Abstract
The expression of the ERα and ERβ estrogen receptors in the hippocampus may be important in the etiology of age-related cognitive decline. To examine the role of ERα and ERβ in regulating transcription and learning, ovariectomized wild-type (WT) and ERα and ERβ knockout (KO) mice were used. Hippocampal gene transcription in young ERαKO mice was similar to WT mice 6 h after a single estradiol treatment. In middle-age ERαKO mice, hormone deprivation was associated with a decrease in the expression of select genes associated with the blood–brain barrier; cyclic estradiol treatment increased transcription of these select genes and improved learning in these mice. In contrast to ERαKO mice, ERβKO mice exhibited a basal hippocampal gene profile similar to WT mice treated with estradiol and, in the absence of estradiol treatment, young and middle-age ERβKO mice exhibited preserved learning on the water maze. The preserved memory performance of middle-age ERβKO mice could be reversed by lentiviral delivery of ERβ to the hippocampus. These results suggest that one function of ERβ is to regulate ERα-mediated transcription in the hippocampus. This model is supported by our observations that knockout of ERβ under conditions of low estradiol allowed ERα-mediated transcription. As estradiol levels increased in the absence of ERα, we observed that other mechanisms, likely including ERβ, regulated transcription and maintained hippocampal-dependent memory. Thus, our results indicate that ERα and ERβ interact with hormone levels to regulate transcription involved in maintaining hippocampal function during aging.
Introduction
The DNA binding domains of α and β estrogen receptors (ERs) are highly homologous (Katzenellenbogen and Korach, 1997; Enmark and Gustafsson, 1999); however, estradiol-induced transcriptional activity differs because of divergence of the ligand binding domain and disparities in the recruitment of coregulators (Nilsson et al., 2001; Harrington et al., 2003). Under low levels of estradiol, ERα transcriptional activity is greater than that of ERβ (Barkhem et al., 1998; Pettersson et al., 2000). Moreover, ERβ can heterodimerize with ERα to inhibit ERα-mediated gene expression (Hall and McDonnell, 1999; Pettersson et al., 2000; A. Gottfried-Blackmore et al., 2007; Gonzales et al., 2008), indicating that regulation of gene expression is dependent upon the ratio of ERα to ERβ and the level of estradiol (Foster, 2012).
Estradiol is neurotrophic (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992; Choi et al., 2003; Akama and McEwen, 2003; Kretz et al., 2004; Rune and Frotscher, 2005; Jelks et al., 2007) and neuroprotective (Nilsen and Diaz Brinton, 2003; Dubal et al., 2006; Garcia-Segura et al., 2006; Aenlle and Foster, 2010), functions that likely underlie the ability of estradiol to preserve cognitive function during aging (Foster, 2012). Interestingly, estradiol's effects on cognitive aging are reduced in aging women (Sherwin, 2006) and rodents (Gibbs, 2000; Markham et al., 2002; Markowska and Savonenko, 2002; Foster et al., 2003; Sherwin, 2006; Bimonte-Nelson et al., 2006; Daniel et al., 2006; Talboom et al., 2008), and temporally correlated with altered hippocampal expression of ERα and ERβ (Tohgi et al., 1995; Adams et al., 2002; Mehra et al., 2005; Sharma and Thakur, 2006; Thakur and Sharma, 2007; Ishunina et al., 2007; Bohacek and Daniel, 2009). Thus, an age-related change in the ratio of ERα/ERβ expression in the hippocampus may contribute to the reduced ability of estradiol treatment to alter gene expression (Aenlle and Foster, 2010) and the reduction in the efficacy of hormone therapy to ameliorate memory loss that often accompanies advanced age (Foster, 2012).
In this study, we used ovariectomized ERαKO and ERβKO female mice to investigate how selective loss of one receptor subtype affects behavior and gene expression profiles in aging mice. Our results are consistent with the idea that, under low estradiol conditions associated with female aging, activation of ERα helps to regulate hippocampal gene expression and function. If ERα levels are reduced, our data suggest that ERβ can compensate to maintain hippocampal function provided that estradiol levels are increased.
Materials and Methods
Mice.
ERα−/− (ERαKO) (Lubahn et al., 1993) and ERβ−/− (ERβKO) (Krege et al., 1998) mice were generated from heterozygous mouse colonies. The genotypes of the mice were screened using PCR amplification as previously described (Lubahn et al., 1993; Krege et al., 1998). Wild-type (WT) littermates produced from the ERαKO and ERβKO breeding colonies were combined into one WT group. Animals were housed 3–5 per cage and maintained on 12:12 light/dark cycle (lights on at 6:00 A.M.). All procedures involving animal subjects were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Florida and were performed in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
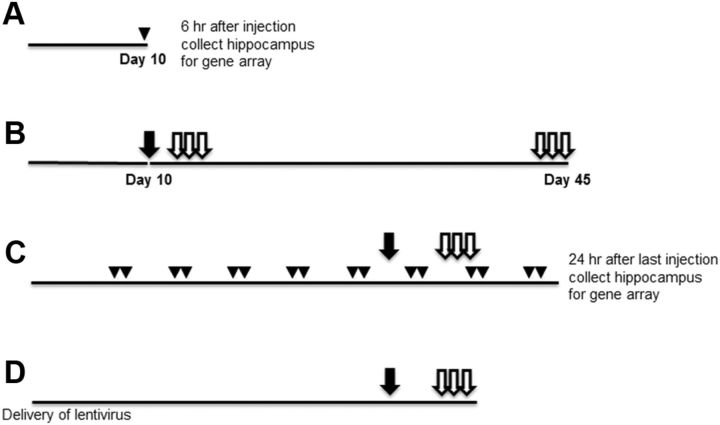
Figure 1 shows the timeline for injections, behavioral training, and tissue collection for each study, starting from the time of surgery. Young mice (WT: n = 27; ERαKO: n = 21; ERβKO: n = 25) were 4 months at the time of surgery. For examination of gene transcription profiles, young mice were ovariectomized, and 10 d after surgery were treated with a single injection of oil or estradiol. The hippocampi of these mice were collected 6 h later (Fig. 1A). The experimental groups included WT-EB (n = 7), WT-oil (n = 7), ERαKO-EB (n = 5), ERαKO-oil (n = 5), ERβKO-EB (n = 7), and ERβKO-oil (n = 5). To determine whether knockout of either ERα or ERβ influences hippocampal function in young mice, ERαKO (n = 11), ERβKO (n = 13), and WT (n = 13) mice were ovariectomized and their performance on the water maze task was examined, starting 10 d after surgery and was repeated 4 weeks later (Fig. 1B).
Figure 1.
Schedule of injections and behavioral testing. All time lines start with surgery to remove the ovaries. A, Young mice were ovariectomized and were treated 10 d later (arrowhead) with a single injection of oil (WT = 7, ERαKO = 5, ERβKO = 5) or estradiol (WT = 7, ERαKO = 5, ERβKO = 7). The hippocampi were collected 6 h after injection for examination of transcription. B, Young mice (WT = 13, ERαKO = 11, ERβKO = 13) were ovariectomized, and their performance on the cue (filled arrow) version of the water maze task was examined 10 d after surgery. Three days of training on the spatial version of the water maze (open arrows) was initiated on day 14 and again on day 43 after surgery. C, Middle-age mice were injected on 2 consecutive days (arrowheads) of every 5 d with oil (WT = 15, ERαKO = 7, ERβKO = 12) or estradiol (WT = 14, ERαKO = 8, ERβKO = 11), starting at the beginning of week 2 after ovariectomy. Arrows indicate the days of training on cue (filled arrow) and spatial (open arrows) tasks during week 5 and week 6, respectively, which was initiated 48 h after injection. At the end of training on week 6, the mice were given two more series of injections and were killed for tissue collection 24 h after the last injection. D, Middle-age ERβKO mice received bilateral hippocampal injections of lentivirus to express ERβ-FLAG and GFP (n = 12) or GFP alone (n = 11) at the time of ovariectomy. Behavior was examined 5 weeks later.
Middle-age mice (WT: n = 15; ERαKO: n = 23; ERβKO: n = 52) were 13–14 months old at the time of surgery. To determine whether ERα and ERβ contribute to estradiol effects on behavior and gene expression during middle age, ERαKO and ERβKO mice were treated with either oil or estradiol. Treatments were initiated 1 week after surgery and were delivered on two contiguous days of a 5 d cycle in accord with our previously published work (Aenlle et al., 2009) (Fig. 1C). The experimental groups included ERαKO-oil (n = 7), ERαKO-EB (n = 8), ERβKO-oil (n = 12), ERβKO-EB (n = 11), WT-oil (n = 15), and WT-EB (n = 14) littermates. Twenty-four hours after the last injection, hippocampi were collected for gene arrays and uterine tissue was collected and weighed. To determine whether expression of ERβ disrupts spatial learning, lentivirus encoding ERβ and GFP (n = 12) or GFP alone (n = 11) was bilaterally injected in the hippocampus of middle-age ERβKO mice at the time of ovariectomy, and behavior was examined starting 5 weeks later (Fig. 1D).
Surgeries.
Female mice (4 months and 13–14 months) were anesthetized (2 mg ketamine and 0.2 mg xylazine per 20 g of body weight), and the ovaries were removed through a small abdominal midline incision. All mice received ad libitum access to food (Purina mouse chow) and water until the surgery, after which time they were placed on casein-based chow (Cincinnati Lab Supply) that has lower levels of phytoestrogens compared with soy-based chow.
Hippocampal lentiviral injections.
To determine whether the behavioral changes observed in middle-age ERβKO mice could be attributed to the absence of ERβ, female ERβKO (13–14 months) were ovariectomized and either pFin-EF1α-GFP-CMV-(FLAG)ERβ-WPRE (ERβ-FLAG) or pFIN-EF1α-GFP-WPRE (GFP) lentivirus (2 × 1012 transducing Us/μl) was injected bilaterally into the hippocampus 1.0 μl per side. Techniques for hippocampal injection of virus have previously been published (Foster et al., 2008; Zeier et al., 2009; Lee et al., 2012).
Estradiol treatments.
Estradiol (17 β-estradiol benzoate, EB) (Sigma) was dissolved (0.1 mg/ml) in light mineral oil (Fisher Scientific) and was injected subcutaneously (5 μg) at the nape of the neck. In rodents, plasma estradiol levels rapidly increased within 1–4 h after a single EB injection (Woolley and McEwen, 1993; Sohrabji et al., 1994), and EB-induced transcriptional changes follow a similar time course (Hammer et al., 1986; Priest et al., 1995; Sohrabji et al., 1995; Too et al., 1999; Aenlle et al., 2009; Aenlle and Foster, 2010). Therefore, for young animals, a single injection was used to examine transcription 6 h after treatment. The dose and time point were selected to maintain consistency with our previous studies examining the effects of estradiol on transcription (Aenlle et al., 2009; Aenlle and Foster, 2010).
To examine the effects of estradiol on behavior and transcription in middle-age mice, injections (5 μg) were delivered on two contiguous days of a 5 d cycle and were initiated 1 week after surgeries in accord with our previously published work (Aenlle et al., 2009). Previous work indicated that repeated injections using these delivery parameters can improve spatial learning and memory in mice (Frick et al., 2002; Xu and Zhang, 2006; Walf et al., 2008; Aenlle et al., 2009).
Water maze.
Behavioral studies involving the water maze were performed in accord with our previously published work (Foster et al., 2008; Aenlle et al., 2009). All mice were first trained on the cue discrimination version of the Morris swim task using four training blocks, with three trials per block. Spatial training was initiated either 3 or 6 d after the completion of the cue discrimination training, using methods that have been previously described (Foster et al., 2008; Aenlle et al., 2009). Briefly, spatial training was performed over a period of three consecutive days and consisted of four training blocks with three trials per block per day. The penultimate trial on days 2 and 3 consisted of a probe trial that served as an index of learning. The probe trial was performed by placing the mouse in the tank for 1 min without the platform and recording both the time the animal spent in each quadrant of the tank and the number of times the animal crossed the location in the tank from which the platform had been removed. The spatial discrimination index was computed using the formula (G − O)/(G + O), where G and O represent the percentage of time spent in the goal quadrant and quadrant opposite the goal, respectively.
RNA isolation and microarray analyses.
Mice were anesthetized with CO2 and decapitated. The brain was quickly removed and placed in ice-cold artificial CSF. Both hippocampi were removed, frozen in liquid nitrogen, and stored at −80°C. Total RNA was isolated from each sample using a Qiagen RNeasy Lipid Tissue Mini Kit (Qiagen). RNA concentration was determined using a spectrophotometer, and a subset of samples was examined using an Agilent 2100 Bioanalyzer (Agilent Technologies) to assess sample quality. Microarray hybridization was performed by the Interdisciplinary Center for Biotechnology Research Microarray Core, University of Florida, following the manufacturer's protocol.
The first study examined the transcriptional response in young (4 months) mice treated with estradiol. Mice were killed 6 h after injection of either estradiol or oil, and hippocampi were prepared for gene transcript analyses using Affymetrix Mouse 430 2.0 arrays (one array per mouse) (Blalock et al., 2003; Aenlle and Foster, 2010). Normalization and computation of gene expression values were performed using dChip (Li and Wong, 2001). The detection of signal (presence/absence) was determined by MAS Version 5.0 (Affymetrix). The number of present calls for each probe was determined across all arrays using the criterion that at least 80% of the chips within any single group (genotype and treatment) had to exhibit a present call for that probe to be included in subsequent analyses. Using this criterion, 24,111 probes were selected for further analyses. Affymetrix GeneChip Mouse Gene 1.0 ST Arrays (one array per mouse) were used for examination of transcription in middle-age mice, after cyclic injections of either estradiol or oil.
Quantitative PCR.
Quantitative PCRs were performed using Gene Expression Assay Mix TaqMan Universal PCR Master Mix (2×), and a 7300 Real-Time PCR System with SDS Software Version 1.3.1 analysis software (Applied Biosystems). The PCR primers were designed to produce short amplified products that crossed exon-exon junctions and minimized amplification of off-target sequences (ESR1: exons 4 and 5, 56 bp, NP_031982.1; ESR2 variant 1: exons 9 and 10, 69 bp, NP_997590.1; ESR2 variant 2: exons 8 and 9, 69 bp, NP_034287.3; BTG2: exons 1 and 2, 102 bp, NP_031596.1; NPAS4: exons 4 and 5, 62 bp, NP_705781.1). Each sample was run in triplicate, and the values were normalized to GAPDH according to our previously described methods (Aenlle et al., 2009; Aenlle and Foster, 2010).
Construction of lentiviral vectors.
The lentiviral vector pFIN-WPRE backbone contained a 2 × 250 bp core chicken β-globin HS4 insulator sequence in the 3′LTR and a woodchuck hepatitis post-transcriptional regulatory element (WPRE) to enhance transgene expression (Semple-Rowland et al., 2007). A dual-promoter vector was constructed by inserting transgenes encoding GFP and FLAG tagged ERβ into the multiple cloning site of the pFIN backbone. The transgenes were arranged head-to-tail so that transcription for both proceeded in the same direction and the transgenes shared the same polyadenylation site located in dl.R region of the 3′ LTR. The EF1α (elongation factor-1 α) promoter was amplified using PCR (5′ (NotI), ATT GCG GCC GCT TTG GAG CTA A; 5′ (NotI), TTA GCG GCC GCC ACG ACA CCT GAA AT); and was ligated into pFIN-WPRE at the NotI site to create pFIN-EF1α-WPRE. GFP was amplified using PCR (5′ (NheI), GCA GCT AGC CGC CAC CAT GAG CAA; 5′ (NheI-MluI-BsiWI), AAT GCT AGC ACG CGT CGT ACG AGA GGC CTC AGT CAG); and was ligated into pFIN-EF1α-WPRE at the NheI site to create pFIN-EF1α-GFP-WPRE. The cytomegalovirus (CMV) promoter was amplified using PCR (5′ (BsiWI), ATA CGT ACG TAG TTC ATA GCC CAT ATA TGG; 5′ (MluI-AsiSI-PacI), TAT ACG CGT GCG ATC GCT TAA TTA AGT AAG CAG TGG GTT CTC TAG T); and was ligated into pFIN-EF1α-GFP-WPRE using the BsiWI and MluI sites to create pFIN-EF1α-GFP-CMV-WPRE. Finally, the open-reading frame encoding human FLAG-tagged ERβ was amplified from pcDNA4/TO-FLAG:hERb (kind gift from Dr. Chegini) using primers that introduced PacI sites on the 5′- and 3′-ends of the coding region (5′-ATA TTA ATT AAA AAC TTA AGC TTA CCG CCA TG; 5′-ATA TTA ATT AAC CCT CTA GAT CAC TGA GAC). The PCR product (1679 bp) was then ligated into pFIN-EF1α-GFP-CMV-WPRE at the PacI site to produce pFin-EF1α-GFP-CMV-(FLAG)ERβ-WPRE. The integrity of FLAG-ERβ in the final vector was verified by sequencing.
The functionality of (FLAG)ERβ was confirmed using previously published methods (Foster et al., 2008). The ability of the receptor to respond to estradiol was examined by cotransfecting HEKT293 cells with pFin-EF1α-GFP-CMV-(FLAG)ERβ-WPRE and ERE-TA-SEAP reporter plasmids and measuring changes in secreted placental alkaline phosphatase after exposure to estradiol. The bicistronic lentiviral vector encoding GFP and (FLAG)ERβ was packaged into lentivirus using a three plasmid packaging system as previously described (Semple-Rowland et al., 2007). Viral titers were estimated using a Lenti-X qRT-PCR kit (Millipore Bioscience Research Reagents) and typically averaged 2 × 1012 viral genomes per milliliters.
Statistical analyses.
For measures of behavior and uterine weight, ANOVAs were used to establish main effects and interactions. Follow-up ANOVAs and Fisher's protected least significant difference post hoc comparisons (p < 0.05) were used to identify significant comparisons. One-tailed Student's t tests (p < 0.05) were used to determine whether the discrimination indices, calculated from the quadrant search behavior (Foster et al., 2003), were different from that expected by chance (i.e., a discrimination index = 0). Two-tailed t tests (p < 0.025) were used to identify differentially expressed probes according to previously published work (Aenlle et al., 2009; Aenlle and Foster, 2010).
Results
Gene expression in the hippocampus of young adult ERαKO and ERβKO mice
We have previously shown that a single 5 μg estradiol injection administered to young mice produces robust changes in hippocampal gene expression 6 h after treatment (Aenlle and Foster, 2010). To examine the role of ERα and ERβ in regulating hippocampal gene expression, we examined the gene expression profiles of 4-month-old ERαKO and ERβKO female mice that were ovariectomized and subsequently treated with estradiol 10 d after surgery and compared them with control animals that were treated with oil vehicle (Fig. 1A).
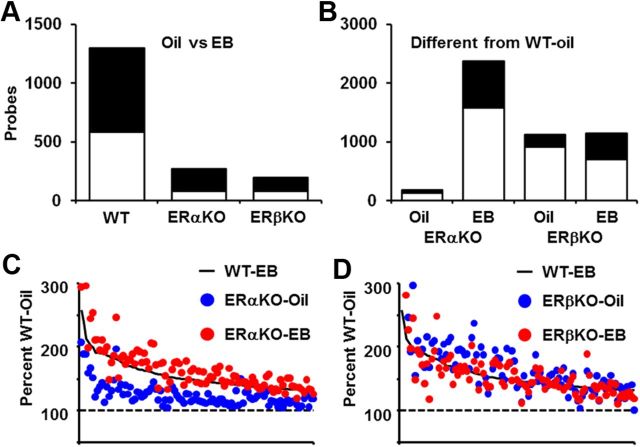
Analysis of differential gene expression after estradiol treatment revealed that there were 1295 probes that were differentially expressed between WT-EB and WT-oil-treated mice (Fig. 2A). Within the set of 1295 probes, 585 (45%) of these increased in expression and 710 (55%) decreased in expression. Only five probes showed a ≥2-fold increase in expression. ERα and ERβ KO mice were less responsive to acute estradiol treatment than WT animals as measured by changes in gene expression. The expression levels of 268 and 195 probes in the ERαKO-EB and ERβKO-EB treatment groups, respectively, were altered compared genotype-matched oil treatment controls. None of the gene expression changes in the estradiol-treated ERαKO and ERβKO mice were ≥2-fold. These results indicate that, compared with genotype-matched and oil-treated animals, young ERα and ERβ KO mice are less responsive to estradiol treatment than WT mice.
Figure 2.
Gene expression in young (4 months) WT and ERKO mice, 6 h after estradiol (WT = 7, ERαKO = 5, ERβKO = 7) or oil (WT = 7, ERαKO = 5, ERβKO = 5) treatment. A, Graphic summary of the total number of probes whose expression either significantly increased (open) or decreased (filled) in WT-EB, ERαKO-EB, and ERβKO-EB mice compared with genotype-matched oil-treated mice. B, Comparison of hippocampal gene expression in oil and estradiol-treated ERKO mice relative to WT-oil mice. ERαKO-oil-treated mice exhibited a negligible shift in basal gene expression and a robust shift in expression after estradiol treatment relative to WT-oil, suggesting that ERαKO mice remain responsive to estradiol treatment. In contrast, ERβKO-oil-treated mice exhibited a relatively large shift in basal gene expression, with the majority of probes (81%) showing increases in expression. The numbers of probes in ERβKO treated with oil or estradiol that differed from WT-oil were similar. C, D, The mean expression for each probe within each group was normalized to the expression level of the probe in WT-oil-treated mice, which was set at 100%. The top 100 genes that showed an increase in expression in WT-EB mice were sorted by percentage change and plotted (solid line) along with their expression in ERαKO-oil (C, blue circles) and ERαKO-EB (C, red circles) and ERβKO-oil (D, blue circles) and ERβKO-EB (D, red circles) mice. In general, expression of probes in for the ERαKO-oil group was slightly greater than WT-oil (i.e., 100%) but less than in WT-EB. Expression increases observed in the ERαKO-EB group were similar to those observed in WT-EB mice, suggesting that ERαKO mice remain responsive to estradiol treatment. For ERβKO-oil and ERβKO-EB mice, probe expression was greater than WT-oil and appeared to track expression in WT-EB mice, results suggesting that knockout of ERβ permits expression of estradiol-sensitive genes in the absence of estradiol treatment.
To determine whether knockout of either the ERα or the ERβ receptor alters gene expression, we examined gene expression profiles in ERαKO and ERβKO animals treated with oil or estradiol and compared them with the gene expression profiles of WT-oil-treated mice (Fig. 2B). ERαKO mice treated with oil exhibited very few differences in gene expression (183 probes) compared with the WT-oil controls. The expression levels of five ERαKO-oil-treated probes increased >2-fold, three of which represented the ERα gene (ESR1 Affymetrix probes: 1453145_at, 1460591_at, and 1457877_at). This result suggests that functional knockout of the ERα, resulting from insertion of a neomycin cassette, induces a compensatory increase in ESR1 transcription to counter loss of ERα receptor activity. Comparison of ERαKO-EB-treated mice with WT-oil revealed 2368 differentially expressed probes. The level of expression of the majority of these probes increased in ERαKO-EB-treated mice (1583 increasing and 785 decreasing) (Fig. 2B). Of the probes that showed an increase in expression, 72 increased at least twofold and included the three probes for ESR1. No probe decreased by ≥2-fold. Collectively, these data show that ERαKO mice are responsive to estradiol treatments, a result that supports the idea that ERβ can compensate for the loss of ERα (Fugger et al., 2000; Foster et al., 2008).
Unlike ERαKO mice, the expression levels of 1125 probes exhibited changes in ERβKO mice treated with oil compared with WT-oil controls (Fig. 2B). The majority of these probes (912, 81%) showed an increase in expression in ERβKO-oil-treated animals. Moreover, the expression of 196 of these probes increased >2-fold, including the probe for the ERβ gene (ESR2: 1426103_a_at); however, ERβ probe was not analyzed further because the number of present calls for this probe across all groups did not reach the criterion. Of the 213 probes that showed a decrease in expression, none showed a decrease ≥2-fold. Comparisons of ERβKO-EB mice with WT-oil controls revealed that 1142 probes were differentially expressed in the ERβKO-EB mice (698 increasing and 444 decreasing; Fig. 2B). Of these, the expression of 3 probes increased and one decreased with magnitudes >2-fold. Thus, in contrast to ERαKO mice whose basal gene expression levels were similar to WT animals, ERβKO mice exhibit a considerable shift in basal gene expression, a shift that may reflect removal of the inhibitory influences of ERβ on ERα-mediated transcription (Pettersson et al., 2000; Williams et al., 2008).
To visualize the changes in basal and estradiol-induced gene expression, the mean expression levels for probes in each group were normalized to the average expression levels of the corresponding probes in WT-oil-treated mice. WT probes whose expression significantly increased in response to estradiol treatment were sorted by the magnitude of their increases, and the percentage change values for the top 100 probes were plotted with the normalized measures for ERαKO (Fig. 2C) or ERβKO (Fig. 2D) mice. The levels of basal gene expression in ERαKO-oil mice were slightly higher than those in WT-oil mice (i.e., 100%). Estradiol treatment of ERαKO mice further increased gene expression so that the levels of gene expression were similar to WT-EB (Fig. 2C). Gene expression levels in ERβKO mice were always higher than those in WT-oil mice regardless of treatment (Fig. 2D) and were similar to those found in WT-EB mice. Indeed, in contrast to the estradiol-mediated increase in gene expression in WT and ERαKO mice, a paired t test of the normalized values revealed that expression of these genes was reduced [(t(99) = 4.94, p < 0.0001) in ERβKO-EB (155 ± 2% of WT-oil) compared with ERβKO-oil mice (172 ± 6% of WT-oil). Our analyses of estradiol-induced changes in hippocampal gene expression are consistent with reports of gene expression changes in other tissues responsive to estradiol (Lindberg et al., 2003; O'Lone et al., 2007) and suggest that, under normal conditions, ERβ inhibits ERα-mediated transcription. The observation that ERβKO-oil mice exhibit a transcription profile similar to WT-EB mice is consistent with this idea.
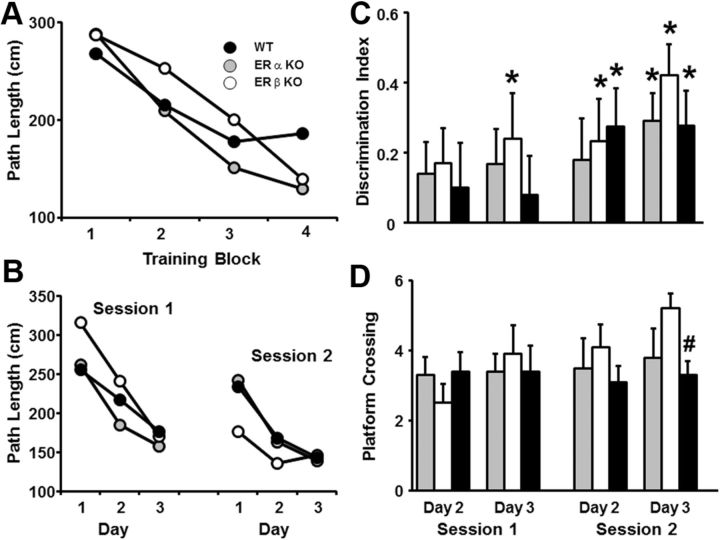
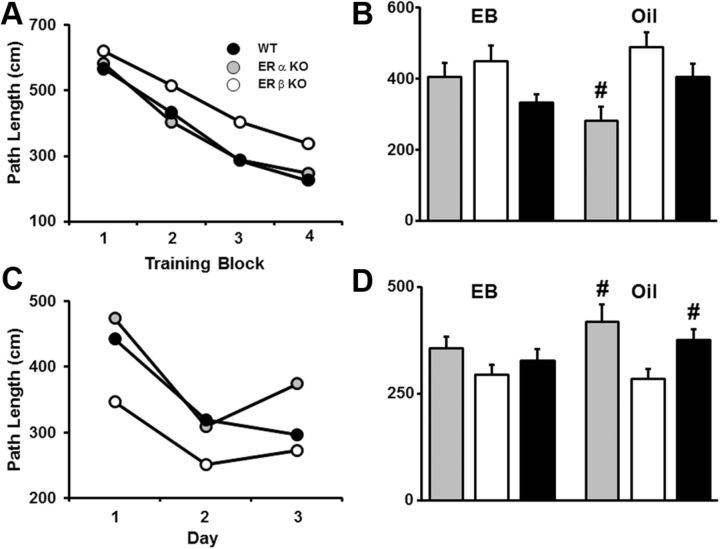
To determine whether basal gene expression patterns in ERαKO and ERβKO mice influence hippocampal function, 4-month-old ERαKO, ERβKO, and WT mice were ovariectomized and their performances on a cue discrimination water maze task were examined 10 d after surgery. A repeated-measures ANOVA indicated that there was a decrease in distance to find the escape platform [F(3,102) = 7.73, p < 0.0001] over the four blocks of training, in the absence of a genotype difference (Fig. 3A). Examination of spatial discrimination over 3 d of training (session 1) indicated that there was a decrease in the escape path length [F(2,68) = 11.62, p < 0.0001] in the absence of a genotype difference (Fig. 3B). Repeated-measures ANOVAs of the probe trial data for the penultimate trial on days 2 and 3 indicated that there was no training effect and no genotype difference for the discrimination index scores or platform crossings (Fig. 3C, D). However, examination of training effects in each group indicated that ERβKO mice did show a training effect for platform crossing [F(1,12) = 5.44, p < 0.05]. To determine whether any group used a spatial search strategy, we compared the discrimination index scores relative to chance (score = 0) performance using one-tailed t tests. Only ERβKO mice exhibited acquisition of a spatial search strategy, with a discrimination index score above chance (p < 0.05) at the end of training on day 3 (Fig. 3C).
Figure 3.
Water maze performance of young ERKO and WT mice. A, Mean path length to reach an escape platform over four training blocks (three trials per block) for WT (filled circles, n = 13), ERαKO (gray circles, n = 11), and ERβKO (open circles, n = 13). A decrease in path length was observed in the absence of a genotype effect. B, Mean path length to reach an escape platform during spatial training. Training was provided in two sessions, starting 13 d after ovariectomy (session 1) and again 30 d after ovariectomy (session 2). For each session, a decrease in path length was observed in the absence of a genotype effect. C, Discrimination index calculated from performance on probe trials for WT (filled bars), ERKO (gray bars), and ERKO (open bars). Probe trials were delivered as the penultimate trial on days 2 and 3 for each session. *Performance above chance and acquisition of a spatial search strategy. D, Platform crossing calculated from probe trial performance. #Significant difference between ERβKO and WT mice.
To determine whether the duration of hormone deprivation resulting from ovariectomy influences spatial learning, ovariectomized ERαKO, ERβKO, and WT mice were retested on the spatial version of the water maze 1 month after the first training session (session 2) using a new spatial location. Similar to the previous learning data, an effect of training [F(2,68) = 13.85, p < 0.0001] on the escape path length was observed in the absence of a genotype difference (Fig. 3B), and no training or genotype differences were observed for the discrimination index scores and platform crossing. Examination of training effects in each genotype indicated that only the ERβKO mice showed a training effect for the discrimination index [F(1,12) = 6.88, p < 0.05] and for platform crossings [F(1,12) = 8.08, p < 0.05]. Unlike the earlier tests in which only the ERβKO acquired a spatial search strategy, one-tailed t tests indicated that ERβKO and WT mice acquired a spatial search strategy by day 2 and all groups performed above chance for day 3 (Fig. 3C). Finally, examination of platform crossings indicated that there was a significant genotype effect on day 3 [F(2,34) = 3.38, p < 0.05]. Post hoc tests indicated that the performances of ERβKO mice were better than those observed in WT mice and that ERβKO mice exhibited a tendency (p = 0.085) to cross the platform more often than ERαKO mice. Together, these results indicate that ERβKO mice exhibited a modest, although significant, improvement in spatial learning compared with the other groups.
ERα and ERβ influences on hippocampal function and gene expression in middle-age mice
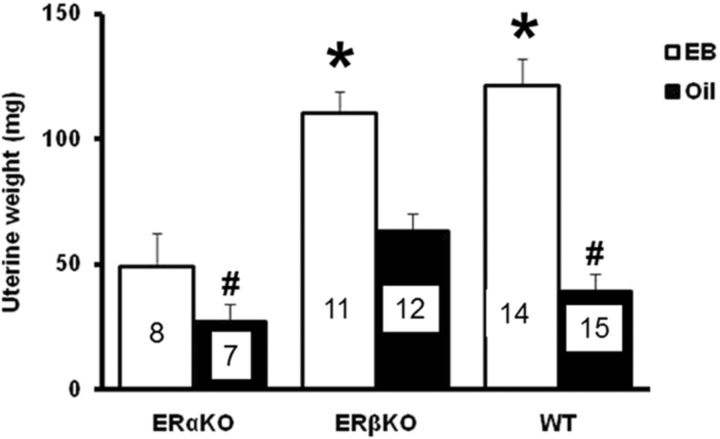
The effect of ovariectomy and estradiol treatment on hippocampal function varies across the life span (Foster, 2005). Previous work indicates that cyclic injections of estradiol can improve spatial learning and memory in mice, including middle-age mice (Frick et al., 2002; Gresack and Frick, 2006; Xu and Zhang, 2006; Walf et al., 2008; Aenlle et al., 2009). To determine whether ERα and ERβ contribute to the observed effects of estradiol on behavior and gene expression during middle age, ERαKO and ERβKO mice were ovariectomized at 13 months of age, treated with oil or estradiol, behaviorally tested, and killed to examine hippocampal and uterine tissues (Fig. 1C). Uterine weight across experimental groups exhibited an interaction of genotype and treatment [F(2,61) = 5.1, p < 0.01] (Fig. 4). Post hoc analyses confirmed that estradiol treatment increased uterine weight in WT and ERβKO mice, but not in ERαKO mice (Lindberg et al., 2002). Furthermore, uterine weight was higher in ERβKO-oil mice compared with ERαKO-oil and WT-oil mice. These results are consistent with the view that ERα induces uterine growth in response to estradiol treatment and that ERβ normally dampens ERα activity (i.e., knockout of ERβ promotes uterine growth in the absence of estradiol treatment).
Figure 4.
Uterine weight was increased by estradiol treatment (open bars) relative to oil treatment (filled bars) in ERβKO and WT mice. The numbers in each bar indicate the number of animals in each group. *Significant (p < 0.05) differences between estradiol and oil treatment. #Significant difference between oil-treated WT and ERαKO animals relative to ERβKO oil-treated mice.
Cue discrimination task training was performed during week 5 after surgery, 48 h after the fifth series of cyclic injections of estradiol or oil (Fig. 1C). Significant effects of training [F(3,183) = 30.28 p < 0.0001] and genotype [F(2,183) = 3.35, p < 0.05] were observed for the distances swam to escape the pool during cue discrimination training (Fig. 5A). Post hoc tests of genotype effects on swim distance collapsed across training blocks, and treatment groups indicated that the distances swum by ERαKO and WT mice were less than those swum by ERβKO mice. Separate ANOVAs within each treatment group indicated a tendency (p = 0.055) for genotype differences between oil-treated mice, and post hoc tests on distance collapsed across training blocks indicated that the swim distances for ERαKO-oil mice were less than those for ERβKO-oil mice (Fig. 5B). Together, these results indicate that, in the absence of estradiol treatment, middle-age ERαKO mice exhibit better cue discrimination learning than ERβKO mice.
Figure 5.
Water maze performance of middle-age ERKO and WT mice treated with estradiol (WT = 14, ERαKO = 8, ERβKO = 11) or oil (WT = 15, ERαKO = 7, ERβKO = 12). A, Mean path length to reach an escape platform plotted over four training blocks during cue discrimination training for ERαKO (gray circles and bars), ERβKO (open circles and bars), and WT mice (filled circles and bars). B, Mean path length (± SEM) across all cue discrimination training blocks grouped by treatment. C, Mean path length to reach an escape platform during spatial training. Spatial discrimination training was initiated on week 6 after surgery, 48 h after the previous injection of either estradiol or oil, and was continued for 3 consecutive days. D, Mean path length (± SEM) across all days of spatial training grouped by treatment. #Significant differences from the ERβKO-oil group.
Spatial discrimination training was initiated on week 6 after surgery, 48 h after the sixth series of cyclic injections of either estradiol or oil, and was continued for 3 consecutive days (Fig. 1C). An ANOVA on escape path length revealed significant training effects [F(2,122) = 22.42 p < 0.0001] and genotype effects [F(2,122) = 3.47, p < 0.05] (Fig. 5C). Post hoc tests of the genotype effects on escape path length collapsed across days and treatment groups indicated that the escape path lengths of ERβKO mice were shorter than those for ERαKO and WT mice (p < 0.05). ANOVAs within each treatment group revealed that there was an effect of genotype in oil-treated mice [F(2,62) = 3.9, p < 0.05], and post hoc tests of these mice indicated that the escape path lengths for ERβKO mice were less than those for ERαKO and WT mice (Fig. 5D). Thus, the effects of genotype on spatial discrimination learning appear to be opposite to those observed for cue discrimination learning; in the absence of estradiol treatment, middle-age ERβKO mice exhibit better spatial discrimination learning than ERαKO and WT mice, a difference that disappeared after estradiol treatment.
The ability of estradiol treatment to restore spatial discrimination learning in ERαKO and WT mice to ERβKO performance levels was confirmed by the probe trial data. Repeated-measures ANOVAs on the discrimination index measures for probe trials delivered as the penultimate trial on days 2 and 3 (Fig. 6A) indicated a training by treatment interaction [F(1,61) = 5.26, p < 0.05] and a trend for an interaction of training and genotype (p = 0.08). Follow-up ANOVAs for each treatment group indicated training effects for estradiol-treated mice [F(1,30) = 9.84, p < 0.005], and an ANOVA for each day indicated a treatment effect on day 3 for the discrimination index (p < 0.05) that reflected improved performance of mice treated with estradiol. Examination of training effects for each genotype indicated that only ERβKO mice showed an improvement in performance [F(1,22) = 5.37, p < 0.05]. Post hoc comparisons indicated that the performance of ERβKO mice on day 3 was significantly better than the performance of the WT (p < 0.05; Fig. 6A). Finally, one-tailed t tests comparing search behavior to chance indicated that all estradiol-treated groups exhibited a search pattern focused on the goal quadrant on day 3 (p < 0.05). For oil-treated mice, only ERβKO-oil mice exhibited performances different from chance (Fig. 6A). A repeated-measures ANOVA on the number of platform crossings for days 2 and 3 indicated an effect of training [F(1,61) = 7.16, p < 0.01] and a trend (p = 0.09) for a genotype effect. Examination of training effects in each genotype indicated that only ERβKO mice improved performance [F(1,22) = 4.81, p < 0.05]. Post hoc comparisons of genotype differences for each day indicated that ERβKO mice made more crossings than WT mice (p < 0.05) on day 3 (Fig. 6B). Thus, all groups treated with estradiol appeared to have acquired a spatial search strategy by the third day of training, and the performances of ERβKO-oil mice were superior to those of the other groups treated with oil (Fig. 6B).
Figure 6.
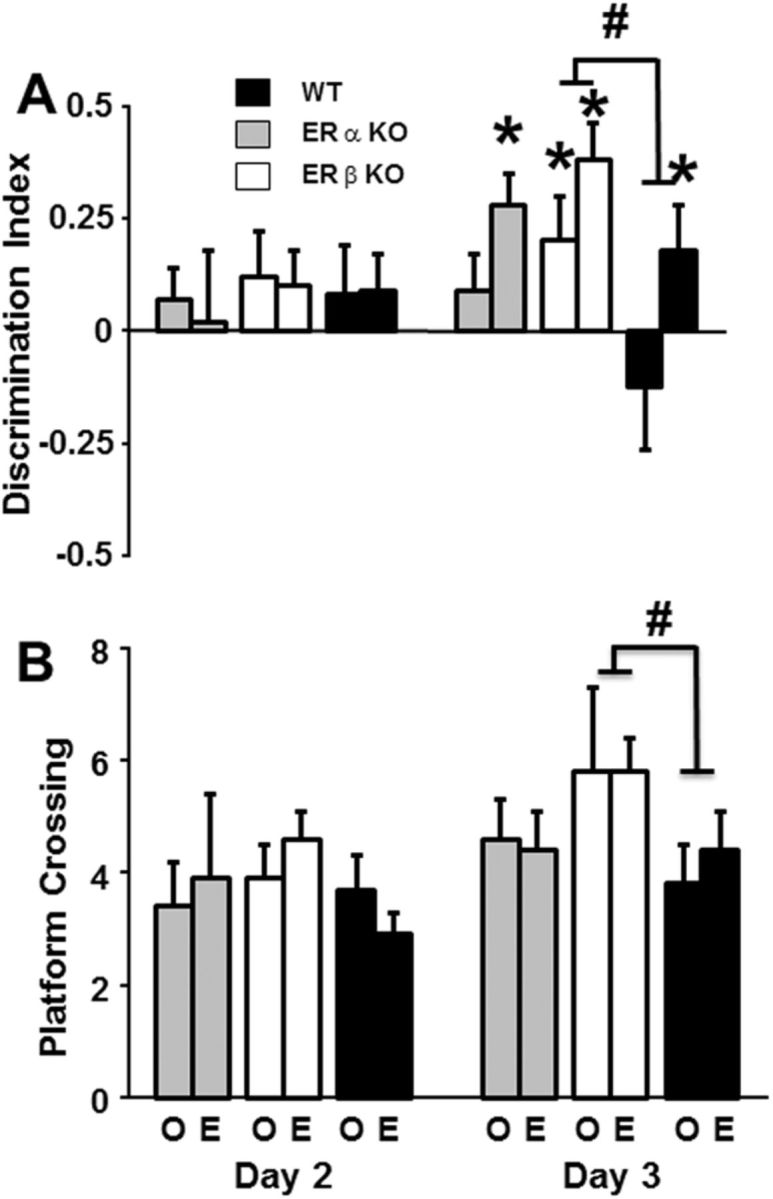
Probe trial measures for middle-age WT (filled bars), ERαKO (gray bars), and ERβKO (open bars) mice treated with oil (O: WT = 15, ERαKO = 7, ERβKO = 12) or estradiol (E: WT = 14, ERαKO = 8, ERβKO = 11). A, Mean (± SEM) discrimination index calculated from probe trial performance. Probe trials were delivered as the penultimate trial on days 2 and 3 for each session. *Performance above chance and acquisition of a spatial search strategy. B, Mean (± SEM) platform crossing calculated from probe trial performance. #Significant genotype difference between ERβKO and WT mice.
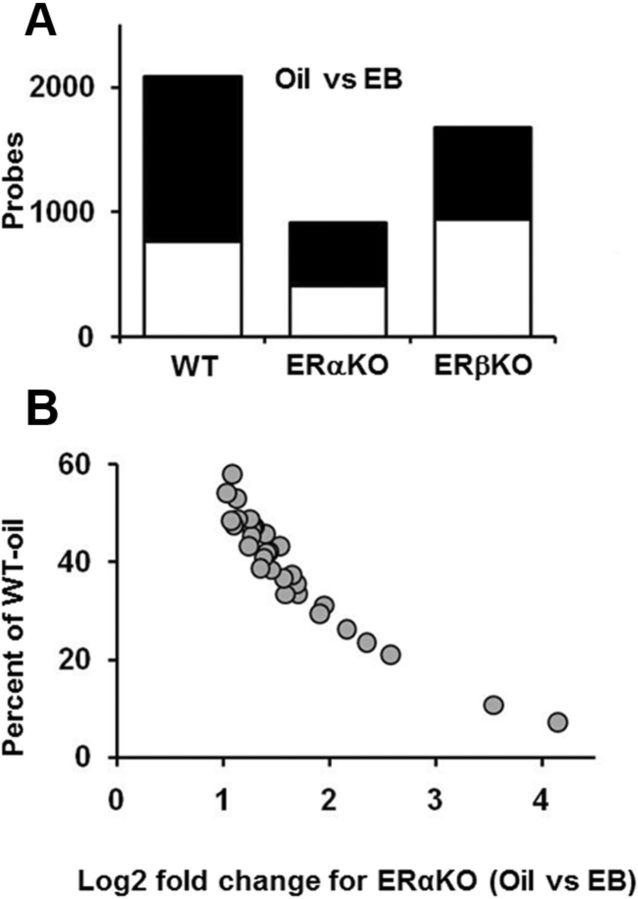
For gene profiling, middle-age mice (4–6 mice per group) were examined 24 h after the eighth series of estradiol or oil injections (Fig. 1C) using Affymetrix GeneChip Mouse Gene 1.0 ST Arrays, one array per mouse. First, we identified differentially expressed genes (p < 0.025) between oil and estradiol-treated mice within each genotype (Fig. 7A). A total of 2089 probes were found to be differentially expressed between WT-EB and WT-oil-treated mice, the majority of which (1324, 63%) exhibited reduced expression. The expression level of one probe was reduced by >2-fold, and the expression of 2 probes increased >2-fold. The number of probes exhibiting differential expression in ERαKO-EB mice compared with ERαKO-oil mice was 916, ∼half the number observed for WT mice. Although the majority of probes for ERαKO-EB mice exhibited reduced expression (509, 56%), no probe was observed whose expression decreased >2-fold. In contrast, 31 probes exhibited a >2-fold increase in expression. Interestingly, the basal expression levels of these 31 probes in ERαKO-oil mice were lower than observed in WT-oil mice (Fig. 7B). These highly responsive probes have been associated with the cells of the blood–brain barrier and choroid plexus (Lein et al., 2007) (Table 1). Thus, our analyses of middle-age ERαKO mice under low estradiol conditions suggest that there is a large shift in the expression of select genes associated with the blood–brain barrier and that their expression is restored to normal levels by estradiol treatment. Finally, the changes in gene expression for ERβKO-EB relative to ERβKO-oil mice were intermediate between WT and ERαKO mice, with 1681 probes showing differential expression. In this case, 938 probes (56%) increased expression, and the increase for only one probe was >2-fold.
Figure 7.
Gene expression in middle-age WT and ERKO mice, 24 h after the last estradiol (WT = 14, ERαKO = 8, ERβKO = 11) or oil (WT = 15, ERαKO = 7, ERβKO = 12) treatment. A, Graphic summary of the total number of probes that increased (open) and decreased (filled) in WT-EB, ERαKO-EB, and ERβKO-EB-treated mice compared with genotype-matched oil-treated counterparts. WT and ERβKO mice exhibited ∼2 times more genes whose expression was altered by estradiol relative to ERαKO mice. B, Expression of 31 probes in ERαKO mice normalized to expression in WT-oil mice. Basal expression for ERαKO-oil mice is plotted on the y-axis, and the increase in expression observed in ERαKO-EB mice is plotted on the x-axis as the log2 fold change.
Table 1.
Genes increased >2-fold by estradiol treatment in middle-aged ERαKO mice
| Affymetrix probe gene ID | Protein | Symbol | Fold |
|---|---|---|---|
| 10356403 | Potassium inwardly rectifying channel, subfamily J, member 13 | Kcnj13 | 17.67 |
| 10454192 | Transthyretin | TTR | 11.63 |
| 10436958 | Chloride intracellular channel 6 | Clic6 | 5.954 |
| 10539393 | Solute carrier family 4, sodium bicarbonate cotransporter, member 5 | SLC4A5 | 5.09 |
| 10419356 | Orthodenticle homolog 2 (Drosophila) | Otx2 | 4.47 |
| 10351224 | Coagulation factor V; similar to murine coagulation factor V | LOC100048143 | 3.86 |
| 10395389 | Sclerostin domain containing 1 | SOSTDC1 | 3.74 |
| 10547191 | Transmembrane protein 72 | TMEM72 | 3.26 |
| 10566034 | Folate receptor 1 (adult) | FOLR1 | 3.22 |
| 10602033 | Claudin 2 | CLDN2 | 3.12 |
| 10599422 | RIKEN cDNA 1110059M19 gene | 1110059M19Rik | 2.99 |
| 10577444 | Defensin β 11 | Defb11 | 2.97 |
| 10402195 | Tandem C2 domains, nuclear | tc2n | 2.89 |
| 10451818 | sulfotransferase family, cytosolic, 1C, member 2 | Sult1c2 | 2.74 |
| 10478048 | Lipopolysaccharide binding protein | lbp | 2.69 |
| 10586118 | Calmodulin-like 4 | calml4 | 2.65 |
| 10527870 | Klotho | KL | 2.63 |
| 10543921 | Solute carrier family 13 (sodium/sulfate symporters), member 4 | Slc13a4 | 2.60 |
| 10569008 | Cytochrome c oxidase, subunit VIIIb | COX8B | 2.55 |
| 10495712 | ATP-binding cassette, subfamily A (ABC1), member 4 | abca4 | 2.44 |
| 10428619 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 | ENPP2 | 2.43 |
| 10381962 | Angiotensin I converting enzyme (peptidyl-dipeptidase A) 1 | ACE | 2.41 |
| 10362104 | Solute carrier family 2 (facilitated glucose transporter), member 12 | SLC2A12 | 2.40 |
| 10344897 | Sulfatase 1 | Sulf1 | 2.37 |
| 10373588 | Retinol dehydrogenase 5 | RDH5 | 2.37 |
| 10517655 | Phospholipase A2, group V; similar to phospholipase A2, group V | LOC100048852 | 2.19 |
| 10542993 | Paraoxonase 3 | PON3 | 2.18 |
| 10440091 | Collagen, type VIII, α 1 | COL8A1 | 2.14 |
| 10436947 | Potassium voltage-gated channel, Isk-related subfamily, gene 2 | KCNE2 | 2.12 |
| 10569344 | Insulin-like growth factor 2 | IGF2 | 2.10 |
| 10584653 | C1q and tumor necrosis factor-related protein 5; membrane-type frizzled-related protein | C1QTNF5 | 2.05 |
Genes in middle-age ERαKO-EB-treated mice that exhibited a >2-fold increase in expression relative to ERαKO-oil mice.
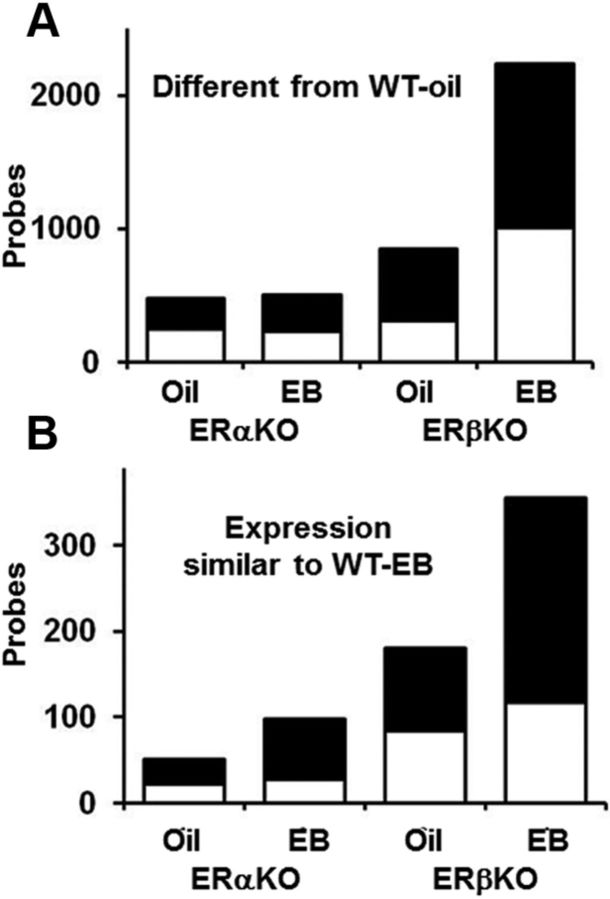
Next, we examined the differences in basal and estradiol-induced gene expression in ERαKO and ERβKO mice relative to WT-oil-treated mice (Fig. 8). ERαKO exhibited little change in the number of differentially expressed genes after either oil or estradiol treatments. ERβKO-oil mice exhibited approximately twice as many differentially expressed probes as ERαKO-oil mice; estradiol treatment induced a fourfold increase in the number of differentially expressed genes in ERβKO mice compared with ERαKO mice (Fig. 8B). Five or fewer probes exhibited a fold change >2 within each group. ERαKO and ERβKO mice do not express the full-length transcripts for ERα and ERβ, respectively, because of a neomycin cassette insert that functionally knocks out the gene. Therefore, it was notable that there was a threefold increase in the level of the transcript encoding the nonfunctional ERβ (ESR2, gene ID 10401035) in ERβKO mice treated with either oil or estradiol, and a 2.5-fold or a twofold increase in the levels of the transcript encoding the nonfunctional ERα (ESR1, gene ID 10367600) in ERαKO treated with estradiol or oil, respectively. The twofold increase in ESR1 in ERαKO-oil mice did not reach our significance cutoff (p = 0.045). The increased transcription of the nonfunctional ESR1 and ESR2 genes in young and middle-age ERKO mice suggests that loss of the functional receptors induces a compensatory transcription increase in these genes.
Figure 8.
Illustration of the total number of probes showing significant increases (open) and decreases (filled) in ERαKO or ERβKO oil (ERαKO = 7, ERβKO = 12) and estradiol-treated (ERαKO = 8, ERβKO = 11) mice compared with WT-oil mice (n = 15). A, Compared with ERαKO mice, ERβKO mice exhibited more genes that were altered under oil conditions. Treatment with estradiol resulted in a further increase in the number of altered genes in ERβKO mice with little evidence of an estradiol effect on gene expression in ERαKO mice. B, Analysis was limited to a subset of genes that showed altered expression in WT-EB compared with WT-oil mice (i.e., estradiol-responsive genes). The number of estradiol-responsive genes is plotted for KO mice. Four times more genes in ERβKO mice than in ERαKO mice exhibited changes that matched those observed in WT-EB under oil and estradiol treatment conditions.
Next, we set out to determine whether the genes that were differentially expressed in KO mice, relative to WT-oil mice, were estradiol-sensitive genes. Estradiol-sensitive genes were defined as the set of 2089 probes that were differentially expressed between WT-oil and WT-EB mice. We used this set of genes as a “seed” to determine whether KO animals expressed an estradiol-sensitive transcription profile similar to that observed in WT-EB mice. For the ERαKO-oil, ERαKO-EB, ERβKO-oil, and ERβKO-EB groups, we determined the number of estradiol-sensitive genes whose change in expression was similar to that observed in the WT-EB group. Of the probes identified, the expression levels of very few were altered in a direction opposite to that observed in WT-EB mice (ERαKO-oil = 3, ERαKO-EB = 4, ERβKO-oil = 0, ERβKO-EB = 5). Figure 8B illustrates the differential expression patterns for probes that were significantly different from WT-oil and exhibited expression changes similar to those observed in WT-EB mice. In general, twice as many estradiol-responsive genes exhibited expression changes in KO mice treated with estradiol than in genotype-matched mice treated with oil, an observation that suggests that knockout of either ERα or ERβ does not completely block estradiol sensitivity in these mice. Furthermore, there were approximately four times more differentially expressed estradiol-responsive genes in ERβKO mice treated with either estradiol or oil compared with ERαKO mice (ERαKO: oil = 51, estradiol = 98; ERβKO: oil = 181, estradiol = 355). This result further supports the idea that one role of ERβ is to suppress ERα driven gene transcription.
RT-PCR
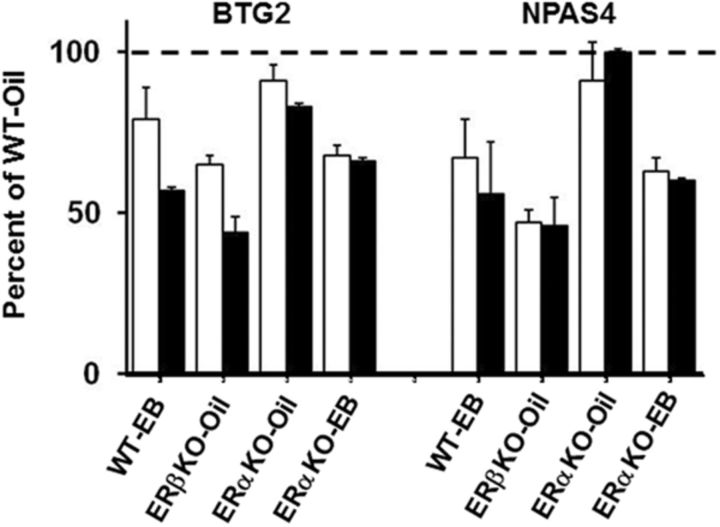
Genes whose expression was altered in WT-EB, ERαKO-EB, and ERβKO-oil mice relative to WT-oil mice were analyzed using RT-PCR. Three mice were analyzed per group. Two genes were examined that had exhibited relatively robust 1.5- to 2.0-fold changes in middle-age WT-EB, ERαKO-EB, and ERβKO-oil mice. BTG2 is an antiproliferative protein whose expression is regulated by ERα and ERβ (Karmakar et al., 2009; Paruthiyil et al., 2010), and NPAS4 is a transcription factor that influences the development of inhibitory synapses (Lin et al., 2008). The expression of these two genes decreased in ERβKO-oil, ERαKO-EB, and WT-EB mice compared with WT-oil mice (Fig. 9A). Pearson correlation for RT-PCR and microarray values for BTG2 and NPAS4 across the groups indicated a significant relationship (r = 0.83, p < 0.01).
Figure 9.
RT-PCR confirmation of the expression patterns observed for a subset of genes. Comparisons of the expression levels of two genes, BTG2 and NPAS4, determined by microarray (open bars) for mice treated with oil (ERαKO-oil: n = 7, ERβKO-oil: n = 12) or estradiol (WT-EB: n = 14, ERαKO-EB: n = 8, ERβKO-EB: n = 11) and RT-PCR (filled bars, n = 3 for each condition), illustrating that expression was decreased by estradiol treatment in WT, ERαKO, and ERβKO-oil mice. Expression is presented as percentage of WT-oil for microarray n = 15 and RT-PCR n = 3.
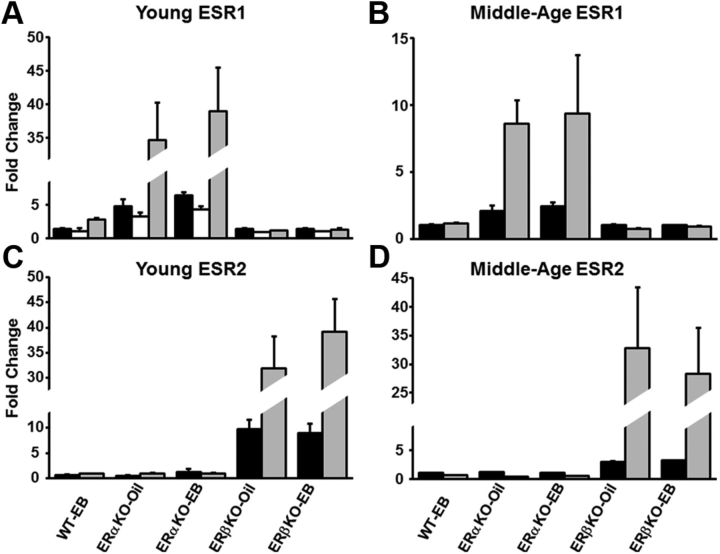
The arrays also suggested that there was an increase in transcription of the functionally disrupted ERs in the ERβKO and ERαKO mice, the magnitudes of which may have been underestimated because the probes flanked the neomycin inserts that were used to disrupt function of the receptors. RT-PCR confirmed that the level of transcription of functionally disrupted ESR1 was increased in ERαKO mice compared with WT-oil mice. Gene arrays revealed a sixfold increase in ESR1 transcription in young ERαKO mice relative to WT-oil, whereas RT-PCR analyses suggested that the increase was 30-fold higher than WT-oil (Fig. 10A). The increases in ESR1 transcript levels were twofold and eightfold higher for middle-age ERαKO mice using gene arrays or RT-PCR, respectively (Fig. 10B). Similarly, ESR2 transcript levels were higher in ERβKO mice (Fig. 10C, D) relative to WT-oil, and the observed increases determined using RT-PCR were greater than those obtained from the gene arrays.
Figure 10.
RT-PCR confirmation of the expression pattern for ESR1 and ESR2 in ERαKO and ERβKO mice. A, The expression of ESR1 was increased in (A) young and (B) middle-age ERαKO mice. Note that the increase in ESR1 was considerably reduced in middle-age ERαKO mice. ESR2 expression was increased in (C) young and (D) middle-age ERβKO mice. A, Data are presented for microarray probes 1435663_at (filled bars) and 1457877_at (open bars) for mice treated with oil (ERαKO-oil: n = 5, ERβKO-oil: n = 5) or estradiol (WT-EB: n = 7, ERαKO-EB: n = 5, ERβKO-EB: n = 7) and RT-PCR (gray bars, n = 3 for each condition). B–D, Data are presented for microarray (filled bars) for mice treated with oil (ERαKO-oil: n = 7, ERβKO-oil: n = 12) or estradiol (WT-EB: n = 14, ERαKO-EB: n = 8, ERβKO-EB: n = 11) and for RT-PCR (gray bars, n = 3 for each condition). All values are expressed as a fold change relative to the condition matched WT-oil (young microarray n = 7 and RT-PCR n = 3, middle-age microarray n = 15 and RT-PCR n = 3). Error bars represent SEM.
Viral expression of ERβ impairs spatial learning in ERβKO mice
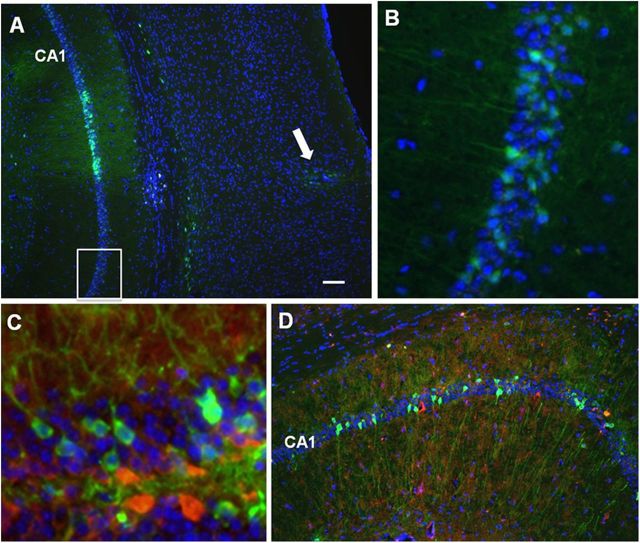
Our experimental data indicate that, in the absence of estradiol treatment, middle-age ERβKO mice exhibit better spatial discrimination learning than ERαKO and WT mice. To further investigate this observation, we ovariectomized and bilaterally injected the hippocampi of middle-age (13–14 months) ERβKO mice with lentivirus encoding both ERβ-FLAG and GFP (n = 12) or GFP alone (n = 11) to determine whether expression of ERβ in the hippocampus of these mice disrupted spatial learning. Training on the cue discrimination task was performed 5 weeks after surgery. Expression of FLAG-tagged ERβ was detected using an anti-FLAG antibody. Consistent with our previous study in which we examined lentiviral-mediated expression of ERα in the hippocampus (Foster et al., 2008), histological examination of the injected hippocampi indicated that the expression of ERβ-FLAG and GFP was primarily localized to the hippocampus with limited expression in the cortex along the needle track (Fig. 11A). Intense expression of the viral transgenes was observed near the site of injection; however, considerable expression was observed several hundred microns on either side of the injection site (Fig. 11B). ERβ-FLAG was largely extranuclear in the cell bodies of pyramidal and granule cells with diffuse expression in the apical dendritic regions (Fig. 11C, D). In contrast, GFP could be observed filling the soma and dendritic processes (Fig. 11C).
Figure 11.
Lentiviral-mediated expression of ERβ and GFP in the hippocampus of ERβKO mice. A, Expression is largely limited to the hippocampus and the needle track (arrow). Intense GFP expression (green) is observed in region CA1 near the injection site and was detectable several hundreds of microns from the injection site (box). B, Enlarged view of the box shown in A. C, ERβ-FLAG (red) and GFP (green) in the dentate gyrus showing perinuclear expression ERβ-FLAG while GFP could be observed filling dendrites. D, Expression of GFP (green) and ERβ-FLAG (red) in region CA1. Scale bar: A, 50 μm.
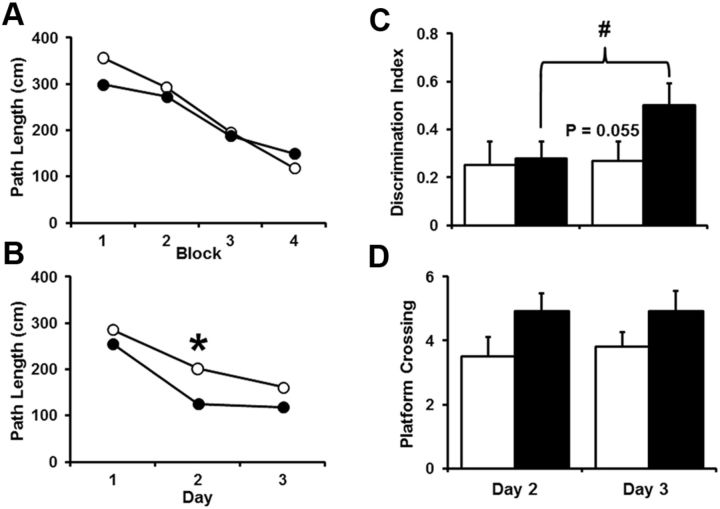
Analyses of the behaviors of these mice showed that there was a significant effect of training [F(3,63) = 15.46, p < 0.0001] for distance to escape the pool during cue discrimination training in the absence of an effect of viral injection (Fig. 12A). Spatial discrimination training was initiated 6 weeks after surgery. An ANOVA on escape path length indicated a significant effect of training block [F(2,42) = 27.28, p < 0.0001] and a tendency (p = 0.06) for an effect of virus injection (Fig. 12B). Post hoc tests for each day indicated that ERβKO mice injected with virus encoding GFP (ERβKO-GFP) exhibited a significantly shorter path length on day 2 than ERβKO mice injected with virus encoding ERβ-FLAG. Examination of the probe trial discrimination index scores indicated a tendency (p = 0.07) for a training effect. The discrimination scores for ERβKO-GFP mice increased across days [F(1,10) = 7.35, p < 0.05], and a tendency for a treatment effect (p = 0.055) was observed for day 3 with ERβKO-GFP mice showing increased learning (Fig. 12C). A repeated-measures ANOVA for platform crossings revealed a treatment effect [F(1,21) = 4.88, p < 0.05] that reflected an higher number of platform crossings by ERβKO-GFP mice compared with ERβ-FLAG-expressing mice (Fig. 12D). Together, these results indicate that expression of ERβ in the hippocampus impaired cognition under conditions of low estradiol, and suggest that hippocampal ERβ expression plays a role in cognition in adults.
Figure 12.
Hippocampal expression of ERβ in middle-age ERβKO mice impairs water maze spatial learning. A, Mean path length to reach an escape platform over four training blocks for ERβKO mice expressing GFP (filled circles and bars, n = 11) or GFP and ERβ-FLAG (open circles and bars, n = 12). A decrease in path length was observed in the absence of a treatment effect. B, Mean path length to reach an escape platform during 3 d of spatial training for ERβKO mice expressing GFP or GFP and ERβ-FLAG. *A tendency for a treatment effect was the result of a decrease in the distance for ERβKO-GFP mice to reach the platform on day 2. C, Spatial learning measured as a discrimination index calculated from probe trial performance. #ERβKO-GFP mice exhibited an increase in the discrimination index score across the 2 d of training. A tendency (p = 0.055) for a treatment effect was observed for day 3, with increased learning for ERβKO-GFP mice. D, Platform crossing calculated from probe trial performance. ERβKO-GFP mice exhibited more platform crossings than ERβKO mice with expression of ERβ limited to the hippocampus.
Discussion
Expression of ERα and ERβ influences spatial learning
The current study indicates that cognitive function is affected by interactions between ERα and ERβ and estradiol exposure history. The learning performances of young (Fig. 3) and middle-age ovariectomized ERβKO-oil mice (Figs. 5 and 6) were superior to those observed for WT-oil and ERαKO-oil mice, indicating that removal of ERβ preserved learning abilities in these hormone-deprived mice. Importantly, we found that expression of ERβ in the hippocampi of ovariectomized, middle-age ERβKO mice reversed the benefits of functional knockout of ERβ.
Daily injections of estradiol benzoate (1–5 μg) improve spatial memory in mice (Frick et al., 2002; Li et al., 2004; Xu and Zhang, 2006); however, higher doses can impair learning (Fugger et al., 1998). We confirmed that estradiol injections (5 μg) can have lasting (48–72 h) effects on cognition (Aenlle et al., 2009). In addition, estradiol treatment induced lasting memory enhancement in middle-age ERαKO mice, a finding that extends previous work showing that estradiol improves cognition in young ERαKO mice (Fugger et al., 2000; Liu et al., 2008). Improved learning in ERαKO-EB mice was observed 72 h after the last treatment, suggesting likely genomic rather than membrane effects. Injection of 5 μg estradiol is expected to produce a supraphysiological rise in serum estradiol, which then declines to basal levels by 72 h after the last injection (Gordon et al., 1986; Woolley and McEwen, 1993; Noppens et al., 2005). Finally, our observations extend previous reports that estradiol treatment does not facilitate memory in young ERβKO mice (Fugger et al., 2000; Liu et al., 2008; Walf et al., 2009) to middle-age ERβKO mice.
ERαKO mice: estradiol treatment required for compensation of a loss of ERα
Young ERαKO and WT mice exhibited a similar pattern in gene expression 6 h after estradiol treatment (Fig. 2), suggesting that there are compensatory mechanisms that regulate gene transcription in response to estradiol in the absence of ERα. Furthermore, estradiol facilitated memory 72 h after treatment in middle-age ERαKO mice, suggesting that these compensatory mechanisms contribute to estradiol effects on memory. However, the ability of ERαKO mice to respond to estradiol may diminish as a function of age because the similarities between the ERαKO-EB and WT-EB transcriptomes were reduced in middle-age ERαKO-EB mice (Fig. 8A).
Middle-age ERαKO-oil mice exhibited reduced expression of genes expressed in capillaries and the choroid plexus, including genes involved in blood–brain barrier transport, several of which (TTR, CLIC6, and KCNJ13) are sensitive to aging in the human hippocampus (Kang et al., 2011). Several of these genes have also been linked to neurodegenerative diseases, including the deposition of amyloid β (Hong-Goka and Chang, 2004; Tang et al., 2004; Quintela et al., 2009) and tau regulation (Woo et al., 2010). These results suggest that blood–brain barrier transport processes may be disrupted by aging and exacerbated by extended loss of ERα activity; nevertheless, our results indicate that ERα-independent and estradiol-sensitive mechanisms exist that can sustain these processes in the presence of hormone replacement.
ERβKO mice: ERβ regulation of transcription and hippocampal function
Superior spatial learning was observed in middle-age ERβKO mice, an advantage that was negated by hippocampal expression of ERβ. This result suggests that knockout of ERβ provided protection against cognitive decline. Furthermore, ERβKO mice exhibited a marked shift in basal transcription of estradiol-responsive genes (Figs. 2 and 8), suggesting that the shift in transcription may have contributed to preserved cognition in ERβKO mice. A similar finding has been reported in bone, such that aging ERβKO mice are protected from bone loss (Windahl et al., 2001) and show increased transcription of estradiol-responsive genes (Lindberg et al., 2003). Furthermore, knockdown of ERβ has been shown to reduce the vulnerability of hippocampal cells to oxidative stress (Yang et al., 2009).
The increased basal transcription of estradiol-responsive genes in the hippocampi of ERβKO-oil mice is likely the result of several factors. Cell culture studies indicate that ERβ antagonizes ERα-mediated transcription (Hall and McDonnell, 1999; Pettersson et al., 2000; Gottfried-Blackmore et al., 2007). Similarly, ERα-associated transcriptional activity in bone (Lindberg et al., 2003) and aortic tissue (O'Lone et al., 2007) is enhanced in ERβKO mice. In brain regions that express both receptors, estradiol-induced transcription declines as the levels of ERα and ERβ shift to favor ERβ (Gonzales et al., 2008). A second factor that influences basal transcription is the affinity of estradiol for ERα and ERβ. Low levels of estradiol are synthesized in the hippocampus (Prange-Kiel et al., 2003; Kretz et al., 2004) and low concentrations of estradiol favor activation of ERα (Kuiper et al., 1997; Tremblay et al., 1997; Barkhem et al., 1998; Pettersson et al., 2000).Together, these results suggest that transcription of estradiol-sensitive genes in ERβKO-oil mice reflects a release of ERβ inhibition of ERα-mediated transcription.
Interaction of ERα and ERβ signaling pathways
The ERs knocked out in our mice were absent during fetal development. However, differences that we observed in cognition cannot be ascribed to developmental effects alone. First, superior performance of the ERβKO mice relative to the ERαKO and WT mice was limited to conditions of hormone deprivation, indicating that changes in hormone levels were required to reveal the interactions between ERβ and ERα. Second, in middle-age ERβKO mice, lentiviral-mediated expression of ERβ in the hippocampus impaired cognition, indicating that hippocampal ERβ expression plays a role in cognition in adults. Indeed, loss of ERβ expression in tissues other than the hippocampus may be a more significant problem for aging animals. At ∼2 years of age, ERβKO mice exhibit excessive weight gain and develop pituitary tumors and myeloproliferative disease, changes that suggest loss of ERβ may induce an excessive growth response (Shim et al., 2003; Fan et al., 2010). Furthermore, there is evidence that brain regions outside the hippocampus may exhibit degeneration in aged ERβKO mice (Wang et al., 2001).
Interactions between ERα and ERβ in younger animals may provide a mechanism for the regulation of hippocampal function and gene expression in an environment of fluctuating hormone levels (Foster, 2005, 2012). In young ERβKO mice, estradiol treatment decreased expression of a number of estradiol-responsive genes, an observation that suggests that excessive ERα activity can produce large-scale transcriptional changes in estradiol responsive pathways. This feedback process may contribute to decreased ERα expression after administration of supraphysiological levels of estradiol (Iivonen et al., 2006). Previous work indicates that young ERβKO mice exhibit learning impairments and a decline in the expression of ERα when chronically treated with estradiol (Rissman et al., 2002), changes that could reflect the absence of ERβ regulation of ERα activity (Foster, 2012). Interestingly, we observed that transcription of nonfunctional ESR1 and ESR2 increased in ERαKO and ERβKO mice, respectively, results consistent with the idea that the activities of these receptors play a role in regulating their transcription.
ERα and ERβ over the life span
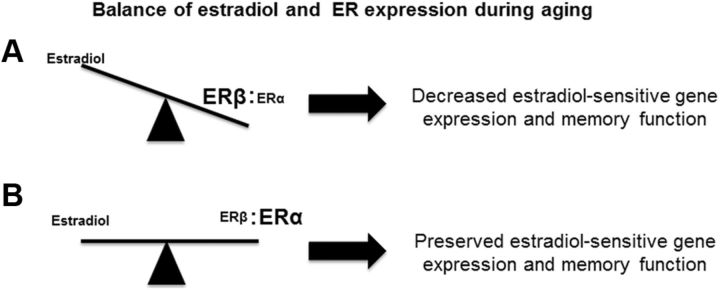
The results indicate that maintenance of hippocampal function depends on a balance between estradiol levels and the relative level of expression of ERα and ERβ (Fig. 13). Aging is associated with decreased expression of hippocampal ERα or the expression of dominant-negative ERα splice variants that could reduce the ability of estradiol treatments to preserve cognition (Tohgi et al., 1995; Adams et al., 2002; Mehra et al., 2005; Ishunina et al., 2007; Bohacek and Daniel, 2009). A decrease in estradiol and reduced expression of ERα relative to ERβ during menopause would be predicted to act in concert to decrease transcriptional processes that normally help preserve cognitive function (Fig. 13A). Indeed, ERα polymorphisms have been associated with greater memory impairment during menopause as estradiol levels decrease (Ji et al., 2000; Corbo et al., 2006; Olsen et al., 2006; Yaffe et al., 2009). Our results indicate that increasing the expression of ERα may preserve transcription and memory, even as estradiol levels decline (Fig. 13B). These results are consistent with mounting evidence that suggest that increased ERα expression is associated with improved learning and memory (Foster, 2012). Treatments to alter the expression of ERs within the hippocampus could provide an alternative to hormone replacement in preserving cognitive function.
Figure 13.
Hippocampal estradiol-sensitive gene expression and memory function depend on the relative level of expression of ERα and ERβ. A, A decrease in estradiol and reduced expression of ERα relative to ERβ during menopause disrupts the transcriptional processes that normally help preserve cognitive function. B, Increasing the expression of ERα relative to ERβ preserves transcription and memory, even as estradiol levels decline.
Footnotes
This work was supported by National Institutes of Health Grants AG014979, AG037984, and AG036800, and the Evelyn F. McKnight Brain Research Foundation. We thank Irina Madorsky, Jose Herrera, Gina Prado, Paul Huang, and Katrina Velez for technical assistance.
The authors declare no competing financial interests.
References
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20:1047–1060. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–945. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The ability of oestradiol administration to regulate protein levels of oestrogen receptor α in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol. 2009;21:640–647. doi: 10.1111/j.1365-2826.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta) Endocrinology. 2003;144:4734–4738. doi: 10.1210/en.2003-0216. [DOI] [PubMed] [Google Scholar]
- Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor α (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer's disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22:67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERα in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Enmark E, Gustafsson JA. Oestrogen receptors: an overview. J Intern Med. 1999;246:133–138. doi: 10.1046/j.1365-2796.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- Fan X, Gabbi C, Kim HJ, Cheng G, Andersson LC, Warner M, Gustafsson JA. Gonadotropin-positive pituitary tumors accompanied by ovarian tumors in aging female ERβ−/− mice. Proc Natl Acad Sci U S A. 2010;107:6453–6458. doi: 10.1073/pnas.1002029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the life span. Front Neuroendocrinol. 2005;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor α and β expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-α to the hippocampus improves spatial learning in estrogen receptor-α knock-out mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERα on spatial learning. Horm Behav. 1998;34:163–170. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Sanz A, Mendez P. Cross-talk between IGF-I and estradiol in the brain: focus on neuroprotection. Neuroendocrinology. 2006;84:275–279. doi: 10.1159/000097485. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) β modulates ERα responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MN, Osterburg HH, May PC, Finch CE. Effective oral administration of 17 β-estradiol to female C57BL/6J mice through the drinking water. Biol Reprod. 1986;35:1088–1095. doi: 10.1095/biolreprod35.5.1088. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Croft G, McEwen BS, Bulloch K. Transcriptional activity of estrogen receptors ERα and ERβ in the EtC. 1 cerebellar granule cell line. Brain Res. 2007;1186:41–47. doi: 10.1016/j.brainres.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Idzerda RL, Brinster RL, McKnight GS. Estrogen regulation of the avian transferrin gene in transgenic mice. Mol Cell Biol. 1986;6:1010–1014. doi: 10.1128/mcb.6.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Hong-Goka BC, Chang FL. Estrogen receptors α and β in choroid plexus epithelial cells in Alzheimer's disease. Neurosci Lett. 2004;360:113–116. doi: 10.1016/j.neulet.2004.01.075. [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puoliväli J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen α and β mRNA levels in ovariectomized female mice. Neuroscience. 2006;137:1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor α and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiol Aging. 2007;28:1670–1681. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-α. J Neurosci. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer's disease, vascular dementia and alcohol-associated dementia. Dement Geriatr Cogn Disord. 2000;11:119–122. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Foster EA, Smith CL. Estradiol downregulation of the tumor suppressor gene BTG2 requires estrogen receptor-α and the REA corepressor. Int J Cancer. 2009;124:1841–1851. doi: 10.1002/ijc.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Korach KS. A new actor in the estrogen receptor drama: enter ER-β. Endocrinology. 1997;138:861–862. doi: 10.1210/endo.138.3.5080. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Lee WH, Kumar A, Rani A, Herrera J, Xu J, Someya S, Foster TC. Influence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 2012;16:339–350. doi: 10.1089/ars.2011.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li J, Wong L. Emerging patterns and gene expression data. Genome Inform. 2001;12:3–13. [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Weihua Z, Andersson N, Movérare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174:167–178. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol Endocrinol (Baltimore) 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor α and β immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Diaz Brinton R. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci U S A. 2003;100:2842–2847. doi: 10.1073/pnas.0438041100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Noppens RR, Kofler J, Hurn PD, Traystman RJ. Dose-dependent neuroprotection by 17β-estradiol after cardiac arrest and cardiopulmonary resuscitation. Crit Care Med. 2005;33:1595–1602. doi: 10.1097/01.ccm.0000169884.81769.f7. [DOI] [PubMed] [Google Scholar]
- O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors α and β mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol (Baltimore) 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Olsen L, Rasmussen HB, Hansen T, Bagger YZ, Tankó LB, Qin G, Christiansen C, Werge T. Estrogen receptor α and risk for cognitive impairment in postmenopausal women. Psychiatr Genet. 2006;16:85–88. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, Cvoro A, Tagliaferri M, Cohen I, Shtivelman E, Leitman DC. Estrogen receptor β causes a G2 cell cycle arrest by inhibiting CDK1 activity through the regulation of cyclin B1, GADD45A, and BTG2. Breast Cancer Res Treat. 2011;129:777–784. doi: 10.1007/s10549-010-1273-5. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Priest CA, Eckersell CB, Micevych PE. Estrogen regulates preproenkephalin-A mRNA levels in the rat ventromedial nucleus: temporal and cellular aspects. Brain Res Mol Brain Res. 1995;28:251–262. doi: 10.1016/0169-328x(94)00213-x. [DOI] [PubMed] [Google Scholar]
- Quintela T, Gonçalves I, Baltazar G, Alves CH, Saraiva MJ, Santos CR. 17β-Estradiol induces transthyretin expression in murine choroid plexus via an oestrogen receptor dependent pathway. Cell Mol Neurobiol. 2009;29:475–483. doi: 10.1007/s10571-008-9339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Eccles KS, Humberstone EJ. Targeted expression of two proteins in neural retina using self-inactivating, insulated lentiviral vectors carrying two internal independent promoters. Mol Vis. 2007;13:2001–2011. [PubMed] [Google Scholar]
- Sharma PK, Thakur MK. Expression of estrogen receptor (ER) α and β in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol Aging. 2006;27:880–887. doi: 10.1016/j.neurobiolaging.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Mäkelä S, Warner M, Gustafsson JA. Disruption of the estrogen receptor β gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci U S A. 2003;100:6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Haslam SZ, Conrad SE, Sisk CL. Estrogen increases brain expression of the mRNA encoding transthyretin, an amyloid β scavenger protein. J Alzheimers Dis. 2004;6:413–420. doi: 10.3233/jad-2004-6409. discussion 443–449. [DOI] [PubMed] [Google Scholar]
- Thakur MK, Sharma PK. Transcription of estrogen receptor α and β in mouse cerebral cortex: effect of age, sex, 17β-estradiol and testosterone. Neurochem Int. 2007;50:314–321. doi: 10.1016/j.neuint.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700:245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- Too CK, Giles A, Wilkinson M. Estrogen stimulates expression of adenine nucleotide translocator ANT1 messenger RNA in female rat hearts. Mol Cell Endocrinol. 1999;150:161–167. doi: 10.1016/s0303-7207(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguere V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol (Baltimore) 1997;11:353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared with diestrous wild-type, but not estrogen receptor β knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor β knockout mice. Proc Natl Acad Sci U S A. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor β on estrogen receptor α signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor β−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001;16:1388–1398. doi: 10.1359/jbmr.2001.16.8.1388. [DOI] [PubMed] [Google Scholar]
- Woo JM, Park SJ, Kang HI, Kim BG, Shim SB, Jee SW, Lee SH, Sin JS, Bae CJ, Jang MK, Cho C, Hwang DY, Kim CK. Characterization of changes in global gene expression in the brain of neuron-specific enolase/human Tau23 transgenic mice in response to overexpression of Tau protein. Int J Mol Med. 2010;25:667–675. doi: 10.3892/ijmm_00000390. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30:607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen receptor β as a mitochondrial vulnerability factor. J Biol Chem. 2009;284:9540–9548. doi: 10.1074/jbc.M808246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier Z, Kumar A, Bodhinathan K, Feller JA, Foster TC, Bloom DC. Fragile X mental retardation protein replacement restores hippocampal synaptic function in a mouse model of fragile X syndrome. Gene Ther. 2009;16:1122–1129. doi: 10.1038/gt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]