Abstract
Background
The management of hilar cholangiocarcinoma has evolved over time and extended liver resection, including the caudate lobe, and major vascular resection and extended lymphadenectomy have become established practice. The benefit of vascular resection has not been investigated.
Methods
A systematic search of the MEDLINE and EMBASE databases was used to identify studies. A systematic review and a meta-analysis of the available studies were conducted according to PRISMA guidelines. Odds ratios were calculated using the Mantel–Haenszel method. Primary outcome variables assessed included morbidity, mortality, vascular complications and the effect of vascular resection on longterm survival.
Results
Of 411 search results, only 24 studies reported the results of vascular resection in hilar cholangiocarcinoma. Meta-analysis showed increased morbidity and mortality with hepatic artery resection. Portal vein resection was achievable with no impact on postoperative mortality. Vascular resection did not improve negative margin rates and had no impact on longterm survival.
Conclusions
Portal vein resection does not preclude curative resection; however, it is not routinely recommended unless there is suspicion of tumour invasion. There was no proven survival advantage with portal vein resection. Arterial resection results in higher morbidity and mortality with no proven benefit.
Introduction
Cancer of the hepatic hilum accounts for 50–80% of biliary malignancies.1,2 Surgical resection of hilar cholangiocarcinoma offers patients the best chance of longterm survival. The first resection was reported by Brown and Myers in 1954.1 Combined portal vein and liver resection for locally advanced hilar cholangiocarcinoma was first successfully performed in 1965 at the Cancer Institute Hospital in Tokyo. The patient in question underwent right hepatectomy, en bloc excision of the bile duct and portal vein with end-to-end reconstruction.2 This was followed by further attempts to perform such radical resections. Improvements in microvascular anastomoses and experience with liver transplants have made it possible to perform resection and reconstruction of both the hepatic artery and portal vein.3,4 These technical advances demonstrate that combined resection of the portal vein improves the rate of radical resection for hilar cholangiocarcinoma and perhaps significantly prolongs survival.5–9 Actual tumour invasion of the portal vein documented on pathological examination is associated with poor survival.8
Median survival following resection is 11–38 months and the 5-year survival rate is 20–40%.10,11 The introduction of hepatectomy with hilar resection has increased the rate of curative resection and the total number of patients who are amenable to surgical resection.5 This radical approach is facilitated by the fact that the left hepatic duct extends 2–5 cm outside the hepatic parenchyma. This makes it possible to achieve a negative margin and to perform an extrahepatic biliary reconstruction. Resectability, negative margin and survival rates are improved when the caudate lobe is resected en bloc with the tumour.12
Since the introduction of portal vein embolization (PVE), preoperative hypertrophy of the future remnant liver, induced by unilateral PVE, has been shown to reduce the risk for postoperative liver failure attributable to a small liver remnant.13
Recently, there has been increasing evidence to support the use of en bloc vascular resection and reconstruction of the main portal vein and the contralateral hepatic artery or both, along with the resected tumour, and a hemi-hepatectomy when the tumour is thought on radiological assessment to involve these vessels.6–9 This is based on the fact that radical surgical resection is the best treatment option for hilar cholangiocarcinoma; negative-margin (R0) resection provides acceptable 5-year survival of 20–40%.14 In addition, experience in vascular reconstruction during liver transplantation has been translated safely to oncological resections.
This review investigates the safety and efficacy of vascular resection and reconstruction in combination with hepatic and bile duct resection, with particular reference to immediate outcome measures and survival benefits.
Materials and methods
Search and study selection
A search was conducted of MEDLINE (1966 to June 2012), PubMed (1950–2011) and EMBASE (1980 to June 2012), using the MESH (medical subject headings) terms ‘hilar cholangiocarcinoma’, ‘vascular resection’, ‘portal vein’ and ‘hepatectomy’; combinations of these terms were also used. Abstracts of the studies identified were reviewed, and studies that addressed vascular resection along with some form of hepatectomy and radical excision of the bile duct were retrieved and assessed for suitability for systematic review.
Eligibility criteria
Clinical reports that dealt with hilar cholangiocarcinoma were reviewed and included only if the management included hepatectomy with radical excision of the bile duct and lymphadenectomy in patients with encasement of the vessels in whom surgery included resection and reconstruction of the portal vein, with or without concomitant resection and reconstruction of the contralateral or main hepatic artery. Studies that reported vascular resection with local excision or pancreatoduodenectomy for lower bile duct cancers were excluded. Because of the absence of randomized trials in this area, the review and meta-analysis were conducted on cohort studies that reported primary outcome measures (mortality, morbidity, 5-year survival, specific complications related to vascular reconstruction), in addition to bile leak and liver failure as significant complications of this radical procedure. The studies were assessed using the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies15 and studies thought to be suitable were included in the meta-analysis.
Search strategy
*Cholangiocarcinoma/or *bile duct neoplasms/
*Hepatic artery/or *portal vein/
*Vascular surgical procedures/
*Klatskin's tumour/or *cholangiocarcinoma/or *bile duct neoplasms/or *hepatectomy/
1 or 4
2 or 3
5 and 6
Hepatectomy
7 and 8.
Data extraction
Relevant data concerned with the outcomes were collected independently by two reviewers from the studies when these data were directly available or were extracted from results that were reported in percentages. These data included details of the number of patients undergoing vascular resection, morbidity, mortality, vascular complications, rates of R0 curative resection, 5-year survival with and without vascular resection, and actual numbers of cases of true invasion of the vessels by the cancer. Differences in the data were resolved by consensus.
Statistical analysis
The relevant outcomes were entered in a meta-analysis using RevMan 5.016 developed by the Cochrane Collaboration. Both random- and fixed-effects models were used. The random-effects model was used for analysis and its results reported as this model was thought to produce more robust results. Data were entered as categorical variables and analysed using the Mantel–Haenszel test. Results were expressed as forest plots and summarized with odd ratios (ORs) and 95% confidence intervals (CIs). A P-value of <0.05 was considered to indicate statistical significance and the results were summarized using the OR. Sensitivity analysis was performed by excluding and including studies whenever heterogeneity was encountered; studies that seemed to be outliers and had significant effects on the results were excluded. Publication bias was assessed using a funnel plot.
Statistical heterogeneity in the results of the meta-analysis was assessed by graphical presentations of the CIs on forest plots and by performing a chi-squared test for heterogeneity. The I2 statistic was used to quantify heterogeneity; P-values of <0.100 were regarded as indicating significance. Funnel plots for publication bias were constructed and outlying studies were excluded.
Results
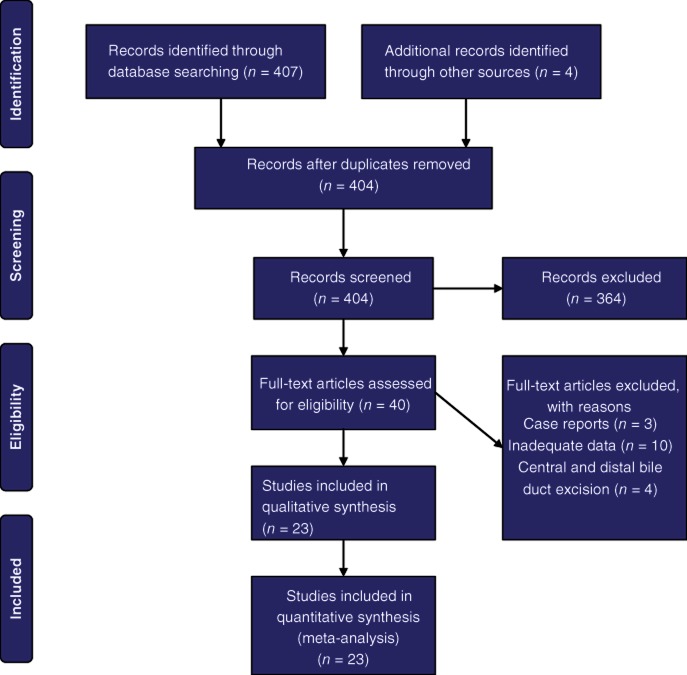
Figure 1 shows the selection of articles according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.17
Figure 1.
Flow chart showing the study selection process
Thirty-seven articles were retrieved and assessed by two reviewers; 24 articles were found to be suitable for inclusion in the review and meta-analysis. These reports covered a total of 2457 patients who underwent resections of hilar cholangiocarcinoma, 669 of whom underwent vascular resection. Five-year survival was 20–56%, morbidity was 22–88%, and mortality was 2–15%. No randomized trials comparing vascular resection with no vascular resection were identified; all studies were cohort studies. Rates of curative R0 resection varied from 36% to 88% (Table 1). Pathological analysis of the resected specimens showed that rates of actual tumour invasion of the vessels were 22–88% (Table 2).
Table 1.
Studies included in the current meta-analysis
| Study | Patients, n | Vascular resection, n | Morbidity, % | Mortality, % | 5-year survival, % | R0, % | R1, % | R0 rate with vascular resection, % |
|---|---|---|---|---|---|---|---|---|
| Muñoz et al. 20027 | 28 | 10 | 25 | 3 | 23 | – | – | – |
| Igami et al. 201022 | 298 | 111 | 43 | 2 | 42 | 52 | 32 | 66 |
| Song et al. 200925 | 259 | 51 | 54 | 9.6 | 29.3 | 29.3 | 17 | 71.8 |
| Young et al. 201023 | 51 | 21 | 75 | 8 | 20 | 40 | 2 | 57 |
| Kondo et al. 200421 | 42 | 14 | 48 | 0 | – | – | – | 95 |
| Miyazaki et al. 201018 | 107 | 25 | – | 2 | – | 33 | 21 | 59 |
| Neuhaus et al. 200331 | 133 | – | – | – | – | 38 | 18 | – |
| Lee et al. 200024 | 111 | 29 | 22 | 6.3 | 24 | 77 | ||
| Nimura et al. 200034 | 142 | 43 | 48.6 | 9 | 25 | 26 | 16 | 61 |
| Nagino et al. 201019 | 261 | 50 | 54 | 2 | 30 | 40.7 | 0 | 54 |
| Ebata et al. 20038 | 160 | 52 | 84 | 9.6 | 37 | – | – | – |
| Hemming et al. 201120 | 95 | 42 | 36 | 5 | 43 | 50 | 0 | 84 |
| Hemming et al. 20059 | 53 | 23 | 40 | 9 | 35 | 45 | 0 | 80 |
| Baton et al. 200730 | 59 | 5 | 42 | 5 | 20 | 28 | 6 | 67 |
| Nagino et al. 200145 | 105 | 33 | 81 | 9.5 | – | – | – | – |
| Magriaga 199828 | 28 | 9 | 32 | 14 | 8 | 11 | 0 | 50 |
| Hidalgo 200814 | 44 | 17 | 66 | 6.8 | 41 | 45 | 26 | 45 |
| Shimada et al. 200326 | 39 | 15 | 71 | 6.7 | 56 | 50 | 10 | 50 |
| Neuhaus et al. 19996 | 66 | 23 | 56 | 3 | 22 | 42 | 9 | 61 |
| Edmond 198943 | 13 | 5 | 69 | 15 | – | – | – | 46 |
| Lygidakis 198844 | 13 | 7 | – | 15 | – | – | – | 46 |
| Klempnauer et al. 199729 | 125 | 41 | 29.8 | 9.9 | 28 | 26 | 6.8 | 73 |
| Miyazaki et al. 200727 | 161 | 43 | 39 | 7 | 36 | 0 | 36 | |
Table 2.
Invasion of the portal vein confirmed by histology
Description of included trials
All included studies reported outcomes in a cohort of patients with hilar cholangiocarcinoma of different stages. Patients who were thought to have involvement of vessels in the hilum underwent vascular resection with reconstruction; venous resection was performed more often than arterial resection and reconstruction. The methodological quality of the studies was considered adequate based on the Newcastle–Ottawa scale for the meta-analysis of cohort studies.15 All included studies reported 5-year survival; data on vascular complications, mortality related to vascular resection and liver failure were variable. The number of studies reporting these outcome measures was suitable for a meta-analysis.
Vascular complications
Occurrences of vascular complications were compared between patients who did and did not undergo vascular resection. These complications included thrombosis of the portal vein or hepatic artery, stenosis of these vessels, and pseudoaneurysms. The meta-analysis revealed higher complication rates in patients who underwent vascular resection (OR 8.8, 95% CI 3.5–22; P < 0.0001) (Fig. 2).
Figure 2.

Vascular complications in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Mortality
Thirteen studies6–8,18–27 reported the difference in mortality between patients who did and did not undergo vascular resection. The meta-analysis showed significantly higher mortality among patients who underwent vascular resection (OR 2.07, 95% CI 1.21–3.57; P = 0.008) (Fig. 3).
Figure 3.

Mortality rates in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Overall morbidity
Only three studies26–28 reported the difference in overall morbidity between patients who did and did not undergo vascular resection. Meta-analysis of these studies showed no difference in morbidity between the two groups; however, the sample size was small. Given that higher mortality occurred in the vascular resection group, a definitive conclusion was not possible (Fig. 4).
Figure 4.
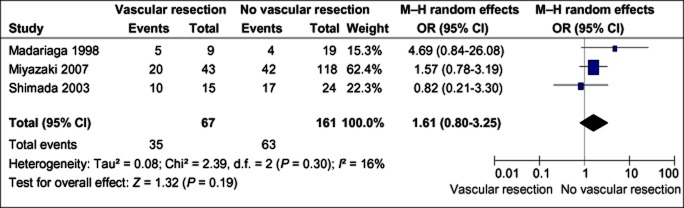
Overall morbidity rates in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Mortality with arterial resection
In order to further analyse the risk for death following arterial resection, mortality rates were compared between patients who underwent arterial resection and reconstruction in addition to portal vein resection, and those who underwent portal vein but not arterial resection.7,19,22–24,26,27 A meta-analysis of data on this outcome showed higher mortality rates in patients who underwent arterial resection (OR 4.48, 95% CI 1.97–10.16; P = 0.0003) (Fig. 5).
Figure 5.

Mortality in patients with and without hepatic artery resection and reconstruction. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Liver failure
A meta-analysis of data from studies that reported differences in liver failure between vascular resection patients and patients without vascular resection showed no significant increase in liver failure after vascular resection.8,19,25,26,27 However, this is subject to variations in definitions of liver failure (Fig. 6).
Figure 6.
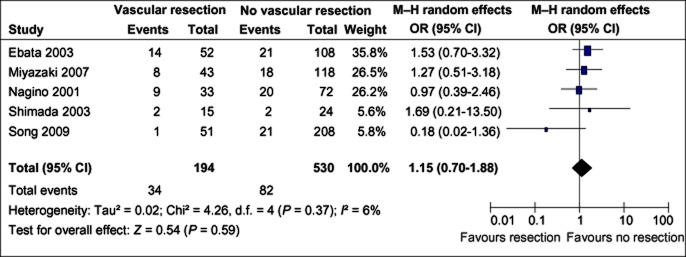
Liver failure rates in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Survival in patients with negative and positive resection margins
Curative resection with negative pathologic margins provides the best chance for longterm survival. Reported 5-year survival rates in patients with a pathological R0 margin in this review were 11–52%, which is significantly better than the 0–32% rates reported for patients who had a pathological R1 margin with no macroscopic residual disease (Fig. 7).
Figure 7.

Five-year survival in patients with R0 resection (negative margins) and R1 resection (microscopically involved margins). M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
It was not clearly evident that adding vascular resection to a radical resection of the bile duct with hepatectomy and lymph node dissection improves longterm survival in hilar cholangiocarcinoma. In fact, the current meta-analysis shows that 5-year survival rates were lower in those who underwent vascular resection (25% versus 39%; OR 1.88, 95% CI 1.22–2.90; P = 0.004) (Fig. 8). A review of the reasons for this established that patients who underwent vascular resection had significantly higher rates of locally advanced disease (T3 and T4). Most of the studies included did not analyse nodal staging and therefore it was not possible to adjust for nodal status in the current analysis, although this factor strongly affects longterm survival. In addition, there was significant statistical heterogeneity among the studies included.
Figure 8.

Five-year survival in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
R0 margin status with vascular resection
Four studies6,8,25,27 reported the pathological margins of specimens resected in patients who did and did not undergo vascular resection. A meta-analysis of these studies showed no significant difference in the rates of R0 resection between the two groups (Fig. 9). Venous invasion was reported to have occurred in 22–88% of patients (Table 2) and it may be that the addition of venous resection increases the rate of R0 resection. However, the relevant data were insufficient to support a meta-analysis.
Figure 9.

Rates of R0 resection in patients with and without vascular resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Arterial resection and leak from hepaticojejunostomy
Three studies8,26,27 reported the difference between patients who did and did not undergo arterial resection in the incidence of leaks from a hepaticojejunostomy. A meta-analysis of these studies showed a higher risk for leak in arterial resection; however, this difference did not reach statistical significance. It is most likely that this reflects the small number of patients included in the meta-analysis (Fig. 10).
Figure 10.

Anastomotic leak from (hepaticojejunostomy) in patients with and without arterial resection. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Survival with portal vein invasion
To assess the impact of actual invasion of vessels on survival, a meta-analysis was performed on the studies that reported this outcome. Five-year survival was similar regardless of whether or not the vessels were invaded (Fig. 11).8,19,21,25,29
Figure 11.

Effect of vascular wall invasion on 5-year survival. M–H, Mantel–Haenszel test; OR, odds ratio; 95% CI, 95% confidence interval
Discussion
The management of hilar cholangiocarcinoma is challenging and it is often hard to achieve negative resection margins because of the proximity of the bile duct to the hilar vessels and liver parenchyma. Surgical treatment has evolved significantly to result in improvements in R0 resection and 5-year survival rates, mainly as a result of the addition of major hepatectomy that includes the caudate lobe.6,30–33 Local resection of the bile duct without liver resection results in a high rate of margin positivity, with local recurrence occurring in up to 76% of patients after extrahepatic bile duct resection.34 Miyazaki et al.35 described an improvement in rates of curative resection from 45% in extrahepatic bile duct resection to 75% in patients in whom liver resection was performed routinely for hilar cholangiocarcinoma. There were no 5-year survivors among patients who underwent extrahepatic bile duct resection only, whereas 5-year survival in patients also subjected to liver resection was 27%.35 Nimura et al., from Nagoya Medical School in Japan, reported similar survival results when liver resection was employed.34 This radical surgical treatment was extended to involve en bloc resection of the portal vein, which is suggested to improve the extent of resection and survival.5,6 Resection of the portal vein was performed in most instances by removing a certain length of vein in conjunction with the tumour and effecting reconstruction by direct end-to-end anastomosis.6,19,36,37
This review shows that resection with clear margins affords the best chance for longterm survival in cholangiocarcinoma and results in a reported 5-year survival of 41%.20 This resection involves a major hepatectomy and radical clearance of the lymph nodes, in addition to extrahepatic bile duct excision, an approach that has proven to enhance longterm survival.6,21,38 This type of resection may entail excision and reconstruction of the portal vein with or without excision and reconstruction of the contralateral or main hepatic artery. Routine portal vein resection may improve survival, but this practice is not universally accepted.6,22 Resection with clear margins is achievable in 36–95% of patients.22,23,36 Although portal vein resection is achievable with a minimal increase in morbidity, resection of the hepatic artery is associated with significant increases in morbidity and mortality, both of which are related to the occurrence of vascular complications arising from arterial resection and reconstruction. Portal vein resection and reconstruction are also associated with the occurrence of vascular complications such as thrombosis requiring reoperation, and late anastomotic strictures that manifest as portal hypertension and variceal bleeding.24
True portal vein invasion in hilar cholangiocarcinoma is difficult to determine preoperatively. On computed tomography, the loss of a clear plane, constriction of the vessel and occlusion are regarded as evidence of venous invasion. Actual rates of venous invasion on histopathological examination after resection vary from 21% to 80%.6–8,18,19,25 Bile duct tumours are often adherent to the surrounding tissues in the hepatoduodenal ligament, which may be related to cholangitis complicating biliary obstruction. In a series of 95 patients with hilar cholangiocarcinoma, Neuhaus et al.6 reported overall 5-year survival of 22%, which increased to 65% in patients in whom clear margins were achieved by extending the liver resection and portal vein resection. It is not possible to attribute this improvement to vascular resection because these patients also underwent liver trisectionectomy, which has been shown to increase survival, probably by increasing R0 resection rates.6,30 Jarnagin et al. reported 5-year survival only in patients subjected to liver resection.39 The impact of portal vein invasion on survival is controversial. Although macroscopic invasion has a negative impact on survival, microscopic invasion proved on multivariate analysis not to have an impact on survival.8 These findings add to the complexity of decision making regarding portal vein resection; however, it is logical to resect the vein if it is suspected to be invaded by tumour. Moreover, en bloc resection that involves vascular resection and reconstruction enables excision with a no-touch technique, which may reduce the shedding of tumour cells and microembolization and is postulated to improve survival with routine portal vein resection.6,19,29
Based on the finding that patients who undergo curative resection achieve better outcomes than those who do not undergo resection, aggressive resection that involves the portal vein with or without resection and reconstruction of the hepatic artery has been introduced to achieve resectability rates of 85%.8,24 Arterial resection, by contrast, has not been proven to add any survival advantage. The present review found vascular resection to be associated with increased risk for vascular complications and mortality. Subgroup analysis established that the increase in mortality occurred as a result of the addition of arterial resection. Complications such as arterial thrombosis, haemorrhage and the formation of a pseudoaneurysm are likely to be responsible for the increase in mortality. Patients subjected to arterial resection experienced higher rates of anastomotic leaks, although the difference did not reach statistical significance.8,26,40 The performance of arterial resection and reconstruction has been previously reported to have dismal outcomes in patients subjected to concomitant hepatectomy and bile duct resection.27,41,42 Gerhards et al. reported death in five of nine patients (55.6%) who underwent hepatic artery resection.41 Miyazaki et al. also reported a high mortality rate of 33% in patients subjected to hepatic artery resection and noted no 3-year survivors in this group.27
Neuhaus et al.6 reported 5-year survival rates of 72% in patients subjected to routine portal vein resection and 52% in patients without portal vein resection. Igami et al. found a similar improvement in survival with routine portal vein resection.22 However, this experience was not reproduced in other studies.8,21,34,39 Ebata et al. have shown convincingly that the requirement for portal vein resection is associated with worse longterm survival; on multivariate analysis, portal vein resection itself did not worsen survival, but transluminal tumour invasion and positive margins were associated with a poorer prognosis.8 In the present analysis, definitive conclusions on the effect of portal vein resection on survival were not possible because of the heterogeneity of the studies and a tendency towards the selective resection of the portal vein in patients with advanced disease (T3, T4) and possibly with nodal metastases. Hepatic failure is a serious complication of extended liver resection and the risk for liver failure in patients undergoing vascular resection and reconstruction is similar to that in patients subjected to liver resection without vascular resection. In all of the studies included, PVE was undertaken when necessary; the threshold for PVE was a future liver remnant of < 30% of estimated total liver volume.20,39
Patients who retained microscopically positive margins appeared to have a survival advantage over patients who did not undergo surgical resection.37 This may lend credence to the suggestion that resection should be attempted in hilar cholangiocarcinoma even if negative margins cannot be achieved and that it may represent a useful palliative operation that results in 5-year survival of up to 45%, provided every attempt is made not to leave any macroscopic disease behind.37
Conclusions
The surgical treatment of hilar cholangiocarcinoma has significantly changed the outcome of this disease in the last two decades. The validity of resection of the involved portal vein is well established and the procedure is likely to increase longterm survival. Routine resection, however, remains controversial and there is little possibility that randomized data will become available. Without randomized trials, it is not possible to conclude whether routine portal vein resection can improve the rate of R0 resection. Although invasion of the portal vein has a negative impact on survival, combined liver and portal vein resection may offer a survival benefit to some patients with advanced cholangiocarcinoma, who would otherwise be considered unresectable.
The addition of arterial resection and reconstruction is associated with increased morbidity and mortality without proven survival benefit or an improvement in the rate of clear margin resections.
Conflicts of interest
None declared.
References
- 1.Brown G, Myers N. The hepatic ducts. A surgical approach for resection of tumours. Aust N Z J Surg. 1954;23:308–312. doi: 10.1111/j.1445-2197.1954.tb05060.x. [DOI] [PubMed] [Google Scholar]
- 2.Kajitani T, Kuno K, Hishida Y, Yamanobe T. Surgical treatment for hepatic hilar carcinoma. Shujutsu. 1966;20:997–1002. [In Japanese.] [PubMed] [Google Scholar]
- 3.Longmire WP, McArthur MS, Bastounis EA, Hiatt J. Carcinoma of the extrahepatic biliary tract. Ann Surg. 1973;178:333–335. doi: 10.1097/00000658-197309000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortner JG, Kinne DW, Kim DK, Castro EB, Shiu MH, Beattie EJ. Vascular problems in upper abdominal cancer surgery. Arch Surg. 1974;109:148–153. doi: 10.1001/archsurg.1974.01360020010003. [DOI] [PubMed] [Google Scholar]
- 5.Nimura Y, Hayakawa N, Kamiya J, Maeda S, Kondo S, Yasui A, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78:727–731. doi: 10.1002/bjs.1800780629. [DOI] [PubMed] [Google Scholar]
- 6.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz L, Roayaie S, Maman D, Fishbein T, Sheiner P, Emre S, et al. Hilar cholangiocarcinoma involving the portal vein bifurcation: longterm results after resection. J Hepatobiliary Pancreat Surg. 2002;9:237–241. doi: 10.1007/s005340200025. [DOI] [PubMed] [Google Scholar]
- 8.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Am J Surg. 2005;241:693–702. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tompkins RK, Saunders K, Roslyn JJ, Longmire WP., Jr Changing patterns in diagnosis and management of bile duct cancer. Ann Surg. 1990;211:614–621. [PMC free article] [PubMed] [Google Scholar]
- 11.Nimura Y, Hayakawa N, Kamiya J, Kondo S, Shionoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]
- 12.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. doi: 10.1007/BF01656368. [DOI] [PubMed] [Google Scholar]
- 13.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 14.Hidalgo E, Asthana S, Nishio H, Wyatt J, Toogood GJ, Prasad KR, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34:787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Ottawa Hospital Research Institute. The Newcastle–Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (last accessed 14 July 2012)
- 16.Cochrane Collaboration. 2008. Review Manager (RevMan) Version 5.0. Nordic Cochrane Centre, Cochrane Collaboration: Copenhagen.
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki M, Kimura F, Shimizu H, Yoshidome H, Otuka M, Kato A, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, and IV between 2001 and 2008. J Hepatobiliary Pancreat Sci. 2010;17:470–475. doi: 10.1007/s00534-009-0207-2. [DOI] [PubMed] [Google Scholar]
- 19.Nagino M, Nimura Y, Nishio H, Ebata T, Igami T, Matsushita M, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 20.Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg. 2011;212:604–613. doi: 10.1016/j.jamcollsurg.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Kondo S, Hirano S, Ambo Y, Tanaka E, Okushiba S, Morikawa T, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igami T, Nishio H, Ebata T, Yokoyama Y, Sugawara G, Nimura Y, et al. Surgical treatment of hilar cholangiocarcinoma in the ‘new era’: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17:449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 23.Young AL, Prasad KR, Toogood GJ, Lodge JP. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centres, Leeds. J Hepatobiliary Pancreat Sci. 2010;17:497–504. doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee SG, Lee J, Park KM, Hwang S, Min PC. One hundred and eleven liver resections for hilar bile duct cancer. J Hepatobiliary Pancreat Surg. 2000;7:135–141. doi: 10.1007/s005340050167. [DOI] [PubMed] [Google Scholar]
- 25.Song GW, Lee SG, Hwang S, Kim KH, Cho YP, Ahn CS, et al. Does portal vein resection with hepatectomy improve survival in locally advanced hilar cholangiocarcinoma? Hepatogastroenterology. 2009;56:935–942. [PubMed] [Google Scholar]
- 26.Shimada H, Endo I, Sugita M, Masunari H, Fujii Y, Tanaka K, et al. Hepatic resection combined with portal vein or hepatic artery reconstruction for advanced carcinoma of the hilar bile duct and gallbladder. World J Surg. 2003;27:1137–1142. doi: 10.1007/s00268-003-6801-6. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–588. doi: 10.1016/j.surg.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Madriaga JB, Iwatsaki S, Todo S, Lee RG, Irish W, Starzl TE. Liver e-section for hilar and peripheral cholangiocarcinoma, a study of 62 cases. Ann Surg. 1998;227:70–79. doi: 10.1097/00000658-199801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klempnauer J, Ridder GJ, Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognosis factors. J Clin Oncol. 1997;15:947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 30.Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204:250–260. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus P, Jonas S, Settmacher U, Thelen A, Benckert C, Lopez-Hanninen E, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg. 2003;388:194–200. doi: 10.1007/s00423-003-0383-5. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki Y, Okamura T, Ozaki A, Todoroki T, Takase Y, Ohara K, et al. Surgical treatment of carcinoma at the confluence of the major hepatic ducts. Surg Gynecol Obstet. 1986;162:457–464. [PubMed] [Google Scholar]
- 33.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma, Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki M, Ito H, Ngakawa K, Ambriu S, Shimizu H, Shimizu Y, et al. Aggressive surgical approach to hilar cholangiocarcinoma: hepatic or local resection. Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- 36.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckles JA, Mirza DF. Surgery for hilar cholangiocarcinoma: a 10-year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Mittal B, Deutsch M, Iwatsuki S. Primary passages of the extrahepatic biliary passages. Int J Radiat Oncol Biol Phys. 1985;11:849–855. doi: 10.1016/0360-3016(85)90320-7. [DOI] [PubMed] [Google Scholar]
- 38.Tsao JI, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, et al. Management of hilar cholangiocarcinoma: comparison of American and Japanese experience. Ann Surg. 2000;232:166–174. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BSJ, et al. Staging, respectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizumoto R, Kawarada Y, Suzuki H. Surgical treatment of hilar carcinoma of the bile duct. Surg Gynecol Obstet. 1986;162:153–158. [PubMed] [Google Scholar]
- 41.Gerhards MF, Gulik TM, de Wit LT, Obertop H, Gouma DJ. Evaluation of morbidity and mortality after resection for hilar cholangiocarcinoma: a single-centre experience. Surgery. 2000;127:395–404. doi: 10.1067/msy.2000.104250. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto Y, Sano T, Shimada K, Kosuge T, Kimata T, Sakuraba M, et al. Clinical significance of reconstruction of the right hepatic artery for biliary malignancy. Langenbecks Arch Surg. 2006;391:203–208. doi: 10.1007/s00423-006-0026-8. [DOI] [PubMed] [Google Scholar]
- 43.Edmond JC, Mayes T, Rouch DA, Thistlethwaite JR, Broelsch Experience with resection in the management of proximal bile duct cancer. HPB Surgery. 1989;1:297–307. doi: 10.1155/1989/37642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lygidakis NJ, van der Heyde MN, van Dongen RJ, Krombout JG, Tytgat GN, Huibregtse K. Surgical approaches for unresectable primary carcinoma of the hepatic hilus. Surg Gynecol Obstet. 1988;166:107–114. [PubMed] [Google Scholar]
- 45.Nagino M, Ando M, Kamiya J, Uesaka K, Sano T, Nimura Y. Liver regeneration after major hepatectomy for biliary cancer. Br J Surg. 2001;88:1084–1091. doi: 10.1046/j.0007-1323.2001.01832.x. [DOI] [PubMed] [Google Scholar]