth analysis sheds new light on the diagnostic significance of PTCL and highlights strategies for best interpretation during the evaluation of allograft biopsies.
PTCL is not a new observation. It was reported by Zollinger et al. (17) nearly 40 years ago and subsequently further studied by Monga et al. (3, 4, 13), Ivanyi et al. (2, 6, 18, 19), and Drachenberg et al. (5). With the recent growing interest in antibody-mediated rejection, the diagnostic significance of PTCL has been re-evaluated. Some authors reported a tight association between severe PTCL and DSA/C4d positivity in late graft biopsies (2, 9, 12, 14, 20–22). PTCL is part of the “Banff definition” of chronic antibody-mediated rejection (23).
Unfortunately, nearly all studies on PTCL thus far have been limited (2, 3, 7–12), and diagnostically useful threshold levels as well as the overall significance of PTCL still remain undetermined. For example, Banff ‘05 and ‘07 reports place emphasis on PTCL for the diagnosis of chronic antibody-mediated rejection without providing strict scoring guidelines (23, 24). The Edmonton group analyzing the impact of antibodies on kidney transplants chose to score findings in the single most affected capillary (25) and, in one recent publication, considered cases with greater than 5 PTCL as “positive” (26). Regele and colleagues (9) using 5 or more circumferential PTCL layers in three capillaries as threshold in a selected cohort of late allograft biopsies found a strong association with the deposition of C4d and presumed antibody induced graft injury. In 2007, Lerut and colleagues (27) followed the original approach of Ivanyi et al. (2, 6, 18, 19) (severe PTCL defined by ≥5 circumferential PTCL layers in 3 capillaries or ≥7 layers in 1 capillary) and failed to find a significant association between DSAs and PTCL. Thus the question, “what can the detection of PTCL really tell us?” remains.
We formed scoring categories based on previous reports (5, 6). We focused on “the Ivanyi” severe PTCL group (termed in our analysis PTCL-C1–3) and additionally studied a new subgroup of very severe PTCL (named subgroup C3 in this report). We showed that PTCL developed in the setting of extended endothelial activation and represented a general mechanism of tissue remodeling that spanned from minimal to marked. In our cohort, PTCL, including the severe and very severe subgroups, did not correlate with patient age, race, or gender, and it was not pathognomonic for any specific type of kidney disease/rejection type. In native kidney specimens, chronic injury with sclerosis was significantly associated with PTCL-C1–3 but not with the very severe PTCL subgroup C3. The degree of multilamination was dependent on the type and duration of the underlying endothelial stimulus. Thus, the most severe forms of PTCL were overall rare in native kidneys and significantly more common in renal allografts after year 1, in particular the very severe PTCL subgroup C3. This observation, to us, suggests that the PTCL subgroup C3 is an important diagnostic category to consider in the evaluation of transplant biopsies. For the first time, we showed that PTCL-C1–3 and subgroup C3 was seen in acute rejection episodes, both T cell and antibody mediated, and in cases with calcineurin inhibitor toxicity. Logistic regression identified graft age, a positive C4d staining status, the presence of transplant glomerulopathy, calcineurin inhibitor toxicity, or acute rejection as “mild risk factors” for severe capillary lamination. The prevalence of PTCL-C/C3 and ORs increased with chronic injury, such as chronic T-cell rejection (Banff category 4). The group with “greatest protracted capillary injury,” that is, cases with mixed chronic T-cell and concurrent presumptive chronic antibody-mediated (Banff category 2) rejection, carried by far the highest OR of very severe PTCL subgroup C3. In general, C4d positivity or the presence of DSAs served as a “PTCL enhancer.” In our study cohort, C4d+ biopsies with confirmed concurrent elevated DSA titers showed PTCL-C1–3 and PTCL subgroup C3 in overall 50% and 39% of cases, respectively. In comparison, biopsies without evidence of circulating DSAs, that is, C4d− and DSA−, showed PTCL-C1–3 and PTCL subgroup C3 in overall 22% and in 9% of cases, respectively. Thus, “antibodies”, while not the only promoters, are definitely strong promoters of PTCL and, in particular, PTCL subgroup C3. This effect becomes most apparent in the setting of chronic allograft injury (Fig. 1), although the prevalence of PTCL-C1–3 or subgroup C3—even in chronic antibody-mediated rejection—never reached 100%. Considering our findings, it is not surprising that neither severe PTCL-C1–3 nor the very severe PTCL subgroup C3 showed high sensitivities for various types of rejection. Overall, we found PTCL subgroup C3 to be most useful in the evaluation of transplant biopsies because only PTCL subgroup C3 correlated with chronic tissue injury and capillaritis in allografts, and it presented with the best positive and negative predictive values for (chronic) antibody-induced graft injury. Based on our overall study approach, our interpretations do not seem to be significantly limited by the relatively small number of patients with known DSA status.
FIGURE 1.
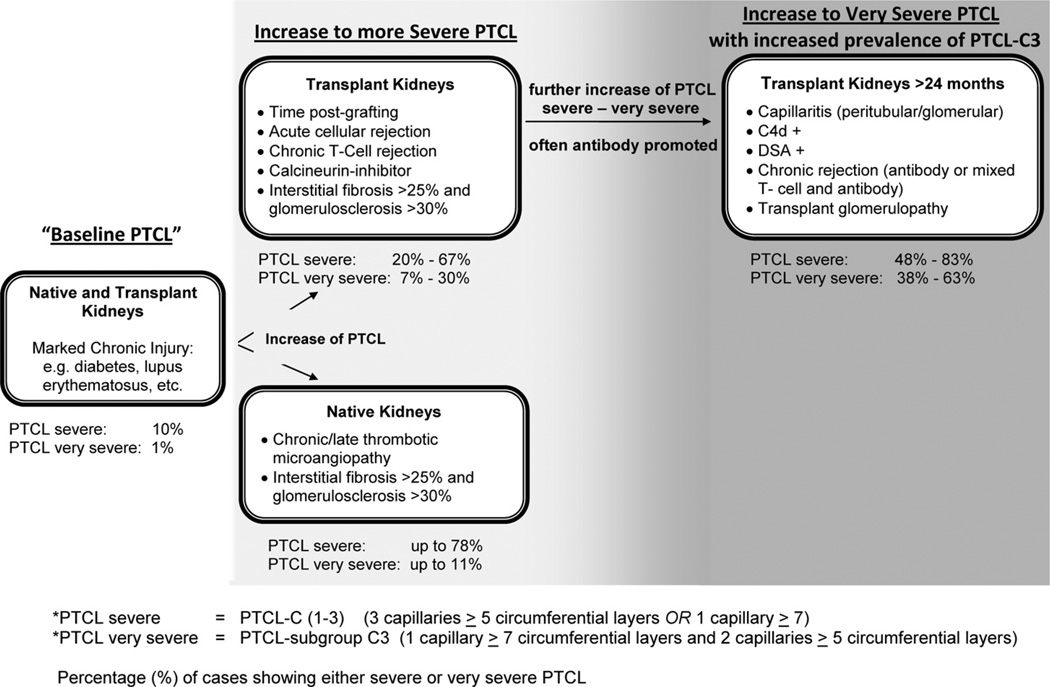
Schematic of changes associated with severe and very severe peritubular capillary basement membrane multilaminations (PTCL). Marked chronic kidney injury, such as seen in diabetes mellitus, can promote severe (so-called PTCL-C(1–3)) and very severe (so-called “PTCL-subgroup C3” in a minority of cases (listed as %). In allografts or native kidneys disease conditions with capillary injury can promote PTCL and the overall rate of severe basement membrane multilaminations increases. Very severe, so-called PTCL-subgroup C3, is primarily seen in renal allografts with protracted, often antibody induced injury that serves as general “PTCL amplifier”.
We conclude that EM and the search for very severe PTCL subgroup C3 is a useful adjunct method in the evaluation of allograft biopsies, in particular after transplantation year 1. We suggest using stricter threshold levels, that is, our PTCL subgroup C3 criteria, for diagnostic purposes. In order to properly identify representative cortical zones without severe scarring or necrosis, it is important to initially scan cases at low magnification (×2500). In appropriate regions, 15–20 peritubular capillaries should be further examined at higher magnification (× 5000–7000), and the three most affected capillaries should be used for scoring, that is, a “three-step EM approach.” The presence of very severe PTCL subgroup C3 is helpful with establishing a diagnosis of rejection-induced injury, possibly antibody mediated and chronic. The absence of very severe PTCL subgroup C3 is a strong indicator to exclude any (chronic) antibody-mediated rejection. Thus, recommendations on the interpretation of very severe PTCL in the evaluation of allograft biopsies should include information on both positive and negative predictive values. Per case, the EM examination of PTCL takes approximately 5 to 10 min, and the subsequent analysis of selected images including PTCL counts approximately 3 min. We believe that scoring attempts based on the evaluation of less than 3 capillaries with less than 5–7 circumferential basement membrane layers each are ill suited for diagnostic purposes, in particular, to evaluate potential antibody induced injury.
Some previous reports suggested that transplant glomerulopathy and PTCL are concurrent findings (21, 24). However, we observed that overall, only half of our cases with PTCL-C or C3 also showed TGL. In particular, severe PTCL was uncommon in biopsies with Banff cg scores 1 and 2, especially in C4d− cases. This finding is in contrast to a previous report (25). Our data illustrate that the pathogenesis of capillary wall remodeling in different tissue compartments is complex and not uniform, even in the presence of antibodies.
For the first time, we report that severe and very severe PTCL can not only be seen in chronic allograft injury, including chronic T-cell–mediated rejection, but also in Banff acute rejection episodes. This finding raises an additional question: What morphologic changes should be used to define chronic rejection? Considering our findings and other recently reported ultrastructural observations (21, 28), currently used light microscopic criteria to mark chronic rejection may be too restrictive, and it seems that EM can help to identify early chronic changes that could potentially benefit from therapy and might even be reversible in some cases (28). Thus, EM studies could help us to revisit current Banff rejection categories.
MATERIALS AND METHODS
This retrospective study was approved by the University of North Carolina institutional review board. Between May 2004 and February 2009, 547 diagnostic renal biopsies and nephrectomy specimens from 507 patients were analyzed: 357 biopsies and 3 nephrectomies from 360 patients with native kidney diseases; 178 allograft biopsies and 9 transplant nephrectomies from 147 transplant recipients. Selected clinical data were obtained from the hospital database: age, sex, race, circulating donor-specific antibodies at time of biopsy from a subgroup of patients, and allograft biopsy diagnoses before May 2004.
Histologic Analysis
All biopsies sufficed for rendering a diagnosis. Sections were prepared for light, immunofluorescence, and EM according to standard protocols. C4d accumulation was evaluated using Banff ‘07 criteria (by immunofluorescence staining in 176 and immunohistochemistry in 7 cases) (23); four cases without C4d data were excluded from some analyses. Diffuse and focal C4d staining was grouped as “positive” in some analyses.
Scoring of Lesions
Lesions were categorized according to published criteria (15,16,23, 29–32). Thrombotic microangiopathy (mainly seen in malignant hypertension and atypical hemolytic-uremic syndromes) were grouped into early exudative and late remodeling phases. Light microscopic scoring was only performed in biopsies containing 10 or more glomeruli or adequate vascular cross sections. In allografts, scoring focused on interstitial fibrosis/tubular atrophy, global glomerulosclerosis, peritubular capillaritis, and TGL (see following).
-
Light microscopy
Interstitial fibrosis/tubular atrophy, arteriosclerosis, arteriolosclerosis, interstitial inflammation, and peritubular capillaritis were graded according to Banff definitions (grade I in our system combines corresponding Banff lesion scores 0 and 1; grade II in our system combines corresponding Banff lesion scores 2 and 3) (23, 32, 33). Transplant glomerulopathy was graded as segmental (combined Banff’97 cgl and cg2 scores) or global (Banff’97 cg3 score) (32). Global glomerulosclerosis was graded as grade I (≤30%); grade II (>30% of glomeruli).
-
Electron microscopy
-
PTCL: Cases were initially scanned at low magnification (×2500) to find representative cortical regions without severe scarring, necrosis, or marked tubular atrophy. In these regions, 15–20 capillaries were examined at higher magnification (× 5000–6300) to identify the three most affected vessels that formed the basis for PTCL scoring modeled after previous reports (5, 6). Segmental PTCL was defined as two or more basement membrane layers/laminations involving less than 50% of a capillary circumference. Circumferential PTCL showed two or more laminations involving 50% or more of a capillary circumference. Basement membrane layers were counted by two observers. Consensus results formed the basis of the PTCL subgrouping.
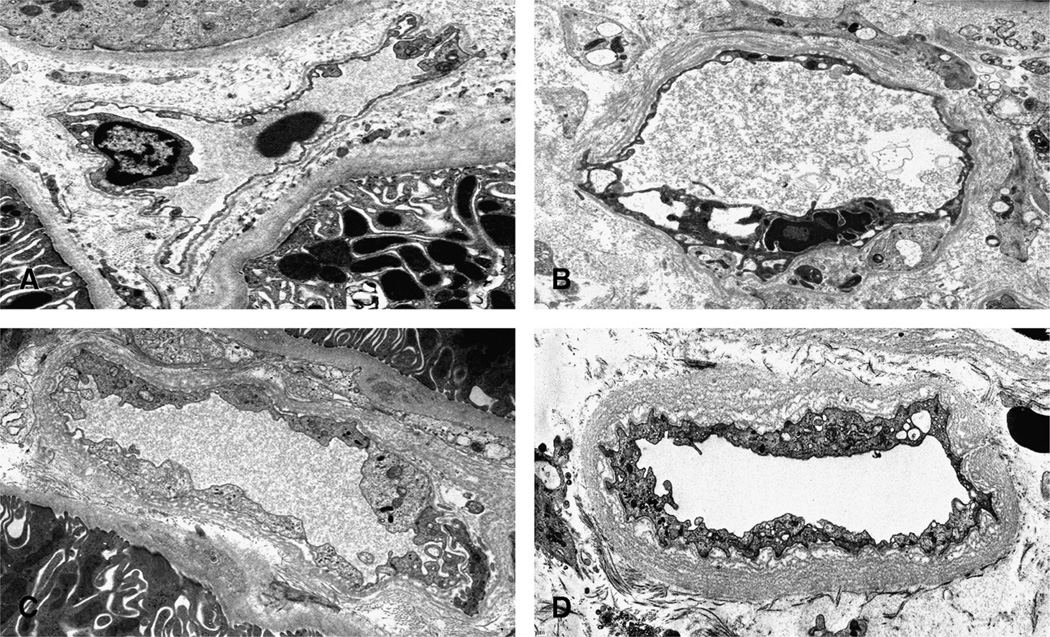
Group A, mild PTCL: three circumferential basement membrane layers or less in all three capillaries (Fig. 2) or any degree of segmental lamination.
Group B, moderate PTCL: four or more but less than seven circumferential layers in one or two capillaries. Any segmental or four circumferential laminations or less in the remaining one or two capillaries (Fig. 2).
Group C, severe PTCL subgroup C1: five or six circumferential layers in three capillaries. Subgroup C2: seven circumferential layers or more in one capillary, five circumferential layers or less or any degree of segmental layering in the second capillary, and any layering in the third capillary. Subgroup C3: seven circumferential layers or more (Fig. 2) in one capillary and five circumferential layers or more in the remaining two capillaries.
The three capillaries used for PTCL scoring were additionally analyzed.
“Ultrastructural capillaritis,” one inflammatory cell or more in one capillary or more.
“Ultrastructural endothelial cell activation,” two endothelial cell nuclei or more in one peritubular capillary cross section or more.
-
FIGURE 2.
Electron microscopy illustrating PTCL using uranyl acetate stains. A, Normal peritubular capillary with one circumferential basement membrane layer (original magnification 6300). B, Segmental peritubular capillary multilayering (original magnification 6300). C, Moderate circumferential peritubular capillary multilayering with four layers (original magnification 8000). D, Severe circumferential peritubular capillary multilayering. The consensus PTCL score for this case was 12 layers (original magnification 6300).
Cross-Matching and DSA Determination
One hundred forty-six of 147 patients received ABO-compatible transplants; all patients were transplanted after a negative T-cell and B-cell flow cytometry crossmatch.
In selected cases, clinical suspicion of antibody-mediated rejection triggered testing for DSAs (42 patients at 54 biopsy time points). Alloantibody profiles were assessed by solid phase testing with flow cytometry or multiplex bead arrays. Sera were screened for class I and II human leukocyte antigen antibodies, and positive cases were further analyzed to determine antibody specificities.
Data Analysis
Data analysis primarily focused on PTCL group C (composed of the three subgroups 1–3) and PTCL subgroup C3 (C3 with most pronounced basement membrane abnormalities). Cases were primarily categorized according to Banff’07 guidelines. C4d positivity resulted in a diagnosis of “presumptive antibody-mediated rejection” (23). Cases grouped as calcineurin inhibitor–induced toxicity by definition lacked evidence of rejection. In the transplant cohort, a comparative control group was defined: specimens with recurrent/de-novo renal disease, acute tubular injury, arterionephrosclerosis, infections, diabetes mellitus, but without signs of rejection or calcineurin inhibitor–induced toxicity. A subanalysis was performed on biopsies from patients with known DSA status.
Differences were analyzed by Fisher exact, Wilcoxon, and chi-square tests. To determine the relative contribution of various histologic findings to PTCL, logistic regression models were used with PTCL-group C as the outcome of interest. Interobserver agreement was determined by Cohen κ statistics.
Acknowledgments
This work was solely supported by departmental funds from the Department of Pathology and Laboratory Medicine at the University of North Carolina.
Footnotes
The authors declare no conflicts of interest.
All authors participated in making the study design, performing the experiment, and preparing the article.
REFERENCES
- 1.Gough J, Yilmaz A, Miskulin D, et al. Peritubular capillary basement membrane reduplication in allografts and native kidney disease: a clini-copathologic study of 278 consecutive renal specimens. Transplantation. 2001;71:1390. doi: 10.1097/00007890-200105270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ivanyi B, Kemeny E, Rago P, et al. Peritubular capillary basement membrane changes in chronic renal allograft rejection: Comparison of light microscopic and ultrastructural observations. Virchows Arch. 2011;459:321. doi: 10.1007/s00428-011-1114-x. [DOI] [PubMed] [Google Scholar]
- 3.Monga G, Mazzucco G, Messina M, et al. Intertubular capillary changes in kidney allografts: a morphologic investigation on 61 renal specimens. Mod Pathol. 1992;5:125. [PubMed] [Google Scholar]
- 4.Monga G, Mazzucco G, Novara R, et al. Intertubular capillary changes in kidney allografts: an ultrastructural study in patients with transplant glomerulopathy. Ultrastruct Pathol. 1990;14:201. doi: 10.3109/01913129009076124. [DOI] [PubMed] [Google Scholar]
- 5.Drachenberg CB, Steinberger E, Hoehn-Saric E, et al. Specificity of intertubular capillary changes: comparative ultrastructural studies in renal allografts and native kidneys. Ultrastruct Pathol. 1997;21:227. doi: 10.3109/01913129709021918. [DOI] [PubMed] [Google Scholar]
- 6.Ivanyi B, Fahmy H, Brown H, et al. Peritubular capillaries in chronic renal allograft rejection: a quantitative ultrastructural study. Hum Pathol. 2000;31:1129. doi: 10.1053/hupa.2000.16677. [DOI] [PubMed] [Google Scholar]
- 7.Morozumi K, Oikawa T, Fukuda M, et al. Electron-microscopic peritubular capillary lesion is a specific and useful diagnostic indicator for chronic rejection of renal allografts showing less specific morphologic lesions in the cyclosporine era. Transplant Proc. 1997;29:89. doi: 10.1016/s0041-1345(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Oikawa T, Koyama K, et al. Multilayering of peritubular capillary is a specific diagnostic criterion for immunologic chronic rejection: does a humoral factor contribute to the pathogenesis of peritubular capillary lesions in chronic rejection? Transplant Proc. 2000;32:306. doi: 10.1016/s0041-1345(99)00965-3. [DOI] [PubMed] [Google Scholar]
- 9.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 10.Kiyici H, Demirhan B, Ozdemir BH, et al. Significance of peritubular capillary basement membrane multilamellation in diagnosis of chronic allograft nephropathy. Transplant Proc. 2003;35:2643. doi: 10.1016/j.transproceed.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 11.Aita K, Yamaguchi Y, Horita S, et al. Thickening of the peritubular capillary basement membrane is a useful diagnostic marker of chronic rejection in renal allografts. Am J Transplant. 2007;7:923. doi: 10.1111/j.1600-6143.2006.01708.x. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with micro-vascular pathology and late graft failure. Am J Transplant. 2009;9:2532. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 13.Mazzucco G, Motta M, Segoloni G, et al. Intertubular capillary changes in the cortex and medulla of transplanted kidneys and their relationship with transplant glomerulopathy: an ultrastructural study of 12 transplantectomies. Ultrastruct Pathol. 1994;18:533. doi: 10.3109/01913129409021895. [DOI] [PubMed] [Google Scholar]
- 14.Vongwiwatana A, Gourishankar S, Campbell PM, et al. Peritubular capillary changes and C4d deposits are associated with transplant glomerulopathy but not IgA nephropathy. Am J Transplant. 2004;4:124. doi: 10.1046/j.1600-6143.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 15.Colvin RB, Nickeleit V. Renal transplant pathology. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. p. 1347. [Google Scholar]
- 16.Nickeleit V. The pathology of kidney transplantation. In: Ruiz P, editor. Transplantation Pathology. New York: Cambridge University Press; 2009. p. 45. [Google Scholar]
- 17.Zollinger HU, Moppert J, Thiel G, et al. Morphology and pathogenesis of glomerulopathy in cadaver kidney allografts treated with antilym-phocyte globulin. Curr Top Pathol. 1973;57:1. doi: 10.1007/978-3-642-65465-7_1. [DOI] [PubMed] [Google Scholar]
- 18.Ivanyi B. Transplant capillaropathy and transplant glomerulopathy: ultrastructural markers of chronic renal allograft rejection. Nephrol Dial Transplant. 2003;18:655. doi: 10.1093/ndt/gfg139. [DOI] [PubMed] [Google Scholar]
- 19.Ivanyi B, Kemeny E, Szederkenyi E, et al. The value of electron microscopy in the diagnosis of chronic renal allograft rejection. Mod Pathol. 2001;14:1200. doi: 10.1038/modpathol.3880461. [DOI] [PubMed] [Google Scholar]
- 20.Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 21.Wavamunno MD, O’Connell PJ, Vitalone M, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7:2757. doi: 10.1111/j.1600-6143.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 22.Liptak P, Kemeny E, Morvay Z, et al. Peritubular capillary damage in acute humoral rejection: an ultrastructural study on human renal allografts. Am J Transplant. 2005;5:2870. doi: 10.1111/j.1600-6143.2005.01102.x. [DOI] [PubMed] [Google Scholar]
- 23.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 24.Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 25.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7:1743. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 26.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 27.Lerut E, Naesens M, Kuypers DR, et al. Subclinical peritubular capillaritis at 3 months is associated with chronic rejection at 1 year. Transplantation. 2007;83:1416. doi: 10.1097/01.tp.0000266676.10550.70. [DOI] [PubMed] [Google Scholar]
- 28.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011;11:2123. doi: 10.1111/j.1600-6143.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 29.Sis B, Mengel M, Haas M, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 30.Mihatsch MJ, Nickeleit V, Gudat F. Morphologic criteria of chronic renal allograft rejection. Transplant Proc. 1999;31:1295. doi: 10.1016/s0041-1345(98)02003-x. [DOI] [PubMed] [Google Scholar]
- 31.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 32.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibson IW, Gwinner W, Brocker V, et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clini-copathological correlates. Am J Transplant. 2008;8:819. doi: 10.1111/j.1600-6143.2007.02137.x. [DOI] [PubMed] [Google Scholar]