New paradigms emerge when existing ones fail to address known factors adequately or are invalidated by new evidence. In this paper, we explore some long-held tenets in the pathogenesis of periodontitis and, in light of emerging data and concepts, challenge their validity.
For decades periodontitis has been considered to be caused by specific bacteria or groups of bacteria and, accordingly, treatment protocols have been based largely on anti-infective therapies. However, close inspection of the available data leads one to question whether overgrowth of bacteria is the cause or the result of periodontitis. Over the same decades considerable evidence was presented which indicated that it is more likely to be the host response to the bacteria that leads to the tissue changes noted in gingivitis and periodontitis. Thus, it seems that it is the host inflammatory and immune responses, and not specific bacteria or their ‘putative’ virulence factors, which determine whether periodontitis develops and progresses. In this review, we highlight aspects of the pathogenesis of periodontitis that we suggest should be re-evaluated in light of the current evidence and discuss emerging treatment paradigms. No longer should the tissue changes noted in this classic chronic inflammatory condition be considered to be solely of microbial origin. In this review, we explore the evidence for a role of bacteria in the initiation, progression and treatment of periodontitis. The title of this review is a rewording of a recent paper entitled ‘Periodontitis: a polymicrobial disruption of host homeostasis’ (21). We present this title in the context of unlearning learned concepts in periodontics and the need for a significant re-evaluation of our understanding of the pathogenesis of periodontitis.
Changing concepts in the etiology of periodontitis
Ever since the first description of oral bacteria by Antonie van Leeuwenhoek in 1683, using a homemade microscope, oral microbiology has been a flourishing field of scientific investigation. It is currently estimated that some 750 microbial species reside in the oral cavity (41, 72). However, despite these 330 years of oral microbiological research, we are still in pursuit of the elusive ‘causative’pathogen(s) for periodontitis. Over the years, three main hypotheses have been proposed to explain the microbial etiology of periodontal disease: nonspecific, specific and ecological.
Nonspecific plaque hypothesis
After the landmark studies of Löe and colleagues implicating bacteria in the pathogenesis of periodontal diseases (50), the nonspecific plaque hypothesis was an early concept put forward to explain the nature of periodontal disease (51, 94). It proposed that the accumulation of bacteria adjacent to the gingival margin led to gingival inflammation and subsequent periodontal destruction. It was a simple proposal based on the premise that plaque mass equated with disease status. Tissue destruction resulted from the production of toxic products by the entire dental-plaque microbiota. It proposed that when only small amounts of plaque were present, the host could neutralize the bacterial by-products. However, it was noted that as the plaque mass increased so did the production of toxic products that would eventually overwhelm the host’s defenses. While this concept is generally valid for the development of gingivitis, it does not adequately describe the development of periodontitis, which is considered to be more of a multifactorial problem (67). Later, the nonspecific plaque-hypothesis concept was questioned on the basis that not all gingivitis lesions progress to periodontitis and that bacterial sampling of periodontitis sites showed specific groups of bacteria. Furthermore, this hypothesis failed to explain why some individuals accumulated high levels of plaque yet demonstrated little overt periodontitis and why the converse also held true, in that some patients with very little visible detectable plaque manifested with aggressive and advanced forms of periodontitis (90). Another complicating issue was the ‘site specificity’ in disease manifestation, whereby some sites were unaffected, yet adjacent sites suffered from considerable periodontal destruction. In the presence of a uniform host response, these observations and findings were inconsistent with the concept that all plaque was equally pathogenic. Recognition of differences in plaque at sites of health and disease resulted in a long and ongoing search for specific pathogens and a paradigm shift in thinking from the nonspecific plaque hypothesis to the specific plaque hypothesis.
Specific plaque hypothesis
The specific plaque hypothesis first emerged in the 1970s and proposed that subgingival plaques differed in their pathogenic potential, which was dependent upon the presence or an increase of specific pathogenic bacteria and their toxic products within the subgingival plaque (51, 94). These specific bacteria were thought to produce noxious products that mediated destruction of the periodontal tissues. Although this concept had been proposed at the turn of the 20th century, it was largely forgotten and later reappeared following studies demonstrating that periodontal disease could be transmitted in experimental animals (42, 45). Subsequently, specific bacteria were identified and associated with periodontitis. For example, studies identified Actinobacillus actinomycetemcomitans (later renamed Aggregatibacter actinomycetemcomitans) as a specific pathogen in localized juvenile periodontitis (63, 64, 86). As a result, considerable attention was focussed on identifying specific microorganisms associated with various periodontal diseases.
Through following the development and maturation of dental plaque, studies identified clear changes in plaque composition in which the presence of gram-negative, obligate anaerobic species were associated with an increase in periodontal pocket depths (61, 92). This was considered to be a transition from a healthy flora to pathogenic plaque and was termed the ‘microbial shift hypothesis’ (6). This hypothesis falls within the specific plaque hypothesis, in which a decrease in the number of beneficial commensal species and an increase in the number of specific pathogens eventually are associated with periodontitis. In this model, the microbial shift (more correctly termed ‘dysbiosis’) dictates that as periodontitis develops, the oral microflora specifically changes from a predominantly gram-positive aerobic configuration, consistent with a symbiotic host– parasite relationship, to one consisting mainly of groups of gram-negative anaerobes (pathogenic flora), which are consistent with disease (54).
These studies culminated with the identification of specific microbial groups within dental plaque (91, 92). Six closely inter-related groups of microbes were reported, with the ‘red complex’ consisting of Bacteroides forsythus (later Tannerella forsythia), Porphyromonas gingivalis and Treponema denticola, and these bacteria were significantly associated with the clinical features of periodontitis (pocket depth and bleeding on probing). It was noted that these groups of bacteria were interdependent and often could not exist in isolation. For example, the yellow, green and purple complexes are early colonizers of tooth surfaces and usually precede colonization with the orange and red complexes. It is unusual to find red-complex bacteria in the absence of the orange complex.
While the specific plaque hypothesis has provided an important conceptual framework for the microbiological etiology of periodontitis, it has been questioned in recent times. For example, the fact that putative periodontal pathogens (such as P. gingivalis and T. forsythia) are frequently found in healthy periodontal sites argues against the case for them being true pathogens (70). With the emergence of yet more bacterial species being identified through sophisticated cloning and sequencing methods, the likelihood of specific bacteria causing periodontitis becomes even more complicated (4).
While periodontology has concerned itself with the specific subgingival microorganisms as the primary etiological agents of periodontal diseases, some have questioned this approach and sought alternative answers. One such alternative hypothesis that has received considerable attention is the ecological plaque hypothesis (54, 57).
Ecological plaque hypothesis
By the early 1990s, the ecological plaque hypothesis was proposed to make sense of the microbiological and pathogenesis data available at that time (54). In this hypothesis, it is proposed that the subgingival environment dictates or selects the specific microbial composition and this, in turn, drives the change from health to disease. More specifically, this hypothesis proposes that the nonspecific accumulation of plaque leads to inflammation within the gingival tissues and to the development of gingivitis. This leads to environmental changes within the gingival sulcus, which in turn favor the growth of gram-negative and proteolytic species of bacteria. These changes lead to further inflammatory and immune-mediated tissue changes, further environmental changes and tissue destruction, culminating in a predominance of periodontal pathogens and a greater degree of tissue damage. Hence, the inflammation within the tissues drives the microbial changes and not vice versa, as is the current dogma. This concept has significant ramifications for our understanding of the pathogenesis and management of periodontitis.
The microflora associated with periodontal health appears to remain stable over time and exists in a state of dynamic equilibrium or ‘microbial homeostasis’. In this context, the host is able to control the subgingival plaque through the innate immune system; there are low levels of gingival crevicular fluid relatively free of tissue-breakdown products that can be used as nutrients by the subgingival flora. However, the host inflammatory and immune response can be overwhelmed by excessive plaque accumulation (nonspecific), by plaque-independent host factors (e.g. immune disorders, changes in hormonal balance or systemic diseases such as diabetes) or by environmental factors (e.g. smoking, diet and stress). Consequently, tissue inflammation, with associated tissue degradation and increased gingival crevicular fluid rich in tissue-breakdown products and other bacterial nutrients, can lead to a shift in the subgingival plaque composition toward a more gramnegative disease-associated flora, culminating in overt periodontitis.
The ecological plaque hypothesis is very compatible with reports that disease-associated bacteria are relatively minor components of the subgingival flora in health and increase significantly with the development of periodontal pockets and periodontitis (30, 58, 71, 76). In health, these organisms seem to be regulated by the interspecies competition of microbial homeostasis. It is also a question of nutrients. Many of the gram-negative anaerobes are asaccharolytic and require amino acids or small peptides for growth. Gingival crevicular fluid enriched with collagen peptides from inflammation selects for these bacteria; therefore, disease is associated with an overgrowth of specific subsets of microbes within the subgingival dental plaque as a result of changes in the microenvironment (54).
Plaque is necessary, but not sufficient, for periodontitis to develop
While bacteria are undoubtedly the principal cause of the initial inflammatory lesion leading to gingivitis, it is the host response, not the type of bacteria, which dictates whether disease progresses (67). Evidence for this first emerged from the landmark paper on the pathogenesis of periodontitis by Page & Schroeder, in 1976 (69). In this paper, the development of gingivitis was generally documented as a nonspecific inflammatory response in the gingival tissue to a nonspecific accumulation of dental plaque adjacent to the gingiva. The stages were termed as initial, early and established lesions. However, it was astutely noted that the established lesion could remain contained and not progress unless some other unknown factor tipped the delicate host–parasite relationship toward further tissue destruction and the development of the advanced lesion. In the subsequent decades, enormous effort and resources were expended on determining the role of specific bacteria in this process. Yet, despite decades of research, we are no closer to defining the specific causative bacteria for the tissue destruction noted as periodontitis. Conversely, overwhelming evidence has accrued to demonstrate that it is uncontrolled inflammatory and immune responses that largely drive the tissue destruction (68).
Microbial specificity and development of periodontitis: is there such a thing as a periodontal pathogen?
A ‘pathogen’ is defined as an agent (especially a microorganism, such as a bacterium, protozoan or virus) that causes a disease. A ‘commensal’ organism is defined as an organism participating in a symbiotic relationship in which one species derives some benefit while the other is unaffected. It has been claimed that these classical definitions do not adequately describe chronic polymicrobial diseases such as periodontitis. However, such definitions are still useful and warrant discussion because the ability of an organism to cause disease, or be associated with disease, is a continuum whereby commensal organisms, usually associated with health, can, under the appropriate environmental conditions, become pathogenic and cause or be associated with disease.
In general, the periodontal microflora can be considered a consortium of commensal organisms (20, 97). While great emphasis has been placed on the possibility that some of these may be of exogenous origin and pathogenic, the evidence to date is overwhelmingly in favor of them being indigenous and commensal. Interestingly, this is not a new debate. As early as 1988, Genco and co-workers published a paper considering the origin of periodontal infections (27). In this paper, the authors described how it is important to determine whether the source of periodontal organisms is indigenous or exogenous. This was a viewpoint that was emphasized a decade later in a review of critical issues in periodontal research (66). If the flora can be shown to be of exogenous origin, then interception of transmission or eradication of an infected individual would prevent colonization. Alternatively, if the infection is indigenous and the organisms behave as opportunistic pathogens, then prevention would be aimed at initial acquisition of the bacteria and treatment would be aimed at lowering their numbers to levels compatible with health. Hence, for individuals with an exogenous periodontal infection, effective treatment would involve eradication of the organism(s) and prevention of reinfection. On the other hand, for individuals with an indigenous opportunistic periodontal infection, there is little hope of eradication of the organism and therefore treatments would be aimed at reducing the levels of these organisms to levels compatible with health.
In general, commensal or indigenous organisms are important and play a primary role in host defence against exogenous pathogens. Their presence makes it very difficult for exogenous pathogens to become established and, in the absence of significant changes in the local environment, exogenous pathogens entering the mouth have difficulty surviving in competition with the established flora.
When considering the data available, it seems apparent that periodontal infections comprise both indigenous commensals and opportunistic pathogens. This becomes even more evident in light of the above conclusions drawn from the ecological plaque studies. Hence, if the periodontal flora is generally of a commensal nature, its function would be one of protection rather than disease, and the association with chronic disease probably only occurs when pathogenicity is increased as a result of host susceptibility or altered host immune and inflammatory responses. Indeed, it is generally accepted that most organisms which colonize humans are commensal and probably beneficial to health, and the oral cavity is no exception (24). However, under certain circumstances some of these organisms can transform from being commensal in nature to pathogenic, for reasons and through mechanisms that are not fully understood (4).
Clinically, these arguments can be supported by observations associated with the development of gingivitis. Some individuals respond quickly and significantly to only minor accumulation of plaque, whereas others show minimal response even to long-term plaque accumulation: whether this is a result of the presence of differing microflora or differences in the host response still remains unresolved. However, because the gingivitis reaction is generally considered to be a nonspecific reaction to a nonspecific accumulation of plaque, the individual differences in clinical response to plaque accumulation could be a result of host or environmental factors, rather than primarily microbiological factors.
The presence of plaque bacteria and gingivitis is very prevalent in humans, affecting in excess of 90% of the adult dentate population (40). However, the same cannot be said for periodontitis where, despite abundant plaque deposits in most people, the prevalence of moderate periodontitis (attachment loss > 5 mm) is relatively low, affecting around 20% of the population; however, it should be noted that this prevalence of periodontitis is not uniformly distributed across different races, ethnicities and socioeconomic groups (2). This being the case, despite the universal presence of plaque, bacteria do not appear to be the major determinants of the progression of gingivitis to periodontitis. Although the popular dogma has been to accept that periodontitis arises from a specific subgingival infection, the concept that periodontitis arises when the periodontal tissues provide an adequate ecological environment for opportunistic bacteria to flourish has been presented, for some time, by eminent oral microbiologists as an alternative proposal (60).
The host–parasite interaction is clearly responsible for the initiation of the gingivitis lesion, but what happens next is less than clear. There is no definitive evidence that specific bacteria are responsible for the progression and manifestation of advanced periodontitis. It can be argued that the specific bacteria noted to date are present as a result of the disease but do not cause the disease. This proposal is no different than for most mucosal biofilms in which the complicating issue, which is still unclear, relates to the relationship between disease and inflammation and which comes first – the host response or the change in the biofilm (23).
Do the bacteria select the disease or does the disease select the bacteria?
The role of bacteria in disease has been studied since the days of Robert Koch, Louis Pasteur and others of the 1800s to the present day. From these studies, it is generally accepted that bacteria can cause disease in humans through three different pathways: (i) as a true pathogen that is generally not found in humans and causes disease upon first exposure, (ii) as part of the indigenous flora in one site, but when translocated to another site, causes disease and (iii) as a commensal organism which can only cause disease if a change occurs in the host that allows it to flourish and cause disease (39). Clearly, the periodontal microflora is found throughout the general population, and not all individuals infected with these organisms develop periodontitis.
There is little evidence to support the notion that periodontal bacteria are true pathogens. Indeed, none of the periodontal pathogens identified to date can fulfill Koch’s postulates for a pathogen, namely:
The microorganism must be found in abundance in all organisms suffering from the disease, but should not be found in healthy organisms.
The microorganism must be isolated from a diseased organism and grown in pure culture.
The cultured microorganism should cause disease when introduced into a healthy organism.
The microorganism must be reisolated from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent.
Because of this, Socransky (88, 89) proposed that these postulates should be modified to suit the commensal / opportunistic infection nature of the periodontal bacteria associated with periodontitis. Accordingly, it was proposed that in order for a bacterium to be considered a periodontal pathogen it needed to fulfill the following criteria:
Association: A pathogen should be found more frequently and in higher numbers in disease states than in healthy states.
Elimination: Elimination of the pathogen should be accompanied by elimination or remission of the disease.
Host response: There should be evidence of a host response to a specific pathogen that is causing tissue damage.
Virulence factors: Properties of a putative pathogen that may function to damage the host tissues should be demonstrated.
Animal studies: The ability of a putative pathogen to function in producing disease should be demonstrated in an animal model.
However, these requirements have been challenged in several areas and question the appropriateness of the terminology ‘pathogen’ for the following reasons (39):
Association: Periodontal bacteria are able to colonize and proliferate in only those sites that meet their nutritional and metabolic requirements.
Elimination: No periodontal treatment can effectively eliminate specific bacteria from deep pockets because the organisms are indigenous commensals.
Host response: The presence of antibodies to components and products of periodontopathogens is consistent with specific bacteria being secondary colonizers of periodontal lesions, and the immune response may be triggered by contact with microbes and / or their products late in the pathogenesis of the disease and after the pocket wall becomes ulcerated. Reduction in antibody titers following treatment indicates a reduced number of bacteria resulting from the treatment and the altered environment.
Virulence factors: Virulence factors enable bacteria to colonize deep pockets by providing nourishment or protection against host defences.
Animal studies: While periodontitis lesions can be induced in experimental animals by oral gavage, the ability to recover and culture the ‘culprit’ organisms has met with varied success.
In addition to the above, relocation of periodontal bacteria from one site to another is generally unsuccessful in inducing successful colonization and periodontitis at the recipient site. Indeed, studies have clearly shown that inoculation of shallow gingival sulci with periodontal pathogens from periodontitis sites failed to result in the establishment and ongoing viability of these organisms past 7 days (14). Probably, the conditions of the shallow pocket did not replicate those of a deeper site and therefore the environment for survival of the periodontal pathogens was not suitable. Other anecdotal evidence for lack of transmissibility of pathogens from one site to another comes from lack of evidence for transmission through kissing, periodontal probing and, even more interestingly, subgingival debridement from site to site during a session of periodontal treatment.
Evidence is strong for the likelihood that the periodontal pathogens identified to date represent commensal organisms that can only cause disease if a change occurs in the host that allows them to flourish, resulting in a microbial shift and leading to association with periodontitis (6). However, whether periodontal pathogens actually cause, or merely associate, with the disease still needs to be determined (23). One way in which commensal bacteria can become associated with disease is when the disease itself alters the environment in favor of the organism. This may well be the case for periodontitis in which the changes in connective tissue, inflammatory conditions and immunological responses create an environment conducive to periodontal pathogen overgrowth and lead to their identification in large numbers at diseased sites.
Similarly, it has been suggested that evidence for a specific bacterial etiology of periodontitis comes from longitudinal studies of subjects infected with A. actinomycetemcomitans. However, these studies also highlight the transition of organisms from commensal to pathogenic status. For instance, an interesting example of how commensal organisms may become opportunistic pathogens is seen in a cohort study of 96 students, 11–17 years of age, that included a test group of 38 A. actinomycetemcomitans- positive students and 58 healthy A. actinomycetemcomitans- negative controls studied longitudinally for 2–3 years (26). Over the study period, seven of 37 A. actinomycetemcomitans-positive (i.e. 18%) subjects developed bone loss compared with none of the A. actinomycetemcomitans-negative subjects. This was interpreted to indicate that A. actinomycetemcomitans is a significant risk marker for the initiation of localized aggressive periodontitis. However, according to our thesis, there is another interpretation of these findings. If only 18% of the cohort with A. actinomycetemcomitans developed bone loss then perhaps there is something special about these individuals and the question arises as to whether it is the presence of A. actinomycetemcomitans or abnormalities of the host response that was responsible for the outcome in these individuals. Moreover, consideration needs to be given to the 82% of subjects who harbored A. actinomycetemcomitans but did not develop bone loss. It could be argued that the host response of these individuals was sufficient to counter any effects of A. actinomycetemcomitans, or did not provide an environment suitable for overgrowth of A. actinomycetemcomitans or to exert any of its potential pathogenic mechanisms. The findings of this study further highlight the commensal and opportunistic nature of periodontal infections and, rather than make a prima facie case for the bacteria, they add further strength to the role of the host in modulating the ultimate outcome of any subgingival infection. Furthermore, this interpretation is entirely consistent with individuals who carry commensal organisms as being in a ‘carrier state’ and only convert to disease when the local environment changes sufficiently to allow the organisms to become opportunistic pathogens (16).
In a subsequent study of the same cohort of students, it was reported that the students who harbored A. actinomycetemcomitans and went on to develop bone loss showed dramatically elevated (up to 20- fold) levels of the chemokine, macrophage inflammatory protein-1α (25). However, it is debatable whether the bacteria in these individuals were the cause of the elevated levels of macrophage inflammatory protein-1α because only 18%of the individuals who harbored A. actinomycetemcomitans developed such a reaction. In our model, we would argue that the 18% of individuals who demonstrated such high levels of macrophage inflammatory protein-1α could represent a hyper-inflammatory phenotype that is well known to be associated with inflammatorymediated tissue destruction (37, 85).
In similar studies it has been suggested that evidence for a specific bacterial etiology of periodontitis comes from longitudinal studies of subjects infected with different genotypes of A. actinomycetemcomitans (22, 33). In these studies a case was made that individuals infected with the JP2, or group II variant, genotype of A. actinomycetemcomitans were more likely to experience conversion from health to disease (aggressive periodontitis). However, the fact that some individuals with the specific A. actinomycetemcomitans phenotype did not develop aggressive disease, and that also a percentage of individuals without this particular A. actinomyce- temcomitans phenotype developed aggressive disease, is of major importance. Therefore, apart from specific infection, there is clearly some other feature of this disease that results in its ultimate clinical manifestation. The inflammatory phenotype of the patient may thus be critical by allowing subgingival changes to occur that are consistent with overgrowth of the A. actinomycetemcomitans ‘pathogenic’ genotype which is already present and without which disease cannot progress. Thus, the initiator of the disease is not the bacteria per se but rather the host inflammatory response initiated by gingivitis (which is unrelated to the presence of periodontal pathogens) that allows the so-called pathogenic subgingival flora to subsequently flourish.
Hence, in periodontitis, we argue that, rather than the disease being caused by the bacteria, the disease selects for the bacteria. This paradigm can explain the overgrowth of periodontal pathogens at periodontitis sites and calls into question the role of microbial pathogenic mechanisms, such as bacterial invasion of tissues, as an important part of the early pathogenesis of periodontitis.
It is recognized that the mutual influence of bacteria and host cannot be used as evidence to suggest that bacteria do not cause periodontitis. Indeed, as detailed above, it has long been considered that bacteria are necessary, but not sufficient, for periodontitis to develop. However, two recent studies have cast doubt on the absolute role of bacteria in this process, whereby if inflammation is induced at a site remote to the periodontium (in both cases this was experimental arthritis), then inflammatory changes can be seen within the periodontal tissues, and associated alveolar bone loss can be seen in the experimental, but not in the control, groups (12, 75). Presumably this occurs in the absence of any periodontal pathogens because no periodontitis was induced in these groups of animals; however, there are clearly bacteria present – the commensal bacteria of the animal.
Bacterial invasion of the gingival tissues: how important is this in the initiation of the disease?
The literature is replete with reference to the role of bacterial toxins and even bacterial penetration of the gingival connective tissue in the early pathogenesis of periodontitis. However, close inspection of the literature indicates that this is a biologically plausible assumption with very little supporting evidence. While it is generally accepted that the subgingival plaque is closely associated with the etiology and pathogenesis of gingivitis and periodontitis, it is remarkable that little comment appears in the literature concerning the presence of these bacteria within the affected gingival tissues. Despite this, the literature is overflowing with comments that periodontal tissue destruction is triggered by ingress of bacteria, bacterial antigens or mitogens through a junctional or pocket epithelium with increased permeability into the connective tissue. However, how this takes place against the tide of gingival crevicular fluid and a barrage of migrating neutrophils toward the subgingival plaque has never been explained adequately. Indeed, the converse is probably more likely, in that there is active prevention of such ingress of bacteria and toxic products. The host has numerous mechanisms to defend itself against entry of subgingival microbes or their products, including: production of antibodies, shedding of epithelial cells, the barrier function of the epithelium, emigration of polymorphs towards the plaque front and the outward flushing of the sulcular fluid.
Furthermore, good evidence for the identification and localization of bacteria and their products in the gingival connective tissues during the initiation of the disease is scant, to say the least. The classic paper of Page & Schroeder (69), on the pathogenesis of periodontitis, gives no indication that bacteria were noted in the tissues of initial, early or established lesions. Even more interesting is that no comment was made of the presence of bacteria in the tissues of advanced lesions. One would think that if bacteria were a main invasive culprit their presence would have been obvious and commented upon. While there are reports of bacterial invasion of gingival connective tissue (3, 15, 53, 73, 78), most of these studies, undertaken in the 1980s, were carried out on biopsies from advanced disease lesions and not from the early stages of periodontal inflammation (e.g. gingivitis). Most recently, it has been reported, and is now well accepted, that tissue invasion by bacteria such as P. gingivalis takes place mainly into epithelial cells and it is very unusual for these bacteria to reach the underlying connective tissue until considerable tissue destruction (as a result of inflammation and not direct action of the ‘invading bacteria’) has occurred (32, 46).
Evidence for the ingress of toxic bacterial byproducts into the gingival tissues is also scant. If one performs a PubMed search using a combination of the search words ‘LPS, immunohistochemistry, localization and gingival connective tissue’, interestingly the result is zero! These findings are extremely surprising because it would seem a very obvious study to undertake would be to use the plethora of antibodies available against periodontal pathogens and their ‘toxic’ products and determine their localization in biopsies from normal and inflamed gingival. The lack of these studies means that either no-one has thought of doing this (which is extremely unlikely) or that it has been performed, but with disappointing negative results.
The above notwithstanding, three important issues should be recognized. First, there is good evidence to support invasion of epithelial cells by a number of subgingival bacteria (17). This provides a mechanism for providing a safe haven for these cells and shelter from the host defenses. It is also a good explanation as to how the epithelium may be a front player in the initiation of the gingival response to subgingival plaque through the release of cytokines, which results in a vascular response and associated inflammatory events (5). Second, and more importantly, if bacterial invasion of the gingival connective tissue occurs, it must take place at a very late stage of the development of the periodontitis lesion. This in itself is interesting and important because it means that the focus on bacterial control is only necessary in late-stage (and not in early-stage) disease. Finally, those researchers who have worked with bacteria such as P. gingivalis know all too well that if live P. gingivalis are injected into the subepithelial connective tissues of experimental animals, the resultant abscess formed is usually fatal within a very short period of time (44). Rarely, if ever, do we encounter such significant fulminating and life-threatening abscess formation in patients with periodontitis; this must also argue against any form of tissue invasion by live organisms such as P. gingivalis.
If there is no reproducible, convincing evidence to support the presence of bacteria and their products in the gingival connective tissues during the early course of the pathogenesis of periodontitis, then several conclusions must be drawn. First, in vitro experiments exposing gingival fibroblasts to ‘toxic’ bacterial products must be called into question for lack of apparent clinical significance of such studies; have any of these substances been shown to be present in the tissues? Second, if bacteria and their products are not to be found in the tissues, how important are they in the overall schema of periodontitis? Does this relegate them to bystander status rather than main players? If this is the case, then clearly our principal focus must be on the host responses rather than on specific bacteria and their products.
Inversion of a treatment paradigm: control (resolve?) the inflammation and you will control (resolve?) the infection?
In light of the above, periodontitis does not demonstrate the accepted attributes of an infectious disease, but rather those of an opportunistic infection that responds (in most cases) to many different forms of treatment, all largely focused on reducing the bacterial load rather than on considering how the infection occurred and dealing with the cause of the overgrowth of some bacteria.
The thesis of this paper is not new. Indeed, robust discussions concerning the etiology of periodontal diseases have been around for a century (90). Over this time, only two thoughts have predominated. One line of thought subscribes to the primary etiologic role of pathogenic bacteria and their products in the pathogenesis of the disease, while another has always subscribed to the notion that bacterial subgingival colonization by pathogenic bacteria is a secondary event to the more important principal driving force of uncontrolled inflammation and immune responses (24, 95). In recent years, greater emphasis has been placed on the host response.
There is a voluminous literature demonstrating high numbers of groups of bacteria in the subgingival environment of periodontitis sites and, through a process of guilt by association, these organisms have been labeled as ‘pathogens’. However, the literature is also replete with studies reporting the presence of these pathogens in periodontally healthy individuals. Curiously, this state of periodontal health can remain for years, decades and even a lifetime, despite ongoing exposure to a massive bacterial burden of which these pathogens form a significant cohort. As detailed above, disease only emerges once there is an environmental change that results in redistribution towards a preponderance of periodontal pathogens. Again, this sequence of events appears to result in a decision of guilt by association rather than proof of causality. This process of environmental change, leading to substantial changes in the microflora and association with disease, has been termed an ‘ecological catastrophe’ (55). That is, the environment change leads to a microbial shift that in late-stage disease may lead to further compounding problems associated with bacterial infection, but in the first instance is clearly a host-mediated response.
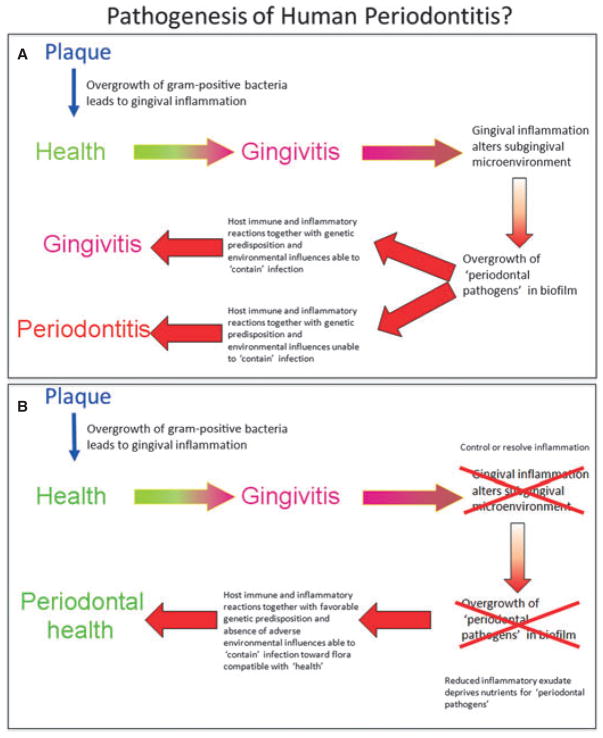
The above observations have led to a slow paradigm shift in the way in which periodontitis might be treated. The change is to move away from a purely mechanistic and antimicrobial approach and consider the driving forces of the disease, namely uncontrolled inflammation. In this emerging paradigm, it is suggested that if the inflammation can be controlled then so can the infection (Fig. 1). The evidence for such an approach is only beginning to emerge. Already, one study has shown that by pushing the inflammatory response of an experimental periodontitis lesion towards a resolution of inflammation, not only do the tissues repair / regenerate, but there is a very significant reversion of the subgingival microflora to one consistent with health (36). This shift in microflora occurred in the absence of any antimicrobial intervention and highlights the potential of resolvins to play an active role in antimicrobial activities in resolving mucosal inflammation (11).
Fig. 1.
(A) Traditional view of the pathogenesis of periodontitis. Gingivitis develops as a nonspecific inflammatory reaction to supragingival plaque accumulation. This leads to an altered subgingival environment owing to the release of inflammatory mediators and gingival connective tissue breakdown products into the gingival sulcus via the gingival crevicular fluid. This altered environment now favors the overgrowth of ‘periodontal pathogens’ in the subgingival biofilm. If the host immune and inflammatory responses are sufficient, and there are favorable genetic and environmental influences, the lesion may be ‘contained’ as gingivitis and not progress to periodontitis. Alternatively, inadequate, or over-responsive, host immune and inflammatory responses, together with unfavorable genetic predisposition and unfavorable environmental influences, result in progression to clinical evidence of periodontitis. (B) Revised treatment strategies for the management of periodontitis. If the inflammatory response occurring at the level of gingivitis can be controlled or resolved (through mechanical debridement and adjunctive chemotherapy) there will be a subsequent change in the subgingival environment, leading to a reduction in nutrient supply to the subgingival microflora (particularly the ‘periodontal pathogens’). The host immune and inflammatory reactions will further subside and this, together with favorable genetic predisposition and absence of adverse environmental influences, will be able to contain the infection and return it toward a commensal flora compatible with periodontal health.
Such approaches are consistent with the principles of the ecological plaque hypothesis and represent a treatment aimed at restricting the nutrient supply to the periodontal pathogens within the commensal subgingival flora through resolving inflammation and promoting healing and health (56). By altering the subgingival environment, the subgingival flora can be manipulated.
Resolution of inflammation
Traditionally, the transition from inflammation to health has been considered to be a passive process resulting from the reduction of proinflammatory mediators over time and the eventual disappearance of the inflammatory response and a return to tissue homeostasis. However, recent studies indicate that effective resolution of inflammation, including efficient removal of leukocytes and return of the resident cells to a ‘noninflammatory’ state, is an active biologic process referred to as catabasis and is thought to be just as complicated as the onset of inflammation (81). The processes of returning tissues to homeostasis is extremely complex, involving restoration to health of chronically inflamed tissues experiencing extensive matrix destruction, fibrosis and frustrated wound healing. In recent years, some important breakthroughs have occurred in our understanding of how inflammation can be naturally resolved.
In periodontitis, the generation of lipid mediators of inflammation, in particular the prostanoids (prostaglandins and thromboxanes), prostacyclins and leukotrienes, are associated with inflammatory cell recruitment, matrix destruction and alveolar bone resorption (74). The resolution of inflammation, which leads to restoration of tissue homeostasis, occurs late in the inflammatory process and is associated with high levels of cyclooxygenases and associated proinflammatory lipid mediators (e.g. prostaglandin E2). At this stage, a ‘class switch’ occurs within neutrophils, leading to the synthesis of proresolving molecules through newly activated pathways separate from the pathways leading to the production of proinflammatory lipid mediators. These molecules are known as lipoxins, resolvins and protectins, and are critical molecular mediators of inflammation resolution that act through a number of complex intracellular processes. This process leads to the release of cytokines, which stop neutrophil migration to the inflammatory site, attract monocytes that do not release proinflammatory mediators, enhance phagocytosis of bacteria and apoptotic cells by macrophages, direct the movement of phagocytes away from the site via the lymphatics and stimulate the synthesis of antimicrobial agents (10, 80, 82).
Hence, in inflammation, the trafficking of leukocytes is controlled by soluble mediators of arachidonic acid metabolism. The proinflammatory mediators are prostaglandins, thromboxanes, prostacyclins and leukotrienes, while the resolving mediators are lipoxins, and with the same enzymes metabolizing omega-3 fatty acids, resolvins and protectins. It is the lipoxins that provide important signals to change the course of inflammation from one of activity associated with neutrophil recruitment and activation to one of resolution and a return to homeostasis. Topical application of lipoxin A4 in an experimental periodontitis lesion in rabbits has been shown to significantly protect experimental sites from developing periodontitis. Furthermore, transgenic animals that overexpress lipoxin appear to be protected from developing periodontitis (83).
The recent identification of resolvins, potent proresolution regulators of inflammation, has been a significant advance (84). Unlike their endogenous counterparts in the lipoxins, resolvins are oxygenated metabolic by-products of omega-3 fatty acid metabolism produced by similar enzymatic pathways used to produce lipoxins. Resolvins are potent mediators and play a significant role in the resolution of inflammation.
Proresolution strategies for managing periodontitis
As detailed above, there is no doubt that bacteria are necessary, but not always sufficient to produce periodontitis. It is well accepted that the development of periodontitis is a multifactorial process through which a bacterial-induced inflammation, modified by environmental, genetic and epigenetic factors, leads to an excessive host response and associated tissue destruction. Understanding that these host-related factors are critical determinants of disease manifestation is leading to a change in treatment focus from principally controlling peri- odontal infection to manipulation of the host inflammatory response (48, 95).
At present, treatments for periodontitis are largely focused toward control of the infection (79). While this is successful to some extent, reinfection generally occurs and control of the inflammatory response is difficult. In some individuals, the inflammatory response may reflect a general systemic dysregulation of inflammation and therefore resolution of inflammation is not always possible using conventional treatment regimes (47, 52).
Recently, the identification of lipoxins, resolvins and protectins, and their ability to stimulate resolution and reduce the magnitude of the inflammatory response, has led to interest in their use as adjuncts to managing inflammatory conditions, such as periodontitis (96). Specifically, the use of these agents would be to target the destructive aspects of inflammation, drive the process down the pathway of resolution of inflammation and return the tissues to homeostasis.
Monotherapies or combined antibacterial and antiinflammatory approaches?
While monotherapy, such as only antibacterial or only anti-inflammatory approaches, for the management of periodontitis have merit and are based on documented scientific literature, the opportunity to combine both is attractive.
A simple example of combined treatments would be the adjunctive use of antibiotics and anti-inflammatory agents in combination with mechanical subgingival debridement (65). While this may be an interesting approach, the logistics of combining individual antibiotics and anti-inflammatory medications presents some clinical issues with regards to patient compliance, correct choice of agents and dispensing.
Recently, considerable interest has developed in the multiple actions of some antibiotics (77, 93). For example, it is well documented that tetracycline, in addition to its antibiotic properties, has potent inhibitory actions against matrix metalloproteinases (77). However, rather than try to combine both of these properties, chemically modified tetracyclines or subantimicrobial dosage regimens have been developed as monotherapy agents for the control of excessive extracellular matrix degradation noted in inflammatory conditions such as periodontitis (31). The clinical results from using such medications have, to date, been equivocal (13, 62).
More recently, interest has focussed on the antibiotic azithromycin. Azithromycin (an azalide) is a synthetic derivative of erythromycin but with better absorption and tissue penetration and a much longer half-life than erythromycin (1). Significant features of azithromycin include its superior acid stability and an ability to penetrate cells, including fibroblasts and phagocytic cells (macrophages and polymorphonuclear leukocytes) (9), which results in higher intracellular concentrations than in serum, and an active release from cells at sites of infection (28). Accordingly, increased concentrations of azithromycin (exceeding the minimal inhibitory concentration) are found in tissues with active infection and inflammation. After administration, the concentration of azithromycin has been reported to exceed the minimal inhibitory concentration in periodontal tissues (7, 29) and to reach high concentrations in the gingival crevicular fluid, which exceeded the minimal inhibitory concentrations for A. actinomycetemcomitans, P. gingivalis and Prevotella intermedia (49).
The current interest in azithromycin relates to its purported method of action, which seems to be twofold. First, it has a bacteriostatic antimicrobial action, achieved by inhibiting bacterial protein synthesis via interactions with specific ribosomal proteins and the 23S rRNA in the peptidyl transferase center (98). Second, it has become evident that macrolides, such as azithromycin, also have antiinflammatory and immunomodulatory properties (19). Using an agent such as azithromycin, we have the opportunity to investigate the therapeutic efficacy of a single agent with both antibiotic and antiinflammatory capabilities (38).
The novel anti-inflammatory properties of azithromycin are related to its ability to inhibit the synthesis of reactive oxygen species and the secretion of proinflammatory cytokines. It also affects leukocyte adhesion by suppressing any increase in the concentration of soluble vascular cell adhesion molecule-1 (18). Furthermore, azithromycin reduces the concentrations of interleukin-1β, granulocyte–macrophage colony-stimulating factor (8) and prostaglandin E2 through inhibition of the cyclooxygenase-1 and cyclooxygenase-2 pathways (59).
For the management of periodontitis, azithromycin has been found to have a beneficial effect on the clinical parameters of periodontal disease when used in conjunction with mechanical subgingival debridement (34, 35, 87). To date, limited studies have investigated the anti-inflammatory effects of azithromycin in periodontal connective tissue. In a recent study, human gingival fibroblasts stimulated with P. gingivalis lipopolysaccharide showed enhanced interleukin-8 production following azithromycin treatment. This increased production of interleukin-8 was considered to be a potential antiinflammatory effect induced by azithromycin, as it may increase the migration of neutrophils to periodontal tissues and phagocytose the periodontopathic bacteria more efficiently (43).
As a result of the anti-inflammatory and immunomodulating actions of macrolides, and specifically azithromycin, the potential exists for azithromycin to have a beneficial effect in the treatment of periodontal disease. It is therefore important to clarify the mechanisms of these immune-modulating actions of azithromycin in periodontal tissues, for validation of its use clinically as an adjunctive treatment with mechanical debridement for aggressive and refractory forms of periodontitis.
Concluding comments
There is no question that host–parasite interactions are responsible for the development and manifestation of the early clinical lesions of gingivitis (50). Within these lesions, so-called periodontal pathogens can be identified, yet the lesions often do not progress. There is still no unequivocal evidence that specific microbes induce any of the later, more advanced, forms of the periodontitis lesion. Rather, there is accumulating evidence to support the hypothesis that a commensal flora can switch to an opportunistic pathogenic flora through complex changes in the local environment, all of which are driven largely by the host and not by the bacteria (56). It is important to stress that such a proposal does not diminish the important role of the subgingival microflora in the etiology and pathogenesis of gingivitis and periodontitis. However, emerging evidence supports the major role of host responses modulated through genetics, immunological and inflammatory responses, stress, smoking, diet, social determinants and general health as being the major determinants of the outcomes of the classic chronic inflammatory condition we know as periodontitis. This rethinking of previously learned concepts will lead to novel treatment protocols focussed more on controlling, redirecting and resolving the host response rather than on solely focussing on the infection, which may well be a result of the disease and not the cause of the disease. If periodontitis is a hostmediated disruption of microbial homeostasis, then it stands to reason that by controlling the inflammation (using both conventional mechanical therapy and pharmacological adjuncts) it should be possible to control the infection.
Acknowledgments
Boston University is assigned patents on resolvins that are licensed for clinical development and are subject to consultant agreements for Dr Thomas E. Van Dyke. USPHS grants DE16191, DE15566 and DE19938 to T. E. Van Dyke and NHMRC grants 1027747 and 627143 to P. M. Bartold.
References
- 1.Addy LD, Martin MV. Azithromycin and dentistry - A useful agent? Br Dent J. 2004;197:141–143. doi: 10.1038/sj.bdj.4811530. [DOI] [PubMed] [Google Scholar]
- 2.Albander JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Allenspach-Petrzilka GE, Guggenheim B. Bacterial invasion of the periodontium; an important factor in the pathogenesis of periodontitis? J Clin Periodontol. 1983;10:609– 617. doi: 10.1111/j.1600-051x.1983.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 4.Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405– 411. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartold PM, Walsh LJ, Narayanan AS. Molecular and cell biology of the gingiva. Periodontol 2000. 2000;24:28–55. doi: 10.1034/j.1600-0757.2000.2240103.x. [DOI] [PubMed] [Google Scholar]
- 6.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blandizzi C, Malizia T, Lupetti A, Pesce D, Gabriele M, Giuca MR, Campa M, Del Tacca M, Senesi S. Periodontal tissue disposition of azithromycin in patients affected by chronic inflammatory periodontal diseases. J Periodontol. 1999;70:960–966. doi: 10.1902/jop.1999.70.9.960. [DOI] [PubMed] [Google Scholar]
- 8.Bosnar M, Bosnjak B, Cuzic S, Hrvacic B, Marjanovic N, Glojnaric I, Culic O, Parnham MJ, Erakovic-Haber V. Azithromycin and clarithromycin inhibit lipopolysaccharideinduced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte macrophage colony-stimulating factor and interleukin-1beta. J Pharmacol Exp Ther. 2009;331:104–113. doi: 10.1124/jpet.109.155838. [DOI] [PubMed] [Google Scholar]
- 9.Bosnar M, Kelneric Z, Munic V, Erakovic V, Parnham MJ. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob Agents Chemother. 2005;49:2372–2377. doi: 10.1128/AAC.49.6.2372-2377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 11.Campbell EL, Serhan CN, Colgan SP. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol. 2011;187:3475– 3481. doi: 10.4049/jimmunol.1100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantley MD, Haynes DR, Marino V, Bartold PM. Preexisting periodontitis exacerbates experimental arthritis in a mouse model. J Clin Periodontol. 2011;38:532–541. doi: 10.1111/j.1600-051X.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- 13.Caton J, Ryan ME. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD) Pharmacol Res. 2011;63:114–120. doi: 10.1016/j.phrs.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Christersson LA, Slots J, Zambon JJ, Genco RJ. Transmission and colonization of Actinobacillus actinomycetemcomitans in localized juvenile periodontitis patients. J Periodontol. 1985;56:127–131. doi: 10.1902/jop.1985.56.3.127. [DOI] [PubMed] [Google Scholar]
- 15.Christersson LA, Wikesjö UM, Albini B, Zambon JJ, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. II. Correlation between immunofluorescence and culture techniques. J Periodontol. 1987;58:540–545. doi: 10.1902/jop.1987.58.8.540. [DOI] [PubMed] [Google Scholar]
- 16.Christersson LA, Zambon JJ, Dunford RG, Grossi SG, Genco RJ. Specific subgingival bacteria and diagnosis of gingivitis and periodontitis. J Dent Res. 1989;68(Sp Iss):1633–1639. [Google Scholar]
- 17.Colombo AV, da Silva CM, Haffajee A, Colombo AP. Identification of intracellular oral species within human crevicular epithelial cells from subjects with chronic periodontitis by fluorescence in situ hybridization. J Periodontal Res. 2007;42:236–243. doi: 10.1111/j.1600-0765.2006.00938.x. [DOI] [PubMed] [Google Scholar]
- 18.Culic O, Erakovic V, Cepelak I, Barisic K, Brajsa K, Ferencic Z, Galovic R, Glojnaric I, Manojlovic Z, Munic V, Novak- Mircetic R, Pavicic-Beljak V, Sucic M, Veljaca M, Zanic Grubisic T, Parnham MJ. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450:277– 289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 19.Culić O, Eraković V, Parnham MJ. Anti-inflammatory effects of macrolide antibiotics. Eur J Pharmacol. 2001;429:209–229. doi: 10.1016/s0014-2999(01)01321-8. [DOI] [PubMed] [Google Scholar]
- 20.Darveau RP. The oral microbial consortium_s interaction with the periodontal innate defense system. DNA Cell Biol. 2009;28:389–395. doi: 10.1089/dna.2009.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 22.DiRienzo JM, Slots J, Sixou M, Sol MA, Harmon R, McKay TL. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect Immun. 1994;62:3058–3065. doi: 10.1128/iai.62.8.3058-3065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dongari-Bagtzoglou A. Pathogenesis of mucosal biofilm infections: challenges and progress. Expert Rev Anti Infect Ther. 2008;6:201–208. doi: 10.1586/14787210.6.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 25.Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Donnelly R, Gunsolley J. Macrophage inflammatory protein-1a: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J Periodontol. 2009;80:106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]
- 26.Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol. 2007;45:3859–3869. doi: 10.1128/JCM.00653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genco RJ, Zambon JJ, Christersson LA. The origin of periodontal infections. Adv Dent Res. 1988;2:245–259. doi: 10.1177/08959374880020020901. [DOI] [PubMed] [Google Scholar]
- 28.Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomi K, Yashima A, Iino F, Kanazashi M, Nagano T, Shibukawa N, Ohshima T, Maeda N, Arai T. Drug concentration in inflamed periodontal tissues after systemically administered azithromycin. J Periodontol. 2007;78:918– 923. doi: 10.1902/jop.2007.060246. [DOI] [PubMed] [Google Scholar]
- 30.Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 1998;36:3292–3242. doi: 10.1128/jcm.36.11.3239-3242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Y, Lee HM, Sorsa T, Salminen A, Ryan ME, Slepian MJ, Golub LM. Non-antibacterial tetracyclines modulate mediators of periodontitis and atherosclerotic cardiovascular disease: a mechanistic link between local and systemic inflammation. Pharmacol Res. 2011;64:573– 579. doi: 10.1016/j.phrs.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Guyodo H, Meuric V, Pottier L, Martin B, Faili A, Pers JO, Bonnaure-Mallet M. Colocalization of Porphyromonas gingivalis with CD4+ T cells in periodontal disease. FEMS Immunol Med Microbiol. 2012;64:175–183. doi: 10.1111/j.1574-695X.2011.00877.x. [DOI] [PubMed] [Google Scholar]
- 33.Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 34.Haas AN, de Castro GD, Moreno T, Susin C, Albandar JM, Oppermann RV, Rosing CK. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696–704. doi: 10.1111/j.1600-051X.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- 35.Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1-year results. J Clin Periodontol. 2007;34:243–253. doi: 10.1111/j.1600-051X.2006.01040.x. [DOI] [PubMed] [Google Scholar]
- 36.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021– 7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 37.Hernichel-Gorbach E, Kornman KS, Holt SC, Nichols F, Meador H, Kung JT, Thomas CA. Host responses in patients with generalized refractory periodontitis. J Periodontol. 1994;65:8–16. doi: 10.1902/jop.1994.65.1.8. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch R, Deeng H, Laohachai MN. Azithromycin in periodontal treatment: more than an antibiotic. J Periodontal Res. 2012;47:137–148. doi: 10.1111/j.1600-0765.2011.01418.x. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch RS, Clarke NG. Infection and periodontal diseases. Rev Infect Dis. 1989;11:707–715. doi: 10.1093/clinids/11.5.707. [DOI] [PubMed] [Google Scholar]
- 40.Hugoson A, Jordan T. Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease. Community Dent Oral Epidemiol. 1982;10:187–192. doi: 10.1111/j.1600-0528.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13:589–595. doi: 10.1016/j.tim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Jordan HV, Keyes PH, Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7:21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- 43.Kamemoto A, Ara T, Hattori T, Fujinami Y, Imamura Y, Wang PL. Macrolide antibiotics like azithromycin increase lipopolysaccharide-induced IL-8 production by human gingival fibroblasts. Eur J Med Res. 2009;14:309–314. doi: 10.1186/2047-783X-14-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesavalu L, Holt SC, Ebersole JL. Virulence of a polymicrobic complex, Treponema denticola and Porphyromonas gingivalis, in a murine model. Oral Microbiol Immunol. 1998;13:373–377. doi: 10.1111/j.1399-302x.1998.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 45.Keyes PH, Jordan HV. Periodontal lesions in the syrian hamster. III. Findings related to an infectious and transmissable component. Arch Oral Biol. 1964;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- 46.Kim YC, Ko Y, Hong SD, Kim KY, Lee YH, Chae C, Choi Y. Presence of Porphyromonas gingivalis and plasma cell dominance in gingival tissues with periodontitis. Oral Dis. 2010;16:375–381. doi: 10.1111/j.1601-0825.2009.01649.x. [DOI] [PubMed] [Google Scholar]
- 47.Kornman KS. Refractory periodontitis: critical questions in clinical management. J Clin Periodontol. 1996;23:293–298. doi: 10.1111/j.1600-051x.1996.tb02092.x. [DOI] [PubMed] [Google Scholar]
- 48.Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79(Suppl):1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 49.Lai PC, Ho W, Jain N, Walters JD. Azithromycin concentrations in blood and gingival crevicular fluid after systemic administration. J Periodontol. 2011;82:1582–1586. doi: 10.1902/jop.2011.110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Löe H, Thielade E, Jensen S. Experimental gingivitis in man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 51.Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976;9:65–107. [PubMed] [Google Scholar]
- 52.Magnusson I, Walker CB. Refractory periodontitis or recurrence of disease. J Clin Periodontol. 1996;23:289–292. doi: 10.1111/j.1600-051x.1996.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 53.Manor A, Lebendiger M, Shiffer A, Tovel H. Bacterial invasion of periodontal tissues in advanced periodontitis in humans. J Periodontol. 1984;55:567–573. doi: 10.1902/jop.1984.55.10.567. [DOI] [PubMed] [Google Scholar]
- 54.Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 55.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 56.Marsh PD. How is the development of dental biofilms influenced by the host? J Clin Periodontol. 2011;38(Suppl 11):28–35. doi: 10.1111/j.1600-051X.2010.01673.x. [DOI] [PubMed] [Google Scholar]
- 57.Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55:16–35. doi: 10.1111/j.1600-0757.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 58.McNabb H, Mombelli A, Gmür R, Mathey-Dinç S, Lang NP. Periodontal pathogens in the shallow pockets of immigrants from developing countries. Oral Microbiol Immunol. 1992;7:267–272. doi: 10.1111/j.1399-302x.1992.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 59.Miyazaki M, Zaitsu M, Honjo K, Ishii E, Hamasaki Y. Macrolide antibiotics inhibit prostaglandin E2 synthesis and mRNA expression of prostaglandin synthetic enzymes in human leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:229–235. doi: 10.1016/s0952-3278(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 60.Moore WEC, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, Ranney RR. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983;42:510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore WEC, Moore LVH. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 62.Needleman I, Suvan J, Gilthorpe MS, Tucker R, St George G, Giannobile W, Tonetti M, Jarvis M. A randomized-controlled trial of low-dose doxycycline for periodontitis in smokers. J Clin Periodontol. 2007;34:325–333. doi: 10.1111/j.1600-051X.2007.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newman MG, Socransky SS. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977;12:120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 64.Newman MG, Socransky SS, Savitt ED, Propas DA, Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976;47:373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- 65.Ng VW, Bissada NF. Clinical evaluation of systemic doxycycline and ibuprofen administration as an adjunct treatment for adult periodontitis. J Periodontol. 1998;69:772–776. doi: 10.1902/jop.1998.69.7.772. [DOI] [PubMed] [Google Scholar]
- 66.Page RC. Critical issues in periodontal research. J Dent Res. 1995;74:1118–1128. doi: 10.1177/00220345950740041301. [DOI] [PubMed] [Google Scholar]
- 67.Page RC, Kornman K. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:10– 19. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 68.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 69.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- 70.Papapanou PN. Population studies of microbial ecology in periodontal health and disease. Ann Periodontol. 2002;7:54–61. doi: 10.1902/annals.2002.7.1.54. [DOI] [PubMed] [Google Scholar]
- 71.Papapanou PN, Baelum V, Luan WM, Madianos PN, Chen X, Fejerskov O, Dahlén G. Subgingival microbiota in adult Chinese: prevalence and relation to periodontal disease progression. J Periodontol. 1997;68:651–666. doi: 10.1902/jop.1997.68.7.651. [DOI] [PubMed] [Google Scholar]
- 72.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 73.Pekovic DD, Fillery ED. Identification of bacteria in immunopathological mechanisms of human periodontal diseases. J Periodontal Res. 1984;19:329–351. doi: 10.1111/j.1600-0765.1984.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 74.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 75.Ramamurthy NS, Greenwald RA, Celiker MY, Shi EY. Experimental arthritis in rats induces biomarkers of periodontitis which are ameliorated by gene therapy with tissue inhibitor of matrix metalloproteinases. J Periodontol. 2005;76:229–233. doi: 10.1902/jop.2005.76.2.229. [DOI] [PubMed] [Google Scholar]
- 76.Riep B, Edesi-Neuss L, Claessen F, Skarabis H, Ehmke B, Flemmig TF, Bernimoulin JP, Göbel UB, Moter A. Are putative periodontal pathogens reliable diagnostic markers? J Clin Microbiol. 2009;47:1705–1711. doi: 10.1128/JCM.01387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan ME, Golub LM. Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy. Periodontol 2000. 2000;24:226–238. doi: 10.1034/j.1600-0757.2000.2240111.x. [DOI] [PubMed] [Google Scholar]
- 78.Saglie FR, Marfany A, Camargo P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J Periodontol. 1988;59:259–265. doi: 10.1902/jop.1988.59.4.259. [DOI] [PubMed] [Google Scholar]
- 79.Sanz M, Teughels W Group A of European Workshop on Periodontology. Innovations in non-surgical periodontal therapy: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35(8 Suppl):3–7. doi: 10.1111/j.1600-051X.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- 80.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O_Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1 / protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 82.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 84.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shapira L, Soskolne WA, Sela MN, Offenbacher S, Barak V. The secretion of PGE2, IL-1 beta, IL-6, and TNF alpha by adherent mononuclear cells from early onset periodontitis patients. J Periodontol. 1994;65:139–146. doi: 10.1902/jop.1994.65.2.139. [DOI] [PubMed] [Google Scholar]
- 86.Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976;84:1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 87.Smith SR, Foyle DM, Daniels J, Joyston-Bechal S, Smales FC, Sefton A, Williams J. A double-blind placebo-controlled trial of azithromycin as an adjunct to non-surgical treatment of periodontitis in adults: clinical results. J Clin Periodontol. 2002;29:54–61. doi: 10.1034/j.1600-051x.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 88.Socransky SS. Microbiology of periodontal disease – present status and future considerations. J Periodontol. 1977;48:497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- 89.Socransky SS. Criteria for the infectious agents in dental caries and periodontal disease. J Clin Periodontol. 1979;6:16–21. doi: 10.1111/j.1600-051x.1979.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 90.Socransky SS, Hafajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 91.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 92.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 93.Sreenivasan PK, Gaffar A. Antibacterials as anti-inflammatory agents: dual action agents for oral health. Antonie Van Leeuwenhoek. 2008;93:227–239. doi: 10.1007/s10482-007-9197-8. [DOI] [PubMed] [Google Scholar]
- 94.Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. 1986;13:905–911. doi: 10.1111/j.1600-051x.1986.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 95.Van Dyke TE. The management of inflammation in periodontal disease. J Periodontol. 2008;79:1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Dyke TE. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. J Clin Periodontol. 2011;38(Suppl 11):119–125. doi: 10.1111/j.1600-051X.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 97.Wade WG. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol. 2011;38(Suppl 11):7–16. doi: 10.1111/j.1600-051X.2010.01679.x. [DOI] [PubMed] [Google Scholar]
- 98.Zuckerman JM, Qamar F, Bono BR. Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am. 2009;23:997– 1026. doi: 10.1016/j.idc.2009.06.013. [DOI] [PubMed] [Google Scholar]