Abstract
Objectives
Selected patients with unresectable colorectal liver metastases (CLM) may be rendered resectable using the two-stage hepatectomy (TSH) approach. This review was conducted with the aim of collating and evaluating published evidence for TSH in patients with initially unresectable CLM.
Methods
Searches of the MEDLINE and EMBASE databases were undertaken to identify studies of TSH in patients with initially unresectable CLM. Studies were required to focus on the perioperative treatment regimen, operative strategy, morbidity, technical success and survival outcomes.
Results
Ten observational studies were reviewed. A total of 459 patients with initially unresectable CLM were selected for the first stage of TSH. Preoperative chemotherapy was used in 88% of patients and achieved partial and stable response rates of 59% and 39%, respectively. Postoperative morbidity and mortality after the first stage of TSH were 17% and 0.5%, respectively. Portal vein embolization (PVE) was used in 76% of patients. Ultimately, 352 of the initial 459 (77%) patients underwent the second stage of TSH. Major liver resection was undertaken in 84% of patients; the negative margin (R0) resection rate was 75%. Postoperative morbidity and mortality after the second stage of TSH were 40% and 3%, respectively. Median overall survival was 37 months (range: 24–44 months) in patients who completed both stages of TSH. In patients who did not complete both stages of TSH, median survival was 16 months (range: 10–29 months). The 3-year disease-free survival rate was 20% (range: 6–27%).
Conclusions
Two-stage hepatectomy is safe and effective in selected patients with initially unresectable CLM. Further studies are required to better define patient selection criteria for TSH and the exact roles of PVE and preoperative and interval chemotherapy.
Introduction
Colorectal cancer is the third most common cancer worldwide.1 Around 25% of patients have synchronous liver metastases at presentation and a further 25–50% subsequently develop metachronous liver disease.2,3 Surgical resection of colorectal liver metastases (CLM) is considered the only curative therapy and achieves 5-year overall survival (OS) rates of up to 58%.4–10,11 Nevertheless, fewer than 25% of patients with CLM are considered to have resectable disease.11–13 In the last decade, considerable efforts were directed towards developing strategies to increase the number of patients with CLM who could benefit from surgical resection. In 2000, Adam et al. published the first series of two-stage hepatectomies (TSHs) in patients with unresectable bilobar CLM that were not amenable to resection in a single operation, even in combination with preoperative chemotherapy and portal vein embolization (PVE).14 Two-stage hepatectomy is conceived as a planned and potentially curative strategy and consists of the resection of CLM in one hemiliver during the first stage, followed by a second resection of CLM in the contralateral hemiliver during the second stage. This systematic review was undertaken to assess the published evidence for the safety and efficacy of TSH in patients with initially unresectable CLM.
Materials and methods
Literature search strategy
A search of the MEDLINE (1966 to June 2012) and EMBASE (January 1974 to June 2012) databases was undertaken. The search terms colorectal cancer or colorectal neoplasm, liver metastases or hepatic metastases tumour, hepatectomy or liver resection or hepatic resection, and stage resection or two-stage hepatectomy were used. These terms were mapped to MESH (medical subject headings) terms and were searched for as text items. Reference lists from relevant articles were searched and the authors’ personal libraries were checked manually for other potentially relevant studies. No search was made of unpublished literature.
Study selection
The study evaluation was performed by two reviewers (VWTL and JML). Reviews, case reports, conference abstracts, non-human studies and case series in which TSH was completed in fewer than 10 patients were excluded. Abstracts of the remaining studies were retrieved and reviewed for relevance. The full texts of previously selected articles were thoroughly reviewed. Studies on which a decision could not be made based upon the abstract were also reviewed. Those studies which described the use of TSH with curative intent in patients with initially unresectable CLM were included for analysis. Studies that adopted hybrid approaches, combining liver resection with ablation techniques, or the resection of extrahepatic metastases with the aim of expanding the criteria for resection of CLM, were also included for review. Only studies reporting both short- and longterm outcomes of TSH were included. When multiple publications were identified from the same or overlapping patient series, only the most complete or recent publication was included. Study methodology quality was assessed according to the Newcastle–Ottawa scale.15 A score of ≥4 was required for inclusion.
Data extraction and critical appraisal
Two reviewers (VWTL and JML) independently appraised each article using predefined criteria. Data extracted included information on methodology, quality criteria, setting of the use of perioperative chemotherapy, response to chemotherapy, proportion of negative margin (R0) resections, disease-free survival (DFS) and OS, and morbidity and mortality in this multimodal approach. Discrepancies were resolved by consensus. Major hepatectomy was defined as the resection of three or more Couinaud segments. Because the reports included lacked control groups and the selected studies were heterogeneous, no meta-analysis could be carried out. A qualitative systematic review was performed without a comparator group by full tabulation of the results. The level of evidence of each article was scored according to the Hierarchy of Evidence table developed by the National Health and Medical Research Council of Australia.16 This systematic review was performed according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.17
Results
The literature search using the strategy described herein identified 226 studies. Duplicated studies, non-human studies, review articles, case reports and conference abstracts were excluded. The manuscripts of the 43 remaining articles were reviewed. Thirty-three articles that did not fulfil the inclusion criteria were excluded. The remaining 10 studies were individually reviewed (Fig. 1). No meta-analyses or randomized controlled trials (RCTs) were identified. Ten observational studies (Level IV evidence) were included for analysis.
Figure 1.
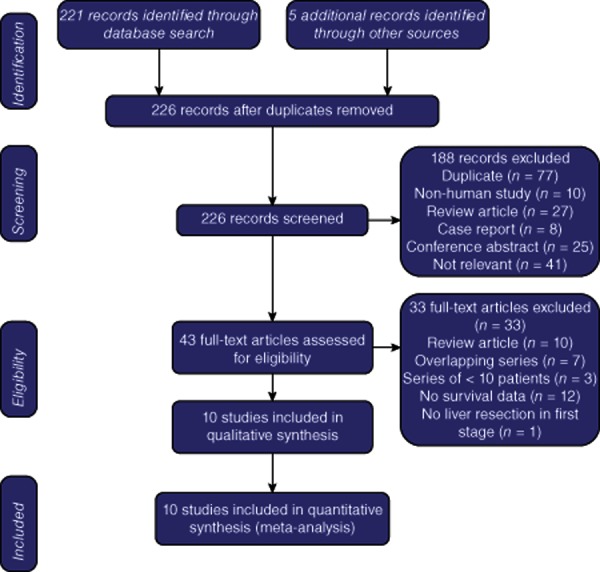
Flow diagram indicating the strategy used to select studies for inclusion in this review
This review pertains to 10 studies covering a combined total of 459 patients with unresectable CLM in whom the first stage of TSH was performed.18–27 One study, reporting TSH for initially unresectable liver metastases in 33 patients with colorectal cancer, three with neuroendocrine tumours, one with a gastrointestinal stromal tumour, one with ocular melanoma and one with salivary gland carcinoma, was included for analysis.22 Three studies with overlapping patient series28,29,30 were excluded, as was one study without longterm survival data.31 Three studies from the same institution reporting the use of ablation of CLM only at the first stage of TSH were excluded.32–34 Criteria used to define patients as initially unresectable were reported in eight studies (Table 1).18–22,24,25,27 Insufficient volume of the future liver remnant (FLR) was the most frequently documented reason for the designation of unresectability (six of eight studies).18–21,22,25 However, these studies did not uniformly identify an adequate FLR volume. Six studies reported patient selection criteria for TSH, but none of them used the same criteria (Table 1).18–20,23,26,27 Five studies included patients with extrahepatic disease in whom total metastasectomy was feasible and planned.20–22,24,27 Ten studies reported the number of patients with synchronous colorectal cancer and liver metastases; collectively, these patients represented 78% (range: 50–96%) of all patients (350 of 451 patients).18,19–27 Preoperative chemotherapy was administered to some patients in all 10 studies: 400 of 457 patients (88%; range: 64–100%) received chemotherapy.18–27 Five studies reported the rate of response to preoperative chemotherapy; partial response was achieved in 130 of 222 patients (59%; range: 43–73%) and stable response was achieved in 86 of 222 patients (39%; range: 19–57%).19,20,23,24,27
Table 1.
Criteria used to define patients as initially unresectable
| Study | Year of publication | Country | Setting | Study period | Criteria for initial unresectability of CLM | Criteria for selection for TSH |
|---|---|---|---|---|---|---|
| Turrini et al.25 | 2012 | France | Retrospective | 2000–2010 | Specific criteria Inability to resect all CLM with tumour-free margins saving 25–30% of remnant liver volume | Not stated |
| Tsim et al.26 | 2011 | UK | Prospective | 2003–2006 | Not stated | Bilobar CLM in which the left hemiliver can be cleared of metastases during stage 1 resection and in which 25% of functional liver volume can be preserved after right PVE and right/extended right hepatectomy |
| Narita et al.27 | 2011 | France | Retrospective | 1996–2009 | Non-specific criteria Multiple and bilobar CLM in which it is impossible to remove all CLM with safe margins during a single hepatectomy procedure | Multiple and bilobar CLM in which: (i) clearance of the left hemiliver is feasible during stage 1; (ii) the left hepatic vein and pedicle are not invaded, and (iii) FLR volume before PVE is < 30–40% of total functional liver volume |
| Muratore et al.24 | 2011 | Italy | Prospective | 1997–2009 | Non-specific criteria Bilobar CLM in which a radical resection by single hepatectomy is not possible | Not stated |
| Brouquet et al.23 | 2011 | USA | Retrospective | 2002–2010 | Not stated | Chemotherapy-responsive bilobar CLM in which limited resection can clear the less affected side of the liver before the patient undergoes a planned extended contralateral liver resection |
| Surgeon must be able to resect all CLM while preserving a sufficient FLR (20% of total liver volume) and adequate vascular inflow and outflow | ||||||
| Bowers et al.22 | 2011 | UK | Retrospective | 2004–2010 | Specific criteria Not possible to resect all disease while retaining an FLR volume ≥ 0.5% of body weight with preserved vascular inflow and outflow and biliary drainage | Not stated |
| Tsai et al.21 | 2010 | USA and Portugal | Retrospective | 1994–2008 | Specific criteria Not possible to resect all CLM while: (i) sparing two adjacent liver segments; (ii) preserving vascular inflow and outflow, and (iii) maintaining adequate remnant liver volume | Not stated |
| Wicherts et al.20 | 2008 | France | Prospective | 1992–2007 | Specific criteria Inability to resect all CLM with tumour-free margins and save 25–30% of liver volume | Non-specific criteria indicating that complete removal of CLM is possible in two sequential resections |
| Pamecha et al.19 | 2008 | UK | Prospective | 1999–2005 | Specific criteria Bilobar CLM in which it is impossible to remove all CLM while leaving remnant liver of 30% | Non-specific criteria indicating that resection of CLM with clear margins can be achieved leaving sufficient FLR volume |
| Tanaka et al.18 | 2007 | Japan | Retrospective | 1992–2004 | Specific criteria with Yamanaka prediction scorea | Specific criteria with Yamanaka prediction score of > 60 |
Yamanaka prediction score = − 84.6 + 0.933A + 1.11B + 0.999C, where A = resection fraction (%) calculated from computed tomography volumetry; B = indocyanine green retention rate at 15 minutes, and C = the age of the patient.
CLM, colorectal liver metastasis; PVE, portal vein embolization; FLR, future liver remnant; TSH, two-stage hepatectomy.
The surgical characteristics and perioperative outcomes of the first stage of TSH are depicted in Table 2. Eight studies reported the proportion of patients undergoing concomitant colorectal resection to give a combined total of 126 of 355 patients (35%; range: 0–50%).20–26,27 Liver resection was performed in all 10 studies in a total of 443 of 453 patients (98%; range: 91–100%).18–27 Concomitant ablation of CLM was reported in all 10 studies in a total of 73 of 435 patients (17%; range: 0–67%). Concomitant portal vein ligation or PVE was reported in all 10 studies in a total of 87 of 435 patients (20%; range: 0–73%). Postoperative morbidity and mortality were reported in all studies and affected a total of 73 of 425 patients (17%; range: 0–26%) and two of 435 patients (0.5%), respectively. One patient died of liver insufficiency and one died of pulmonary embolus.
Table 2.
Surgical characteristics and perioperative outcomes of the first stage of two-stage hepatectomy
| Study | Patients, n | Extrahepatic disease | Preoperative chemotherapy, % | Response ratea, % | Synchronous colorectal resection, % | Liver resection, % | Ablation, % | Operative portal vein ligation/embolization, % | Postoperative morbidity, % | Postoperative mortality, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Turrini et al.25 | 48 | No | 100 | NR | 37 | 87 | 67 | 0 | 10 | 0 |
| Tsim et al.26 | 38 | No | 97 | NR | 0 | 100 | 0 | 0 | 11 | 0 |
| Narita et al.27 | 80 | Yes | 84 | 100 | 40 | 99 | 31 | 4 | 14 | 0 |
| Muratore et al.24 | 47 | Yes | 79 | 92 | 50 | 100 | 0 | 23 | 19 | 0 |
| Brouquet et al.23 | 65 | No | 100 | 100 | 29 | 100 | 3 | 0 | 25 | 0 |
| Bowers et al.22 | 39b | Yes | 74 | NR | 31 | 100 | 8 | 0 | 23 | 0 |
| Tsai et al.21 | 45 | Yes | 71 | NR | 50 | 91 | 29 | 73 | 26 | 4c |
| Wicherts et al.20 | 59 | Yes | 97 | 100 | 20 | 100 | 5 | 56 | 20 | 0 |
| Pamecha et al.19 | 14 | No | 100 | 79 | NR | 100 | 0 | 0 | 0 | 0 |
| Tanaka et al.18 | 24 | No | 64 | NR | NR | 100 | 0 | 71 | 13 | 0 |
| Total | 459 | 88 | 97 | 35 | 98 | 17 | 20 | 17 | ||
| Range | 64–100 | 79–100 | 0–50 | 87–100 | 0–67 | 4–71 | 0–26 | |||
Includes partial or stable radiological response to chemotherapy.
Series included patients with liver metastases from colorectal cancer (n = 33), neuroendocrine tumours (n = 3), gastrointestinal stromal tumour (n = 1), ocular melanoma (n = 1) and salivary gland carcinoma (n = 1).
Two postoperative deaths: one from liver insufficiency and one from pulmonary embolus.
NR, not reported.
The surgical characteristics and perioperative outcomes of the second stage of TSH are depicted in Table 3. Interval PVE was reported in all 10 studies in a total of 262 of 430 patients (61%; range: 0–100%). In total, 349 of the initial 459 patients (76%) underwent portal vein ligation or embolization. Interval chemotherapy was reported in all 10 studies in a total of 154 of 412 patients (37%; range: 13–100%). A total of 107 patients did not progress to the second stage of TSH. The main reasons for the non-completion of the second stage of TSH were interval disease progression (94 of 107 patients, 88%), followed by inadequate FLR volume (four of 107 patients, 4%), portal vein injury or thrombosis (four of 107 patients, 4%) and death after the first stage of TSH (two of 107 patients, 2%). Of the initial 459 patients, 352 (77%) ultimately underwent the second stage of TSH. Liver resection was performed in 346 of 352 patients (98%), 290 (84%) of whom underwent major liver resections. Seven studies reported R0 resection rates; R0 margins were achieved in a total of 158 of 210 patients (75%; range: 52–100%). Concomitant ablation of CLM was reported in nine studies in a total of 39 of 314 patients (12%; range: 0–59%). Postoperative morbidity and mortality were reported in all 10 studies and affected a total of 146 of 365 patients (40%; range: 20–56%) and 11 of 347 patients (3%), respectively. All 11 postoperative deaths were secondary to liver insufficiency.
Table 3.
Surgical characteristics and perioperative outcomes of the second stage of two-stage hepatectomy
| Study | Interval portal vein embolization, % | Interval chemotherapy, % | Patients, n | Proportion of patients selected for stage 2, % | Liver resection, % | Major liver resection, % | R0 resection rate, % | Ablation, % | Postoperative morbidity, % | Postoperative mortality, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Turrini et al.25 | 100 | 29 | 34 | 71 | 100 | 91 | 100 | 59 | 20 | 6 |
| Tsim et al.26 | 95 | 13 | 33 | 87 | 100 | 85 | 58 | NR | 33 | 0 |
| Narita et al.27 | 92 | 31 | 61 | 76 | 100 | 95 | NR | 8 | 54 | 0 |
| Muratore et al.24 | 57 | 53 | 36 | 77 | 100 | 94 | 86 | 0 | 44 | 0 |
| Brouquet et al.23 | 70 | 19 | 47 | 72 | 100 | 85 | 64 | 0 | 49 | 6 |
| Bowers et al.22 | 72 | 15 | 32 | 82 | 84 | 59 | 52 | 7 | 56 | 4 |
| Tsai et al.21 | 4 | 62 | 35 | 78 | 97 | 80 | NR | 20 | 26 | 6 |
| Wicherts et al.20 | 20 | 78 | 41 | 69 | 100 | 76 | NR | 12 | 59 | 7 |
| Pamecha et al.19 | 14 | 100 | 11 | 79 | 100 | 73 | 100 | 0 | 27 | 0 |
| Tanaka et al.18 | 0 | 0 | 22 | 92 | 100 | 67 | 87 | 0 | 23 | 0 |
| Total | 61 | 37 | 352 | 77 | 98 | 84 | 75 | 12 | 40 | 3 |
| Range | 0–100 | 0–100 | 69–92 | 84–100 | 59–95 | 52–100 | 0–59 | 20–59 | 0–7 | |
NR, not reported.
Thus, TSH was completed in 346 patients. Survival outcomes after completion of TSH are depicted in Table 4. Eight studies reported median follow-up time, giving a median value of 27 months (range: 12–50 months). Survival outcomes were reported in all 10 studies. Median DFS was reported in four studies (141 of 346 patients), which gave values of 8 months, 9 months, 12 months and 18 months, respectively. Three-year DFS was reported in seven studies (272 of 346 patients) to give a median value of 20% (range: 6–27%), whereas 5-year DFS was reported in three studies (122 of 346 patients) at values of 13%, 14% and 20%, respectively. Median OS in patients who did not complete both stages of TSH was reported in eight studies (106 of 113 patients) to give a median value of 16 months (range: 10–29 months). Median OS in patients in whom both stages of TSH were completed was reported in eight studies (277 of 346 patients) to give a median value of 37 months (range: 24–44 months). Three-year OS post-TSH was reported in all 10 studies to give a median value of 59% (range: 28–84%), whereas 5-year OS post-TSH was reported in five studies (194 of 346 patients) to give a median value of 42% (range: 32–64%).
Table 4.
Survival outcomes after completion of two-stage hepatectomy
| Study | Median follow-up, months | Median DFS post-TSH, months | 3-year DFS post-TSH, % | 5-year DFS post-TSH, % | Median OS in non-completion of TSH, months | Median OS post-TSH, months | 3-year OS post-TSH, % | 5-year OS post-TSH, % |
|---|---|---|---|---|---|---|---|---|
| Turrini et al.25 | 41 | NR | 24 | 14 | 19 | 44 | 59 | 35 |
| Tsim et al.26 | 19 | 18a | 27a | NR | 29 | 35 | 50a | NR |
| Narita et al.27 | 30 | NR | 15b | 8b | 19 | 40 | 59 | 32 |
| Muratore et al.24 | 24d | 8 | 10 | NR | 12 | 38 | 65 | NR |
| Brouquet et al.23 | 50 | NR | 20 | 20 | 25 | NR | 84 | 64 |
| Bowers et al.22 | 12 | NR | NR | NR | 10c | 24c | 28c | NR |
| Tsai et al.21 | NR | NR | NR | NR | 10 | 36 | 58 | NR |
| Wicherts et al.20 | 24 | NR | 26 | 13 | 11 | 39 | 60 | 42 |
| Pamecha et al.19 | 43 | 12 | NR | NR | NR | 33 | 70 | 50 |
| Tanaka et al.18 | NR | NR | 6 | NR | NR | NR | 33 | NR |
| Median | 27 | 20 | 16 | 37 | 59 | 42 | ||
| Range | 12–50 | 6–27 | 10–29 | 24–44 | 28–84 | 32–64 | ||
In patients with R0 liver resection.
In patients who underwent preoperative chemotherapy.
Survival outcomes in patients with CLM only.
Mean value.
TSH, two-stage hepatectomy; CLM, colorectal liver metastases; NR, not reported; DFS, disease-free survival; OS, overall survival.
Discussion
This systematic review demonstrates that TSH in selected patients with initially unresectable CLM is associated with low perioperative morbidity and mortality and acceptable survival outcomes. Median OS was 36 months. These outcomes are comparable with those of liver resection in patients with resectable CLM.4–11 These survival outcomes are encouraging when compared with the poor survival outcomes of systematic chemotherapy alone in patients with unresectable CLM.35 This study focused on the TSH approach, in which liver resections are performed in each of two separate stages of a planned procedure and which differs from two-step liver surgery in which ablation but no resection of CLM is performed in the first stage of the procedure.32–34 In addition, this study differs from a previous review36 in its addition of three more recent studies and exclusion of three studies that included overlapping data,28–30 one study that did not report survival data31 and one study that reported two-step liver surgery.33
Although the value of liver resection in CLM has never been demonstrated in a prospective RCT, numerous surgical series have demonstrated the possibility of longterm survival. Additionally, no treatment other than liver resection has shown a survival plateau. Recently, a number of case series describing 10-year actual survival rates after liver resection of CLM have been published.37–39 These results support liver resection as standard practice as well as the only curative treatment for CLM. However, only 10% of patients were candidates for liver resection when traditional criteria for the resectability of CLM were applied (namely, up to three unilobar metastases that are resectable with a generous margin of healthy liver tissue40). Over the past decade, considerable effort has been focused on the development of innovative approaches to improve resectability of CLM.41 These include downstaging chemotherapy followed by rescue liver resection, preoperative PVE followed by liver resection, the use of ablative therapy and TSH.14,42–44
The studies included in this systematic review are not uniform in their definitions of the technical unresectability of CLM. There is a significant lack of agreement among liver surgeons on what constitutes resectability and the appropriate use of adjuvant modalities in the treatment of CLM.45 Similarly, up to a third of patients initially labelled as unresectable are reclassified as resectable following review by a multidisciplinary oncology team.46 This lack of agreement even among experts significantly limits the critical evaluation of outcomes of TSH in patients with initially unresectable CLM. Standardization of technical resectability criteria will clearly facilitate a better understanding of the roles of preoperative and interval chemotherapy, as well as that of PVE, in patients with initially unresectable CLM. In 2006, a consensus group proposed that there should be a paradigm shift in the definition of resectability and that the consideration of resectability should focus on what will remain after resection rather than on what is to be removed. Three specific criteria for resectability of CLM were proposed: (i) the preservation of two contiguous liver segments; (ii) the preservation of adequate vascular inflow and outflow and biliary drainage, and (iii) the preservation of an adequate FLR (> 20% in a healthy liver).47 The widespread adoption of uniform definitions will clearly facilitate the interpretation of future results.
Another limitation to the interpretation of the data included in this systematic review concerns the lack of uniform criteria for patient selection for TSH. All of the studies included were reported from high-volume hepatobiliary cancer surgery centres and thus an aggressive surgical approach could reasonably be assumed. Parameters based on the sizes, numbers and locations of CLM lesions were not used as contraindications for TSH. Three of the studies included suggested that TSH should be considered in patients with unresectable bilobar CLM in whom one affected side of the liver can be resected during the first stage and all remaining CLM can be resected in a planned second hepatectomy, preserving sufficient FLR volume. Nevertheless, patients scheduled for TSH are highly selected and represent only a small proportion of patients with CLM. Two-stage hepatectomy should be considered only if a one-stage hepatectomy cannot be performed even with the use of preoperative PVE and ablative therapies. Adam et al. proposed a classification of multinodular CLM depending on the number, size and location of metastases.48 It was suggested that TSH should be considered in selected patients with bilobar multinodular CLM in whom a right hepatectomy would leave more than three metastases or any metastasis of >3 cm in the FLR.
Modern chemotherapy regimens using the combination of 5-fluorouracil (5-FU) plus oxaliplatin and/or irinotecan have produced impressive response rates in patients with CLM. Partial response rates of up to 50% and median survival approaching 2 years have been reported.49,50 The addition of biological agents such as bevacizumab and cetuximab has been shown to further improve response rate.46,51 One systematic review demonstrated that downstaging chemotherapy could convert almost a quarter of patients with initially unresectable CLM into resection candidates.43 Despite the increased efficacy of chemotherapy in patients with CLM, its use is not without risk. There is concern that chemotherapy-associated liver injury might have a negative impact on perioperative outcomes in patients undergoing resection of CLM. Liver steatosis and steatohepatitis have been shown to be associated with 5-FU and irinotecan exposure, respectively, whereas sinusoidal liver injury is a potential consequence of oxaliplatin treatment.52 Previous studies have shown increased perioperative mortality in patients with chemotherapy-associated steatohepatitis. Increased perioperative bleeding has been associated with chemotherapy-associated sinusoidal liver injury.53 Vauthey et al. therefore advocated a ‘20/30/40’ rule to ensure the safety of liver resection: an FLR representing a minimum of 20% of liver volume is required in normal liver; an FLR representing 30% of liver volume is required in chemotherapy-damaged liver, and an FLR representing 40% of liver volume is required in cirrhotic liver.54 In this analysis, preoperative chemotherapy was used in 88% of patients selected for TSH. This high rate of chemotherapy administered before resection is probably related to the extent and perceived unresectability of CLM in these patients. The rationale for preoperative chemotherapy includes an opportunity to demonstrate chemotherapy-specific efficacy as well as a lower rate of positive margins.55 The objective response to preoperative chemotherapy has also been shown to be a strong predictor of survival after resection of CLM.56,57 One study included in this systematic review reported response to preoperative chemotherapy on imaging as part of its patient selection criteria for TSH.23 In the present analysis, the dropout rate after the first stage of TSH was 24%, mainly as a result of disease progression. Previous experimental studies have shown that both liver resection and PVE stimulated increased expression of growth factors and thus residual tumour growth.58,59 Interval chemotherapy has thus been proposed as a strategy to halt tumour progression between the first and second stages of TSH.24 In the studies analysed in this review, interval chemotherapy was used in 37% of cases. One study examined the impact of interval chemotherapy according to the rate of disease progression and dropout between the first and second stages of TSH.24 However, the use of interval chemotherapy with 5-FU plus oxaliplatin or irinotecan did not significantly affect rates of disease progression and dropout.
Both postoperative morbidity (40% versus 17%) and mortality (3% versus 0.5%) were higher in the second than the first stage of TSH. All of the 11 postoperative fatalities that occurred after the second stage of TSH were related to liver insufficiency. These deaths occurred despite the high rate of PVE used (76% of the initial 459 patients selected for TSH). Previous reports have demonstrated that the uniform delivery of PVE before major liver resection does not influence postoperative morbidity or mortality.60 Portal vein embolization is therefore indicated only in patients in whom liver resection is technically feasible but would leave an insufficient FLR volume.
In this study, a pooled analysis in which the data from observational studies were combined as if they were derived from a single sample was conducted. The application of any formal meta-analytic methods, particularly simple pooling, to observational studies has been considered controversial.61 Although combining data by meta-analytic methods is preferable, it was not feasible in this study.62 It should be acknowledged that the studies in this review each included a small number of resectable patients and that considerable heterogeneity was evident across designs. Nevertheless, the pooling of data provides an indication of real-world outcomes, but the subsequent results should be interpreted with caution.
In conclusion, this systematic review demonstrates that TSH is associated with acceptable perioperative and survival outcomes in patients with initially unresectable CLM. These results are comparable with those in patients with initially resectable disease. Further prospective controlled studies are required to better define criteria for the selection of patients for TSH and the exact roles of preoperative and interval chemotherapy and PVE.
Conflicts of interest
None declared.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Steele GJ, Ravikumar TS. Resection of hepatic metastases from colorectal metastases: biologic perspectives. Ann Surg. 1989;210:127–138. doi: 10.1097/00000658-198908000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, Dibartolomeo M, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in longterm survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. discussion 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: longterm results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 10.Rees M, Tekkis PP, Welsh FKS, O'Rourke T, John TG. Evaluation of longterm survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 11.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 12.Adson MA. Resection of liver metastases – when is it worthwhile? World J Surg. 1987;11:511–520. doi: 10.1007/BF01655817. [DOI] [PubMed] [Google Scholar]
- 13.Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, et al. Resection of non-resectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–522. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumours. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Third Symposium on Systematic Reviews: Beyond the Basics. Oxford, 3–5 July 2000.
- 16.National Health and Medical Research Council. A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines. Canberra, ACT: AusInfo; 1999. [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Shimada H, Matsuo K, Ueda M, Endo I, Togo S. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007;33:329–335. doi: 10.1016/j.ejso.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 19.Pamecha V, Nedjat-Shokouhi B, Gurusamy K, Glantzounis GK, Sharma D, Davidson BR. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolization and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig Surg. 2008;25:387–393. doi: 10.1159/000176063. [DOI] [PubMed] [Google Scholar]
- 20.Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan L-A, et al. Longterm results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. [DOI] [PubMed] [Google Scholar]
- 21.Tsai S, Marques HP, De Jong MC, Mira P, Ribeiro V, Choti MA, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB. 2010;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowers KA, O'Reilly D, Bond-Smith GE, Hutchins RR. Feasibility study of two-stage hepatectomy for bilobar liver metastases. Am J Surg. 2011;203:691–697. doi: 10.1016/j.amjsurg.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083–1090. doi: 10.1200/JCO.2010.32.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muratore A, Zimmitti G, Ribero D, Mellano A, Viganò L, Capussotti L. Chemotherapy between the first and second stages of a two-stage hepatectomy for colorectal liver metastases: should we routinely recommend it? Ann Surg Oncol. 2011;19:1310–1315. doi: 10.1245/s10434-011-2069-5. [DOI] [PubMed] [Google Scholar]
- 25.Turrini O, Ewald J, Viret F, Sarran A, Goncalves A, Delpero JR. Two-stage hepatectomy: who will not jump over the second hurdle? Eur J Surg Oncol. 2012;38:266–273. doi: 10.1016/j.ejso.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Tsim N, Healey AJ, Frampton AE, Habib NA, Bansi DS, Wasan H, et al. Two-stage resection for bilobar colorectal liver metastases: R0 resection is the key. Ann Surg Oncol. 2011;18:1939–1946. doi: 10.1245/s10434-010-1533-y. [DOI] [PubMed] [Google Scholar]
- 27.Narita M, Oussoultzoglou E, Jaeck D, Fuchschuber P, Rosso E, Pessaux P, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg. 2011;98:1463–1475. doi: 10.1002/bjs.7580. [DOI] [PubMed] [Google Scholar]
- 28.Karoui M, Viganò L, Goyer P, Ferrero A, Luciani A, Aglietta M, et al. Combined first-stage hepatectomy and colorectal resection in a two-stage hepatectomy strategy for bilobar synchronous liver metastases. Br J Surg. 2010;97:1354–1362. doi: 10.1002/bjs.7128. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka K, Kumamoto T, Nojiri K, Takeda K, Ichikawa Y, Endo I. Timing of two-stage liver resection during chemotherapy for otherwise unresectable colorectal metastases. World J Surg. 2012;36:1832–1841. doi: 10.1007/s00268-012-1578-0. [DOI] [PubMed] [Google Scholar]
- 30.Togo S, Nagano Y, Masui H, Tanaka K, Miura Y, Morioka D, et al. Two-stage hepatectomy for multiple bilobular liver metastases from colorectal cancer. Hepatogastroenterology. 2005;52:913–919. [PubMed] [Google Scholar]
- 31.Homayounfar K, Liersch T, Schuetze G, Niessner M, Goralczyk A, Meller J, et al. Two-stage hepatectomy (R0) with portal vein ligation – towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis. 2008;24:409–418. doi: 10.1007/s00384-008-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lygidakis NJ, Bhagat AD, Vrachnos P, Grigorakos L. Challenges in everyday surgical practice: synchronous bilobar hepatic colorectal metastases – newer multimodality approach. Hepatogastroenterology. 2007;54:1020–1024. [PubMed] [Google Scholar]
- 33.Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoridis J, et al. Two-stage liver surgery for advanced liver metastasis synchronous with colorectal tumour. Hepatogastroenterology. 2004;51:413–418. [PubMed] [Google Scholar]
- 34.Lygidakis NJ, Vlachos L, Raptis S, Rassidakis G, Balaskas C, Sgourakis G, et al. New frontiers in liver surgery. Two-stage liver surgery for the management of advanced metastatic liver disease. Hepatogastroenterology. 1999;46:2216–2228. [PubMed] [Google Scholar]
- 35.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 36.Chua TC, Liauw W, Chu F, Morris DL. Summary outcomes of two-stage resection for advanced colorectal liver metastases. J Surg Oncol. 2012 doi: 10.1002/jso.23170. (In Press) [DOI] [PubMed] [Google Scholar]
- 37.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 38.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Longterm survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 39.Pulitanò C, Castillo F, Aldrighetti L, Bodingbauer M, Parks RW, Ferla G, et al. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB. 2010;12:244–249. doi: 10.1111/j.1477-2574.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828–4833. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 41.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 42.Elias D, Baton O, Sideris L, Boige V, Malka D, Liberale G, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42. doi: 10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 43.Lam VWT, Spiro C, Laurence JM, Johnston E, Hollands MJ, Pleass HCC, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–1301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 44.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of non-resectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohammad WM, Martel G, Mimeault R, Fairfull-Smith RJ, Auer RC, Balaa FK. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB. 2012;14:291–297. doi: 10.1111/j.1477-2574.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Folprecht G, Gruenberger T, Bechstein WO, Raab R, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximan: the CELIM randomized phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 47.Charnsangavej C, Clary B, Fong Y, Grothey A, Pawlik TM, Choti MA. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 48.Adam R, Miller R, Pitombo M, Wicherts DA, de Haas RJ, Bitsakou G, et al. Two-stage hepatectomy approach for initially unresectable colorectal hepatic metastases. Surg Oncol Clin N Am. 2007;16:525–536. doi: 10.1016/j.soc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 49.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 50.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 51.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 52.Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 53.Morris-Stiff G, Tan Y-M, Vauthey JN. Hepatic complications following preoperative chemotherapy with oxaliplatin or irinotecan for hepatic colorectal metastases. Eur J Surg Oncol. 2008;34:609–614. doi: 10.1016/j.ejso.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Vauthey JN, Abbott DE. Commentary on ‘Feasibility study of two-stage hepatectomy for bilobar liver metastases’. Am J Surg. 2012;203:698–699. doi: 10.1016/j.amjsurg.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385–2390. doi: 10.1245/s10434-009-0492-7. [DOI] [PubMed] [Google Scholar]
- 56.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumour progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. discussion 1061–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome endpoint after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 58.Kollmar O, Corsten M, Scheuer C, Vollmar B, Schilling MK, Menger MD. Tumour growth following portal branch ligation in an experimental model of liver metastases. Br J Surg. 2010;97:917–926. doi: 10.1002/bjs.7003. [DOI] [PubMed] [Google Scholar]
- 59.Meredith K, Haemmerich D, Qi C, Mahvi D. Hepatic resection but not radiofrequency ablation results in tumour growth and increased growth factor expression. Ann Surg. 2007;245:771–776. doi: 10.1097/01.sla.0000261319.51744.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farges O, Belghiti J, Kianmanesh R, Regimbeau J-M, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28:1–9. doi: 10.1093/ije/28.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Bravata DM, Olkin I. Simple pooling versus combining in meta-analysis. Eval Health Prof. 2001;24:218–230. doi: 10.1177/01632780122034885. [DOI] [PubMed] [Google Scholar]


