Abstract
Objective
Cholesteryl ester transfer protein (CETP) and phospholipid transfer protein (PLTP) are two genetically-related plasma proteins involved in the exchange of cholesteryl esters and phospholipids between high-density lipoproteins (HDL) and other lipoproteins. Although low CETP and high PLTP activity both result in higher concentrations of plasma HDL-cholesterol (HDL-C), there is no evidence that either of these changes is associated with a decrease in cardiovascular disease (CVD) in a general population.
Methods
Plasma CETP and PLTP activities, measured by homogenous fluorometric assays using synthetic donor particle substrates, were related to the incidence of a first CVD event in Framingham Heart Study Offspring participants without CVD (n = 2679, mean age 59 y, 56% women) attending the 6th examination cycle (1995–98). Because of an effect modification by sex for both CETP and PLTP, analyzes were stratified by sex.
Results
During follow-up (mean 10.4 years) 187 participants experienced a first CVD event. In sex-specific Cox models, both CETP and PLTP as continuous and as binary variables were associated with significantly increased CVD in men, but not women. In men compared to a referent group with CETP ≥ median and PLTP < median, the multivariable-adjusted hazard ratio (HR) for new CVD events was significantly greater with either the combination of high CETP and high PLTP (HR 2.27, 95% CI 1.23–4.20); low CETP and low PLTP (HR 2.23, 95% CI 1.19–4.17); or low CETP and high PLTP (HR 2.85, 95% CI 1.53–5.31). In contrast, in women the multivariable-adjusted HR for new CVD events was non-significant and virtually equal to “1.0” with all combinations of high and low CETP or PLTP values.
Conclusions
Lower plasma CETP or higher PLTP activity was each associated with a significantly increased risk of CVD. Inexplicably, the increase in CVD associated with both lipid transfer proteins was confined to men.
Keywords: High density lipoproteins, Cholesteryl ester transfer protein, Phospholipid transfer protein
1. Introduction
Low plasma concentrations of high-density lipoprotein-cholesterol (HDL-C) have long been recognized as a major, independent cardiovascular disease (CVD) risk factor. Two closely related plasma proteins, cholesteryl ester transfer protein (CETP) and phospholipid transfer protein (PLTP), are known to have a major and potentially favorable effect on the concentration and composition of plasma HDL particles. CETP catalyzes the transfer of cholesteryl esters from HDL to apolipoprotein (apo) B-containing plasma lipoproteins in exchange for triglycerides, and pharmacologic inhibition of CETP has been shown to result in a substantial increase in plasma HDL-C concentrations [1]. PLTP promotes both HDL formation and remodeling in the circulation [2] that in predominately preclinical studies is associated with an increase in plasma HDL concentrations [3] and the potential for enhanced HDL-mediated efflux of cholesterol from body stores.
At the present time there is no direct evidence from clinical studies to suggest that either low levels (or inhibition) of CETP activity or an increase in PLTP activity will result in a reduction of CVD events. Indeed, with respect to CETP, there have been three recent large population-wide studies [4–6] that have similarly reported that low levels of CETP activity or mass, which were associated with higher levels of plasma HDL-C, were not associated with a lower incidence of CVD. In addition, and irrespective of the toxicity associated with torcetrapib, both this first developed pharmacologic inhibitor of CETP and dalcetrapib, a second and nontoxic inhibitor of CETP, failed to reduce coronary events in large, controlled clinical trials in spite of substantial increases in plasma HDL-C [7,8].
Clinical evidence linking a change in PLTP activity to the occurrence of CVD is far more limited than for CETP. However, two modest-sized studies suggest that higher levels of PLTP activity, irrespective of a seemingly favorable increase in plasma HDL-C, are associated with an increase in cardiovascular events; in one study in people with coronary artery disease (CAD) treated with statins [9], in another in cases with CAD compared to controls without CAD [10]. More recently, a larger analysis in 3 European populations has found using a gene score based on several single nucleotide polymorphisms of PLTP associated with low PLTP activity, that CVD was lower with a lower overall PLTP gene score [11]. To our knowledge, the relation of plasma PLTP activity to CVD incidence in a general population has not been prospectively directly determined.
The current analysis was undertaken in the Framingham Heart Study (FHS) to determine in a large community-based setting, the consequences of different activity levels of plasma CETP and PLTP alone and together for the development of a CVD event.
2. Methods
2.1. Study sample
The design and selection criteria of the Framingham Offspring Study have been previously detailed [12]. Participants in this second FHS generation are evaluated at the Heart Study clinic approximately every four years starting in 1971. At each Heart Study examination, attendees undergo laboratory testing for CVD risk factors, and a standardized medical history and physical examination targeted at detection of CVD. For the present investigation, we included attendees at the sixth examination cycle (1995–1998) with both plasma CETP and PLTP activity measured. Of 3532 individuals attending this examination, we excluded 853 individuals hierarchically for the following reasons: prevalent CVD (n = 421), plasma CETP or PLTP activity not measured (n = 428), or plasma triglycerides that were in a distinctly outlier range of greater than 800 mg/dL (n = 4). After these exclusions, 2679 participants remained eligible for analyzes. All participants provided written informed consent, and the study was approved by the Institutional Review Board at the Boston University Medical Center.
2.2. Plasma CETP and PLTP activity assays
All attendees at the sixth examination cycle underwent phlebotomy after an overnight fast and plasma was separated with centrifugation and frozen at −80 °C until assayed. Plasma CETP and PLTP activities were measured by Roar Biomedical (New York, NY) using commercially available fluorometric assay procedures that are described in detail in Supplement 1. In brief, the CETP assay uses a synthetic fluorescent CE donor particle and apo-B-containing lipoprotein acceptor particles. CETP-mediated transfer is determined by an increase in fluorescent intensity in the acceptor. The plasma PLTP assay uses a fluorescent phospholipid donor and a synthetic acceptor and, again, PLTP-mediated transfer is measured by an increase in fluorescent intensity. Intra- and inter-assay coefficients of variation for both assays ranged from 12 to 15%. Plasma total cholesterol, HDL-C, triglyceride, and C-reactive protein (CRP) concentrations were measured using automated, standardized assays and low-density lipoprotein cholesterol (LDL-C) was calculated.
2.3. Definitions of CVD events
All FHS participants are under continuous surveillance for the incidence of CVD events; an endpoint adjudication team reviews all relevant medical information, hospitalization records and physician office visits to ascertain CVD incidence using standardized criteria. A separate neurology review panel group adjudicates all suspected cerebrovascular events. For the present investigation, a diagnosis of a major CVD event included fatal or non-fatal coronary heart disease (recognized and unrecognized myocardial infarction and coronary heart disease death) and stroke (ischemic or hemorrhagic).
2.4. Statistical analyzes
Given the approximate normal distributions and symmetry of plasma CETP and PLTP activity, we used untransformed values for all analyzes. In assessing the relationship of CETP and PLTP activities to CVD incidence in the models discussed below, we observed a statistically significant effect modification by sex and hence all analyzes were stratified by sex. For the test of the interaction, alpha level of 0.10 was considered statistically significant due to the low power of this test [13]. We modeled plasma CETP and PLTP activity as continuous and binary variables (dichotomized at the sex-specific median value). We also performed additional sex-specific analyzes classifying individuals into 4 categories according to median plasma CETP and PLTP activity (high CETP and low PLTP; high CETP and high PLTP; low CETP and low PLTP; low CETP and high PLTP) and assessed the relationship between these categories and CVD incidence.
We estimated the sex-specific cumulative incidence of CVD for groups with plasma CETP and PLTP activity at or above versus below the median values. We confirmed that the assumption of proportionality of hazards was met in the primary models and then used sex-specific Cox proportional hazards regression to relate plasma CETP and PLTP activity to incidence of a first CVD event on follow-up. Three sets of sex-specific models were constructed hierarchically: A. models evaluating continuous CETP and PLTP (simultaneously) adjusting for age, systolic blood pressure, hypertension treatment, obesity (defined as BMI ≥30), smoking, diabetes, and plasma lipids (LDL-C, HDL-C, and triglycerides). Models evaluating CETP were adjusted for PLTP and vice versa. B. models evaluating CETP and PLTP as categorical variables dichotomized at the median. C. model using the 4 category variable according to median plasma CETP and PLTP activity as described above (with the group with CETP above median and PLTP below median serving as referent). D. Models evaluating continuous CETP/PLTP Ratio. Models B, C and D were adjusted for the same covariates as A. To gain insights into potential nonlinearity of associations between plasma CETP and PLTP activity and incidence of CVD, we examined generalized additive Cox models using penalized splines. These analyzes also facilitate assessment of the dose–response relation between plasma CETP and PLTP activity and CVD incidence.
We tested for effect modification by age at by incorporating corresponding interaction terms in models with the binary plasma CETP or PLTP activity variable (P < 0.1 was considered statistically significant) [13]. Additional adjustments for CRP, lipid therapy, and body mass index at the baseline exam did not improve the models and therefore these results are not presented.
The discriminatory ability of the Cox models was assessed with the use of the C statistic [14]. Specifically, C statistic from multivariate models with and without the transfer proteins are presented and descriptively compared. Also, the continuous net reclassification index, assessing the change in discriminatory ability of the multivariate risk factor model when the activity of transfer proteins is added, is presented. The net reclassification index is calculated as in Pencina et al. [15]. A statistical test assessing whether the NRI is 0% was carried out at the two-sided 0.05 level of significance. A two-sided P-value <0.05 indicates the NRI is significantly different from 0%.
All analyzes were conducted using SAS (SAS, Inc) version 9.2 and a two-sided P-value < 0.05 was used to indicate statistical significance unless otherwise specified.
3. Experimental results
Both mean plasma CETP and PLTP activity levels were higher in women (CETP 59 ± 17 nmol/L/h; PLTP 9 ± 4 nmol/L/h) than in men (CETP 54 ± 17 nmol/L/h; PLTP 7 ± 3 nmol/L/h); P < 0.0001 for both CETP and PLTP. Overall, CETP and PLTP activities were only weakly correlated (r = −0.04, P = 0.04), but more strongly in men (r=−0.10, P = 0.001) than in women (r=−0.05, P = 0.07). Baseline characteristics of the study group are shown in Table 1 by sex, and separately for plasma CETP and PLTP activities below versus above the sex-specific median value for each of the two transfer proteins. Of note, HDL-C concentrations were significantly correlated with CETP both in men and women (r=−0.25 in men, −0.24 in women) and were significantly lower in both men and women with higher CETP than with lower CETP activity (P < 0.001). In contrast, HDL-C concentrations were higher in both men and women with higher PLTP than in those with lower PLTP activity (P < 0.0001) and again were significantly correlated with PLTP in both sexes (r = 0.25 in men, r = 0.21 in women). Furthermore, as shown in Table 2, across increasing CETP or decreasing PLTP quartile values in men, plasma HDL-C decreased and triglyceride values increased. In contrast, while across increasing PLTP quartile values in women plasma HDL-C increased, triglyceride levels remained stable. In both men and women neither a difference in plasma PLTP nor CETP activity was associated with a notable difference in plasma LDL-C concentrations but with an increase in either PLTP or CETP there was a significant increase in serum levels of C-reactive protein (CRP).
Table 1.
Baseline characteristics by sex of study population by plasma cholesteryl ester transfer protein activity and phospholipid transfer protein activity.
| Cholesterol ester transfer protein activity | Phospholipid transfer protein activity | |||||
|---|---|---|---|---|---|---|
| Men (N = 1188) | ||||||
| <median N = 593 | ≥median N = 595 | P value | <median N = 611 | ≥median N = 577 | P value | |
| Age, yr | 58.0 ± 9.5 | 58.6 ± 9.7 | 0.26 | 58.1 ± 9.6 | 58.5 ± 9.6 | 0.55 |
| Body mass index, kg/m2 | 28.4 ± 4.4 | 28.7 ± 4.5 | 0.25 | 28.4 ± 4.3 | 28.8 ± 4.6 | 0.08 |
| Systolic blood pressure, mm Hg | 129.7 ± 16.8 | 130.0 ± 17.3 | 0.76 | 129.1 ± 17.1 | 130.6 ± 16.9 | 0.15 |
| Diastolic blood pressure, mm Hg | 77.9 ± 9.0 | 77.8 ± 9.3 | 0.75 | 77.6 ± 9.0 | 78.1 ± 9.3 | 0.30 |
| Blood pressure therapy, % | 26 | 27 | 0.58 | 25 | 28 | 0.29 |
| Smoking, % | 13 | 15 | 0.33 | 14 | 15 | 0.37 |
| Diabetes, % | 8 | 10 | 0.43 | 8 | 10 | 0.13 |
| HDL-C, mg/dl | 46.6 ± 13.2 | 41.0 ± 10.6 | <0.001 | 41.4 ± 11.0 | 46.3 ± 13.1 | <0.001 |
| LDL-C, mg/dl | 127.2 ± 33.2 | 128.9 ± 32.4 | 0.39 | 126.9 ± 34.3 | 129.3 ± 31.2 | 0.21 |
| Triglycerides, mg/dl | 136.3 ± 80.4 | 144.5 ± 93.4 | 0.10 | 146.7 ± 96.3 | 133.7 ± 75.8 | 0.01 |
| Lipid drug therapy, % | 11 | 10 | 0.56 | 12 | 10 | 0.45 |
| Plasma CETP activity, pmol/L/h | 41.1 ± 8.4 | 66.8 ± 12.2 | <0.001 | 54.8 ± 15.4 | 53.1 ± 17.8 | 0.08 |
| Plasma PLTP activity, pmol/L/h | 7.6 ± 3.4 | 7.0 ± 3.3 | 0.006 | 4.8 ± 1.2 | 9.9 ± 2.9 | <0.001 |
| Women (N = 1491) | ||||||
| <median N = 745 | ≥median N = 746 | P value | <median N = 763 | ≥median N = 728 | P value | |
| Age, yr | 59.1 ± 9.3 | 58.2 ± 10.0 | 0.08 | 58.4 ± 9.9 | 58.8 ± 9.4 | 0.46 |
| Body-mass index, kg/m2 | 26.9 ± 5.4 | 27.7 ± 5.9 | 0.007 | 27.2 ± 5.6 | 27.4 ± 5.6 | 0.57 |
| Systolic blood pressure, mm Hg | 126.1 ± 19.1 | 126.7 ± 20.6 | 0.57 | 125.6 ± 19.3 | 127.3 ± 20.5 | 0.09 |
| Diastolic blood pressure, mm Hg | 74.0 ± 8.7 | 73.4 ± 9.4 | 0.19 | 73.5 ± 9.0 | 74.0 ± 9.1 | 0.35 |
| Blood pressure therapy, % | 23 | 23 | 0.89 | 21 | 24 | 0.17 |
| Smoking, % | 15 | 15 | 0.89 | 15 | 14 | 0.72 |
| Diabetes, % | 7 | 7 | 0.68 | 6 | 8 | 0.39 |
| HDL-C, mg/dl | 61.2 ± 17.2 | 55.1 ± 14.7 | <0.001 | 55.4 ± 15.3 | 61.0 ± 16.8 | <0.001 |
| LDL-C, mg/dl | 126.9 ± 34.5 | 127.0 ± 34.7 | 0.98 | 128.6 ± 35.7 | 125.2 ± 33.3 | 0.06 |
| Triglycerides, mg/dl | 124.3 ± 69.9 | 134.7 ± 76.6 | 0.006 | 129.8 ± 73.5 | 129.21 ± 73.5 | 0.88 |
| Lipid drug therapy, % | 9 | 8 | 0.78 | 9 | 8 | 0.27 |
| Estrogen Treatment, % | 26% | 28% | 0.31 | 25% | 30% | 0.05 |
| Plasma CETP activity, pmol/L/h | 45.4 ± 9.1 | 71.9 ± 12.4 | <0.001 | 59.7 ± 16.8 | 57.6 ± 17.5 | 0.02 |
| Plasma PLTP activity, pmol/L/h | 8.8 ± 3.9 | 8.7 ± 3.7 | 0.56 | 5.9 ± 1.51 | 11.7 ± 3.2 | <0.001 |
Data are means ± SD unless indicated; Median values of CETP activity for men 52.7 pmol/L/h, for women 57.6 pmol/L/h; Median values of PLTP activity for men 6.8 pmol/L/h, for women 8.2 pmol/L/h.
Table 2.
Means of plasma lipids and C-reactive protein by quartiles of cholesteryl ester transfer protein (CETP) and quartiles of phospholipid transfer protein (PLTP) activity.
| CETP | N | CETP | PLTP | HDL-C | TG | LDL-C | CRP |
|---|---|---|---|---|---|---|---|
| Men | |||||||
| Q1 | 297 | 86.9 | 9.9 | 47.9 | 133.9 | 125.3 | 3.2 |
| Q2 | 296 | 118.8 | 9.0 | 45.2 | 138.7 | 129.1 | 2.6 |
| Q3 | 299 | 144.1 | 8.8 | 41.8 | 141.5 | 128.2 | 4.9 |
| Q4 | 296 | 190.2 | 8.8 | 40.1 | 147.6 | 129.6 | 4.5 |
| P for linear trenda | – | 0.001 | <0.0001 | 0.053 | 0.153 | 0.020 | |
| PLTP | |||||||
| Q1 | 297 | 140.6 | 4.7 | 39.4 | 150.1 | 125.6 | 3.1 |
| Q2 | 297 | 134.1 | 7.2 | 43.3 | 142.6 | 128.1 | 3.8 |
| Q3 | 297 | 134.8 | 9.7 | 44.8 | 136.1 | 128.9 | 3.6 |
| Q4 | 297 | 130.5 | 14.9 | 47.6 | 132.8 | 129.6 | 4.8 |
| P for linear trenda | 0.005 | – | <0.0001 | 0.010 | 0.128 | 0.055 | |
| Women | |||||||
| Q1 | 372 | 95.9 | 11.4 | 62.3 | 122.3 | 126.6 | 3.8 |
| Q2 | 373 | 131.2 | 10.6 | 60.1 | 126.3 | 127.2 | 4.2 |
| Q3 | 374 | 157.7 | 11.0 | 57.1 | 128.0 | 127.8 | 4.4 |
| Q4 | 372 | 202.0 | 10.7 | 53.1 | 141.8 | 126.1 | 6.0 |
| P for linear trenda | – | 0.115 | <0.0001 | 0.001 | 0.926 | 0.001 | |
| PLTP | |||||||
| Q1 | 372 | 152.2 | 5.9 | 53.9 | 128.2 | 127.0 | 4.3 |
| Q2 | 374 | 146.7 | 8.9 | 56.6 | 131.7 | 130.3 | 4.9 |
| Q3 | 372 | 143.6 | 11.6 | 59.6 | 129.0 | 125.8 | 4.6 |
| Q4 | 373 | 144.3 | 17.4 | 62.5 | 129.1 | 124.5 | 4.7 |
| P for linear trenda | 0.007 | – | <0.0001 | 0.870 | 0.113 | 0.809 |
Abbreviations: Q, quartile; N, number; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein. Values for CETP and PLTP activities are shown as pmol/L/h; for HDL-C, TG, and LDL-C as mg/dL; and for CRP as mg/L.
Age-adjusted P-value for linear trend.
During a mean follow-up of 10.4 years (range 2 days–14.2 years), 187 participants experienced a new CVD event. There were 69 myocardial infarctions or CHD deaths in men and 39 in women while there were 39 strokes in men and 40 in women.
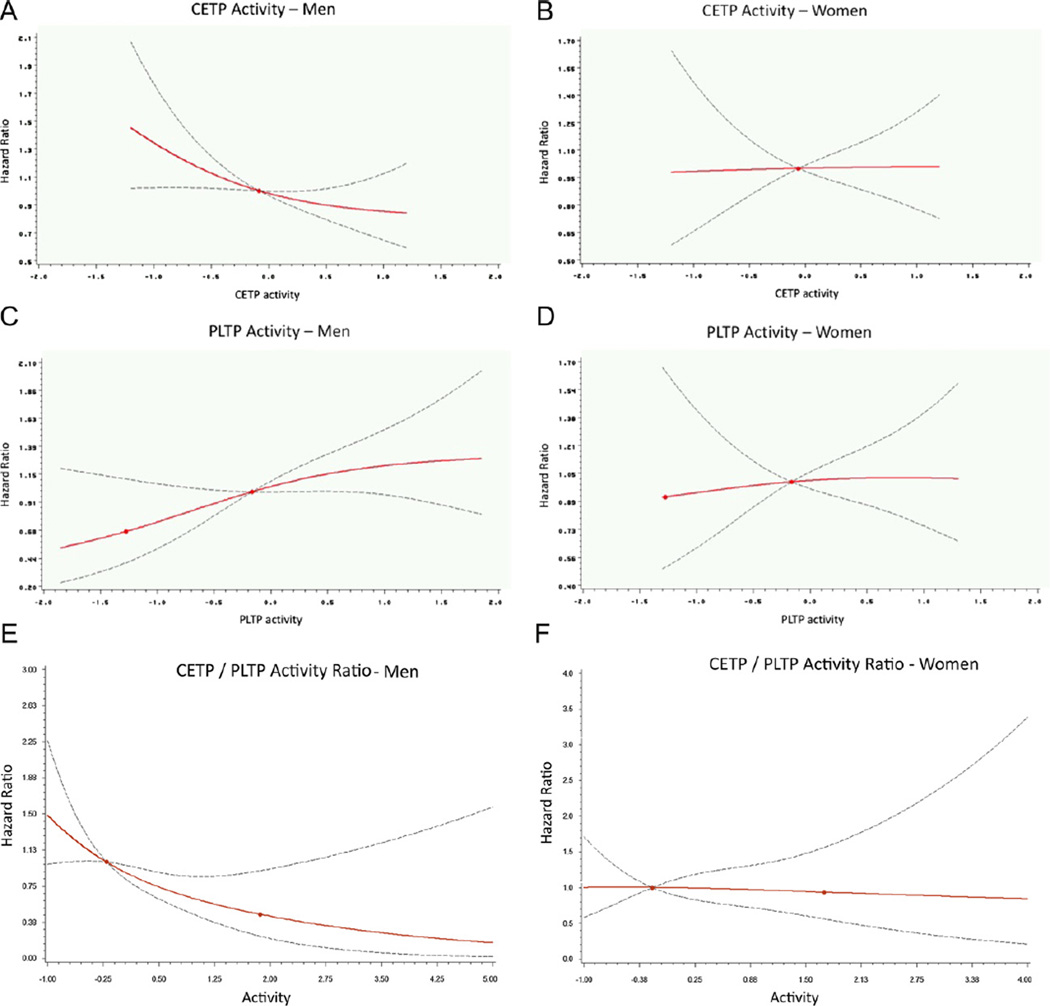
As shown in Fig. 1 by multivariable-adjusted regression splines, the pattern of risk for a new CVD event was distinctly different in men and women over the range of CETP and PLTP activity values as well as the ratio for CETP/PLTP values. Whereas in men the incidence of CVD was inversely related to CETP and positively related to PLTP, in women the relationship of each of these transfer proteins to the occurrence of a new CVD event was essentially flat.
Fig. 1.
Population-wide relation of CVD incidence by gender to plasma CETP and PLTP activities as shown by penalized regression splines. Incidence of CVD is shown by multivariable-adjusted, continuous hazard ratios (y axis) for men and women separately in relation to plasma CETP and PLTP activities and the ratio of CETP to PLTP activities (x axis; standardized units). Solid lines indicate the point estimate of the hazard ratio and dashed lines indicate the 95% CI. Hazard ratios were adjusted for age, smoking, diabetes, systolic blood pressure, blood pressure therapy, plasma HDL-C, triglycerides, and LDL-C as well as for PLTP activity with CETP activity as the variable of interest and for CETP activity with PLTP activity as the variable of interest.
Statistically significant interactions were observed between sex and “CETP/PLTP four level variable” (P = 0.05), between sex and dichotomized CETP (P = 0.06) and between sex and quantitative CETP (P = 0.07). Borderline significant interactions were observed between sex and dichotomized PLTP (P = 0.12) and sex and quantitative PLTP (P = 0.13). Therefore, all further analyzes were stratified by gender.
Only in men were decreasing CETP activity levels significantly associated with increasing risk of CVD; increasing PLTP activity significantly associated with increasing risk of CVD; and decreasing CETP/PLTP Ratio significantly associated with increasing risk of CVD (Table 3, P < 0.05); for women there was no significant relationship between CETP or PLTP activities and CVD incidence. In men, the CETP/PLTP Ratio had the smallest P-value as compared to other predictors, all adjusted for the same set of covariates. Similarly, only in men with a referent group consisting of those with both CETP ≥ median and PLTP < median was the multivariable-adjusted hazard ratio for a new CVD event significantly greater with either the combination of a high CETP and high PLTP (HR = 2.27 (1.23, 4.20), P = 0.009); low CETP and low PLTP (HR = 2.23 (1.19, 4.17), P = 0.012); or low CETP and high PLTP (HR = 2.85 (1.53, 5.31), P = 0.001).
Table 3.
Association of plasma CETP activity and PLTP activity with incidence of CVD events by sex: results of multivariable Cox regression.
| Model | Men | Women | ||
|---|---|---|---|---|
| Hazards ratio (95% CI) | P | Hazards ratio (95% CI) | P | |
| Plasma CETPb as a continuous variable, adjusted for baseline risk factorsa | 0.79 (0.64, 0.98) | 0.032 | 1.01 (0.81, 1.27) | 0.914 |
| Plasma CETPb as a continuous variable, adjusted for baseline risk factorsa and PLTP | 0.80 (0.65, 0.99) | 0.036 | 1.01 (0.81, 1.27) | 0.920 |
| Plasma CETP activity as a binary variable, adjusted for baseline risk factorsa and PLTP; hazards ratio for CETP activity ≥median (<median as referent) | 0.64 (0.43, 0.95) | 0.026 | 0.94 (0.59, 1.49) | 0.789 |
| Plasma PLTPb as a continuous variable, adjusted for baseline risk factorsa | 1.20 (1.00, 1.45) | 0.046 | 1.03 (0.81, 1.32) | 0.796 |
| Plasma PLTPb as a continuous variable, adjusted for baseline risk factorsa and CETP | 1.20 (1.00, 1.44) | 0.052 | 1.03 (0.81, 1.32) | 0.799 |
| Plasma PLTP activity as a binary variable, adjusted for baseline risk factorsa and CETP; Hazards Ratio for CETP activity ≥median (<median as referent) | 1.64 (1.10, 2.46) | 0.016 | 0.95 (0.60, 1.51) | 0.838 |
| CETP/PLTP activity ratiob | 0.66 (0.51, 0.87) | 0.003 | 0.97 (0.75, 1.25) | 0.806 |
Adjustments: age, smoking, diabetes, obesity (defined as BMI ≥ 30), systolic blood pressure, use of antihypertensive medications, plasma HDL-C, triglycerides, and LDL-C.
Hazard ratio is per 1 standard deviation.
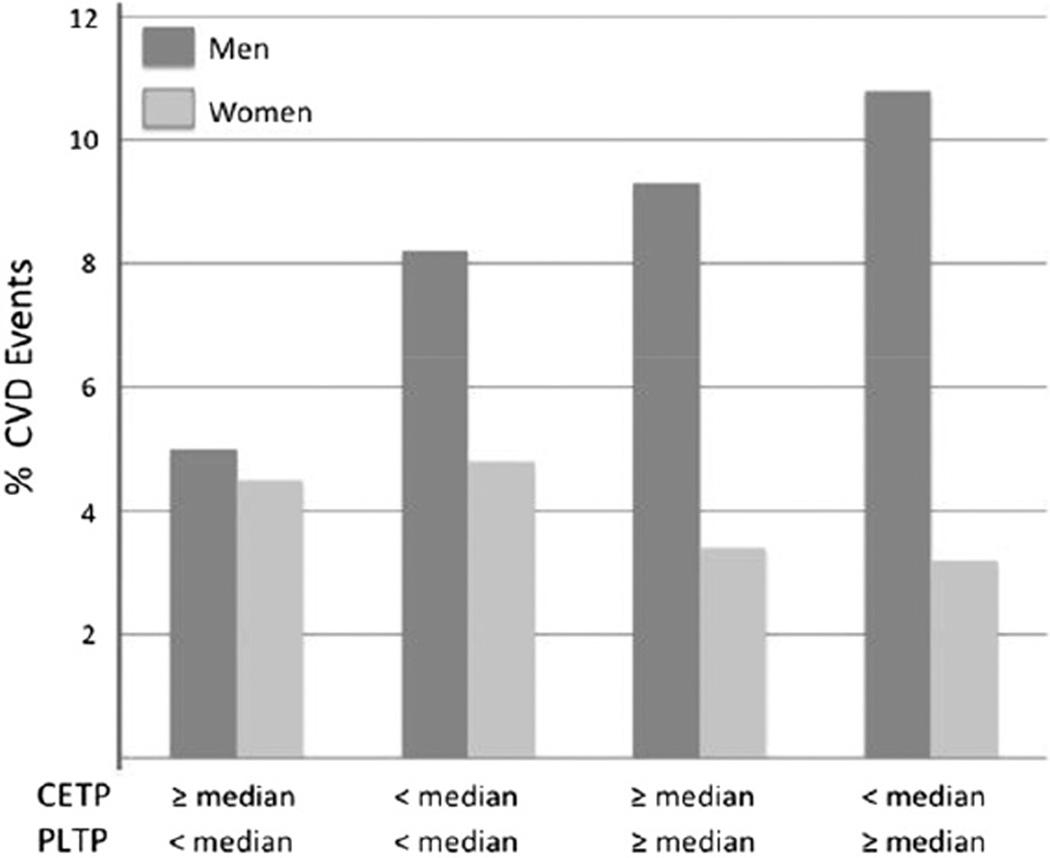
Furthermore, as shown in Fig. 2, for combinations of lower or higher CETP and PLTP activity levels, in men (but not in women) with a lower CETP (<median) and/or a higher level of PLTP (≥median) there was an increase in the 10-year age-adjusted cumulative incidence of CVD. In addition, when ranked by increasing CVD risk, the combination of a lower CETP and higher PLTP in men was associated with a significantly greater CVD event rate than other combinations of these transfer proteins (P = 0.005, P = 0.47 for the differences in CVD event rate trend in men and women, respectively).
Fig. 2.
Cardiovascular event rates in men and women by levels of lower and higher plasma CETP activity and PLTP activity. The 10-year age-adjusted cumulative incidence of new CVD events in conjunction with plasma values of lower (<median) and/or higher (≥median) values of CETP activity and PLTP activity in men (dark bars) and women (light bars). Median value of CETP activity in men, 52.7 pmol/L/h; in women 57.6 pmol/L/h; median value of PLTP activity in men, 6.8 pmol/L/h; in women 8.2 pmol/L/h.
The baseline C statistic for the multivariate CVD model in men was 0.74, increasing to 0.75 when both continuous CETP and continuous PLTP activities were added (net reclassification 0.36 (95% CI of 0.15–0.57)). In women, the baseline C statistic for the multivariate CVD model was 0.82, remaining at 0.82 when both continuous CETP and continuous PLTA were added (net reclassification −0.03 (95% CI of −0.32 to 0.26).
4. Discussion
This analysis was undertaken to determine in a community-wide setting the effect of different levels of activity of two closely related plasma lipid transfer proteins, CETP and PLTP, on the development of CVD events. We had previously reported that in the FHS a lower level of plasma CETP activity was associated with an increase in CVD [4]. In an extension of our previous study we have now not only confirmed these findings using a different CETP activity assay but also demonstrated that high PLTP activity has an independent and significant effect on the development of CVD. In our present analysis, the relation of both CETP and PLTP activity to the development of CVD was clearly sex-specific, being of clinical consequence only in men. Indeed, as shown by continuous splines (Fig.1) whereas the relation of both CETP and PLTP to CV events was decidedly curvilinear in men this relation was essentially flat for both transfer proteins in women. Moreover, again as shown by sex-specific splines (Fig. 1E), throughout a broad range of values a change in the ratio of CETP to PLTP activity, a parameter with the smallest P-value (Table 3), was related to CVD risk only in men.
Our present results showing an increase in plasma PLTP activity that is associated with an increase in CVD events in a general population appear to be distinctly novel. We have, however, no explanation for the sex-specific nature of the PLTP-associated CVD risk nor for a similar sex-specific increase in CVD risk which is associated with a decrease in plasma CETP activity. In the previously published analysis from the FHS [4] that related CETP activity to CVD events this gender difference was also recognized but not utilized to separately analyze results by sex.
Although plasma HDL-C appears to be central to the mechanism of action of both PLTP and CETP, HDL-C concentrations did not vary with varying activity levels of either of these transfer proteins in a direction that is usually consistent with increased (or decreased) CVD risk. That is, over a quartile range, with increased PLTP or decreased CETP activity, that in this analysis was associated with an increase in CVD events in men, plasma HDL-C levels were highest. Furthermore, this direction in change in plasma HDL-C with a change in CETP or PLTP activity was similar in men and women and approximately of the same magnitude even though not apparently of clinical consequence in women. We also have found that with an increase in CETP there was an increase in serum CRP concentrations in men and women alike. This is unexplained and seemingly contrary to the finding in some recent clinical trials with CETP inhibitors [8,16] of small yet significant increases in serum CRP levels.
We believe our findings related to CETP activity measurements in relation to CVD events are not only confirmatory of our earlier study in the FHS [4] but concordant with two other recently published population studies that have reported that lower levels of CETP mass predicted an increase in CVD events [5,6]. In accordance with these findings in a population-wide setting, it is notable that several smaller studies have also shown that higher levels of CETP mass, when measured with either an exogenous [17,18] or an endogenous [19,20] substrate, are associated with a decreased risk of CVD.
In these studies one important variable, as demonstrated by both Boekholdt et al. [17] and Borggreve et al. [18], is the concentration of plasma triglycerides: These studies show when plasma triglycerides are elevated in the presence of a high plasma CETP there is an increase in CVD events; but when not increased, as largely the case in our present study, higher plasma CETP is associated with a reduced CVD event rate. Case-control studies with CETP as a focus have also demonstrated that endogenous CETP activity appears to better predict CVD events compared to CETP mass [19], which is only modestly correlated with CETP activity [20], and when increased at a young age, correlated with both increased blood levels of LDL-C and low levels of HDL-C [20].
Our results with respect to PLTP activity largely support findings in case–control studies in predominantly male groups that have reported that high levels of plasma PLTP activity are associated with coronary artery stenosis [10] as well as with coronary events in individuals with known coronary artery disease who were being actively treated with statins [9]. Smaller case–control studies have also shown an increase in PLTP activity is related to an increase in left ventricular systolic dysfunction [21] and an increase in carotid intima-media thickness in diabetes [22] while, in contrast, another has found that lower PLTP activity was associated with peripheral artery disease [23]. In addition to these several studies in which PLTP activity has been measured, a lower incidence of CVD has been indirectly associated with a lower PLTP activity in 3 large European populations with an increased prevalence of several PLTP single nucleotide polymorphisms that have been shown to be associated with lower activity levels of plasma PLTP [11].
Both CETP and PLTP, from studies largely in mice, have been viewed as clinically relevant mediators of cholesterol exchange between HDL and other plasma lipoprotein particles and, in the case of PLTP, as a catalyst for HDL remodeling in the circulation that will result in an increase in plasma HDL and the potential for enhanced efflux of cholesterol from body tissues. Studies in PLTP-deficient mice have shown that plasma HDL-C and apolipoprotein A1 levels are decreased [24] whereas with PLTP overexpression plasma HDL-C is increased [25,26], especially preβ-HDL particles that are thought to be the principal acceptor particles for “effluxed” cellular cholesterol. In addition to these HDL-related changes PLTP deficiency has been associated in mice with impaired hepatic apoB synthesis and secretion [27]. In our current study although we did not measure plasma apoB, concentrations of plasma LDL-C, the principal apoB-containing lipoprotein, were unchanged across a quartile range of either PLTP or CETP activity levels.
We have found that although increased PLTP activity was associated with higher levels of plasma HDL-C an increase in PLTP activity was nonetheless associated with an increased rate of CVD events. Studies in mice have also suggested that PLTP may enhance the pro-inflammatory properties of HDL and that an inflammatory response might be lower with a reduction in PLTP activity [28,29]. In our current study this possibility was not a specific focus of study but it is of note that plasma CRP concentrations, as a general marker of inflammation, were lower at the lowest compared to the highest quartile of plasma PLTP activity in men but not women (Table 2).
With respect to CETP our present results, using a relatively new activity assay that avoided a large dilution of plasma, reinforce our earlier findings [4] that lower CETP activity (which is associated with higher concentrations of HDL-C) was not associated with fewer CVD events, but, rather, with a significant increase in events. As we have previously noted [4], this finding seems clearly at-odds with efforts to pharmacologically block CETP to increase cholesteryl ester concentrations in plasma HDL. However, our present results are consistent with evidence from compartmental analysis in humans [30] showing that there is negligible direct transport of plasma cholesteryl ester from plasma HDL to the liver to be excreted from the body in bile. Our results are also consistent with a study in transgenic mice [31], showing more directly that neither biliary cholesterol secretion nor fecal bile acid excretion was increased with increased hepatic expression of CETP and increased hepatic uptake of cholesteryl esters.
5. Conclusions
Our present results demonstrate that the activities of plasma CETP and PLTP, two proteins that are known to be integral in the exchange of lipids between plasma HDL and other lipoproteins, had a major effect on the development of CVD events in the FHS. However, we have also found that the clinical consequences of a difference in the activity level of both of these plasma proteins is restricted to men and that while either a decrease in CETP activity or an increase in PLTP activity in men may result in a significant increase in CVD events independent of conventional CVD risk factors, comparable changes in these plasma transfer proteins in women do not change CVD events.
Supplementary Material
Acknowledgments
Acknowledgments: None.
Sources of funding: This research was supported through the National Institute of Health/National Heart, Lung, and Blood Institute (NHLBI/NIH Contract N01-HC-25195).
Footnotes
Disclosures: Robert W. Brocia is Chairman of Roar Biomedical, Inc. (New York, NY) in whose laboratory the assays of CETP and PLTP activity were performed. None of the other authors have any financial disclosures or conflicts of interests to disclose relative to the material presented in this manuscript.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2013.01.046.
Contributor Information
Sander J. Robins, Email: sjrobins@bu.edu.
Asya Lyass, Email: asya@bu.edu.
Robert W. Brocia, Email: rbrocia@roarbiomedical.com.
Joseph M. Massaro, Email: jmm@bu.edu.
Ramachandran S. Vasan, Email: vasan@bu.edu.
References
- 1.Clark RW, Sutfin TA, Ruggeri RB, et al. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler Thromb Vasc Biol. 2004;24:490–497. doi: 10.1161/01.ATV.0000118278.21719.17. [DOI] [PubMed] [Google Scholar]
- 2.Settasatian N, Duong MN, Curtiss LK, et al. The mechanism of the remodeling of high density lipoproteins by phospholipid transfer protein. J Biol Chem. 2001;276:26898–26905. doi: 10.1074/jbc.M010708200. [DOI] [PubMed] [Google Scholar]
- 3.Jiang X, Francone OL, Bruce C, et al. Increased prebeta-high density lipoprotein, apolipoprotein AI, phospholipid in mice expressing the human phospholipid transfer protein and human apolipoprotein AI transgenes. J Clin Invest. 1996;98:2373–2380. doi: 10.1172/JCI119050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasan RS, Pencina MJ, Robins SJ, et al. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation. 2009;120:2414–2420. doi: 10.1161/CIRCULATIONAHA.109.872705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritsch A, Scharnagl H, Eller P, et al. Cholesteryl ester transfer protein and mortality in patients undergoing coronary angiography: the Ludwigshafen Risk and Cardiovascular Health Study. Circulation. 2010;121:366–374. doi: 10.1161/CIRCULATIONAHA.109.875013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khera AV, Wolfe ML, Cannon CP, Qin J, Rader DJ. On-statin cholesteryl ester transfer protein mass and risk of recurrent coronary events (from the pravastatin or atorvastatin evaluation and infection therapy – thrombolysis in myocardial infarction 22 [PROVE-IT-TIMI 22] Study) Am J Cardiol. 2010;106:451–456. doi: 10.1016/j.amjcard.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 7.Nissen SE, Tardif J-C, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 9.Schlitt A, Blankenberg S, Bickel C, et al. PLTP activity is a risk factor for subsequent cardiovascular events in CAD patients under statin therapy: the AtheroGene Study. J Lipid Res. 2009;50:723–729. doi: 10.1194/jlr.M800414-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlitt A, Bickel C, Thumma P, et al. High plasma phospholipid transfer protein levels as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2003;23:1857–1862. doi: 10.1161/01.ATV.0000094433.98445.7F. [DOI] [PubMed] [Google Scholar]
- 11.Vergeer M, Boekholdt M, Sandhu MS, et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122:470–477. doi: 10.1161/CIRCULATIONAHA.109.912519. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Selvin S. Statistical analysis of epidemiologic data. 3rd ed. New York, NY: Oxford Univ. Press; 1996. p. 208. [Google Scholar]
- 14.Pencina MJ, D’Agostino RB., Sr Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr, Steyerbergd EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 17.Boekholdt SM, Kuivenhoven J-A, Wareham NJ, et al. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women. Circulation. 2004;110:1418–1423. doi: 10.1161/01.CIR.0000141730.65972.95. [DOI] [PubMed] [Google Scholar]
- 18.Borggreve SE, Hillege HL, Dallinga-Thie GM, et al. High plasma cholesteryl ester transfer protein may favour reduced incidence of cardiovascular events in men with low triglycerides. Eur Heart J. 2007;28:1012–1018. doi: 10.1093/eurheartj/ehm062. [DOI] [PubMed] [Google Scholar]
- 19.Kappelle PJWH, Perton F, Hillege HL, Dallinga-Thie GM, Dullaart RPF. High plasma cholesteryl ester transfer but not mass predicts incident cardiovascular disease: a nested case-control study. Atherosclerosis. 2011;217:249–252. doi: 10.1016/j.atherosclerosis.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Zeller M, Masson D, Farnier M, et al. High serum cholesteryl ester transfer rates and small high-density lipoproteins are associated with young age in patients with acute myocardial infarction. J Am Coll Cardiol. 2007;50:1948–1955. doi: 10.1016/j.jacc.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 21.Cavusoglu E, Marmur JD, Chhabra S, Chopra V, Eng C, Jiang X-C. Relation of baseline plasma phospholipid transfer protein (PLTP) activity to left ventricular systolic dysfunction in patients referred for coronary angiography. Atherosclerosis. 2009;207:261–265. doi: 10.1016/j.atherosclerosis.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deVries R, Dallinga-Thie GM, Smit AJ, Wolffenbuttel BHR, van Tol A, Dullaart RPF. Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima-media thickness in type 2 diabetes mellitus. Diabetologia. 2006;49:398–404. doi: 10.1007/s00125-005-0088-0. [DOI] [PubMed] [Google Scholar]
- 23.Schgoer W, Mueller T, Jauhiainen M, et al. Low phospholipid transfer protein is a risk factor for peripheral atherosclerosis. Atherosclerosis. 2008;196:219–226. doi: 10.1016/j.atherosclerosis.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X-C, Bruce C, Mar J, et al. Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels. J Clin Invest. 1999;103:907–914. doi: 10.1172/JCI5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Haperen R, van Tol A, Vermeulen P, et al. Human plasma phospholipid transfer protein increases the antiatherogenic potential of high density lipoproteins in transgenic mice. Arterioscler Thromb Vasc Biol. 2000;20:1082–1088. doi: 10.1161/01.atv.20.4.1082. [DOI] [PubMed] [Google Scholar]
- 26.Jaari S, van Dijk KW, Olkkonen VM, et al. Dynamic changes in mouse lipoproteins induced by transiently expressed human phospholipid transfer protein (PLTP): importance of PLTP in preβ-HDL generation. Comp Biochem Physiol Part B. 2001;128:781–792. doi: 10.1016/s1096-4959(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X-C, Qin S, Qiao C, et al. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat Med. 2001;7:847–852. doi: 10.1038/89977. [DOI] [PubMed] [Google Scholar]
- 28.Schlitt A, Liu J, Yan D, Mondragon-Escorpizo M, Norin AJ, Jiang XC. Anti-inflammatory effects of phospholipid transfer protein (PLTP) deficiency in mice. Biochim Biophys Acta. 2005;1733:187–191. doi: 10.1016/j.bbalip.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Cheung MC, Brown BG, Larsen EKM, Frutkin AD, O’Brien KD, Albers JJ. Phospholipid transfer protein activity is associated with inflammatory markers in patients with cardiovascular disease. Biochim Biophys Acta. 2006;1762:131–137. doi: 10.1016/j.bbadis.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz CC, VandenBroek JM, Cooper PS. Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res. 2004;45:1594–1607. doi: 10.1194/jlr.M300511-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Harada LM, Amigo L, Cazita PM, et al. CETP expression enhances liver HDL-cholesteryl ester uptake but does not alter VLDL and biliary lipid secretion. Atherosclerosis. 2007;191:313–318. doi: 10.1016/j.atherosclerosis.2006.05.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.