Abstract
Background
Obesity has been associated with poor oncologic outcomes following pancreatoduodenectomy for pancreatic cancer. However, there is a paucity of evidence on the impact of obesity on postoperative complications, oncologic outcome and survival in patients with hepatocellular carcinoma (HCC) undergoing orthotopic liver transplantation (OLT).
Methods
From a database of over 1000 patients who underwent OLT during 1996–2008, 159 patients with a diagnosis of HCC were identified. Demographic data, body mass index (BMI), perioperative parameters, recurrence and survival were obtained. Complications were grouped according to Clavien–Dindo grading (Grades I–V).
Results
There were increased incidences of life-threatening complications in overweight (58%) and obese (70%) patients compared with the non-obese patient group (41%) (P < 0.05). Furthermore, the incidence of recurrence of HCC was doubled in the presence of overweight (15%) and obesity (15%) compared with non-obesity (7%) (P < 0.05). Time to recurrence also decreased significantly. Differences in mean ± standard deviation survival in the overweight (45 ± 3 months) and obese (41 ± 4 months) groups compared with the non-obese group (58 ± 6 months) did not reach statistical significance.
Conclusions
These findings indicate that BMI is an important surrogate marker for obesity and portends an increased risk for complications and a poorer oncologic outcome following OLT for HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related mortality worldwide.1 Moreover, a recent study looking at the Surveillance, Epidemiology and End Results (SEER) database demonstrated that incidence rates for HCC have tripled in the last three decades.2 Furthermore, despite advances in medical care, 1-year survival rates remain under 50% overall.2 Orthotopic liver transplant (OLT) represents a potentially curative procedure for patients with tumours not amenable to resection or ablative therapies. Yao et al. have shown that with the application of Model for End-stage Liver Disease (MELD) scores for organ allocation, the number of patients with HCC undergoing transplantation is rising.3 However, according to the United Network for Organ Sharing (UNOS) website, 16 868 patients are currently listed for a liver transplant (http://www.unos.org). Therefore, assessing recipient characteristics that have negative effects on perioperative and oncologic outcomes is critical to improve results in OLT for HCC.
Over the last decade, obesity has become a pandemic: more than 1.1 billion people worldwide are estimated to be overweight.4,5 Furthermore, over the last decade nearly 54% of patients undergoing OLT in the USA have been either overweight or obese.6 Epidemiologic evidence suggests that obesity is associated with an increased incidence of multiple cancers, including HCC and pancreatic cancer.7 Mathur et al. recently showed that increased visceral fat was associated with an inferior oncologic outcome and decreased survival in patients with pancreatic cancer who underwent pancreatoduodenectomy.8,9 Furthermore, obesity has been associated with increased perioperative morbidity and mortality in patients undergoing major surgical procedures.10,11 However, there is a paucity of evidence evaluating the impact of body mass index (BMI), used as a surrogate measure for obesity, on the occurrence of postoperative complications and oncologic outcomes in patients with HCC undergoing OLT. This study hypothesized that BMI is an important surrogate marker for obesity and portends an increased risk for complications and an inferior oncologic outcome in patients with HCC undergoing OLT.
Materials and methods
Approval to conduct this study was obtained from the University of South Florida Institutional Review Board. From a database of over 1000 patients who underwent OLT during 1996–2008, 159 patients with a diagnosis of HCC were identified. All transplants were performed using organs sourced from deceased donors, in orthotopic fashion, and were conducted in patients with disease within the Milan Criteria. Immunosuppression consisted of tacrolimus-based therapy in 90% and cyclosporine-based therapy in 10% of patients, with or without the addition of mycophenolate mofetil. Patients were weaned from prednisone at 3–6 months, except those with autoimmune liver disease. Demographic data including age, BMI and sex were obtained. Patients were categorized as non-obese (BMI < 25 kg/m2), overweight (BMI ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2). Preoperative MELD scores and biochemical parameters including serum bilirubin, creatinine and α-fetoprotein levels were documented. Perioperative parameters including cold and warm ischaemia times were recorded. Postoperative length of stay (LoS), complications, recurrences and survival were noted. Furthermore, tumour characteristics on final pathology were recorded, including tumour size and lymphovascular invasion. Additionally, complications were grouped according to the Clavien–Dindo system (Grades I–V).12 Briefly, complications of Grades III, IV and V are life-threatening: Grade III complications require surgical, radiologic or endoscopic interventions; Grade IV complications include organ failure that requires intensive care unit (ICU) management, and Grade V complications result in patient death.
Statistical analysis
Statistical analyses were performed using Sigma Stat Version 4.0 (Jandel Corp., San Jose, CA, USA). Data were expressed as the median or as the mean ± standard deviation (SD) as appropriate. Data were analysed using analysis of variance (anova), Student's t-test and Fisher's exact test, as appropriate. Survival analyses were performed using Kaplan–Meier methods. Significance was accepted with 95% confidence.
Results
Patient demographic data are depicted in Table 1. Of the 159 patients, 47 were non-obese (BMI < 25 kg/m2), 112 were overweight (BMI ≥ 25 kg/m2) and 58 were obese (BMI ≥ 30 kg/m2). The average age was 55 years and the majority (91%) of patients were male. Importantly, no differences in patient race were found across the three groups. The aetiology of HCC is depicted in Table 2. Hepatitis C was the most common aetiology. No differences existed among the three groups with respect to aetiology. Serum biochemical parameters are shown in Table 3. No differences existed in serum bilirubin, serum creatinine or serum α-fetoprotein levels across the three groups. Preoperative mean ± SD MELD scores did not differ among non-obese, overweight and obese patients (17 ± 8, 18 ± 7 and 19 ± 7, respectively; P > 0.05). Similarly, cold ischaemia time (437 ± 166 min, 461 ± 168 min and 455 ± 187 min, respectively; P > 0.05) and warm ischaemia time (43 ± 9 min, 42 ± 10 min and 42 ± 11 min, respectively; P > 0.05) did not differ statistically significantly among the non-obese, overweight and obese groups. Data from explant pathology evaluation with respect to tumour size (3.1 ± 2.0 cm, 2.8 ± 1.4 cm and 2.7 ± 1.0 cm, respectively; P > 0.05), lymphatic invasion (3%, 2% and 2%, respectively) and vascular invasion (7%, 9% and 11%, respectively) did not differ among the non-obese, overweight and obese groups. Furthermore, tumour burden did not differ among the non-obese, overweight and obese groups (1.6 ± 1.0, 2.2 ± 2.0 and 2.4 ± 2.0, respectively; P > 0.05). Rates of preoperative locoregional therapy (LRT) were also similar among the three groups. Among non-obese patients, 70% underwent LRT [64% transarterial chemoembolization (TACE), 6% radiofrequency ablation (RFA)]; among overweight patients, 62% underwent LRT (58% TACE, 4% RFA) and among obese patients, 55% underwent LRT (48% TACE, 7% RFA). Postoperative LoS is shown in Fig. 1. Length of stay was significantly greater in obese patients than in non-obese patients (P < 0.05). Incidences of Clavien–Dindo Grade I–V complications are presented in Table 4. There was an increased incidence of life-threatening complications (Grades III–V) (Fig. 2) in both overweight (58%) and obese (70%) patients compared with non-obese patients (41%) (P < 0.05). Rates of 90-day mortality were 3% in non-obese patients (n = 2 of sepsis), 5% in overweight patients (n = 2 of cardiac events, n = 3 of sepsis), and 4% in obese patients (n = 1 of cardiac events, n = 1 of sepsis). Additionally, primary graft non-function rates were 2% in non-obese, 4% in overweight and 3% in obese patients (P > 0.05). Percentages of recurrence of HCC and months to recurrence are demonstrated in Figs 3 and 4, respectively. Overweight and obesity deleteriously affected both of these parameters compared with outcomes in non-obese patients. Furthermore, recurrences occurred in the transplanted allograft in 33% of non-obese, 50% of overweight and 29% of obese patients (P > 0.05). Survival is depicted in Fig. 5. Despite a trend towards decreased survival in overweight (45 ± 3 months) and obese (41 ± 4 months) compared with non-obese (58 ± 6 months) patients, differences did not achieve statistical significance.
Table 1.
Demographic data for patients undergoing orthotopic liver transplantation for hepatocellular carcinoma
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | BMI ≥ 30 kg/m2 | |
|---|---|---|---|
| Patients, n | 47 | 112 | 58 |
| BMI, kg/m2, mean ± SD | 23 ± 2 | 31 ± 4a | 34 ± 3a |
| Age, years, mean ± SD | 55 ± 7 | 55 ± 7 | 55 ± 8 |
| Male gender | 87% | 92% | 91% |
| White | 63% | 76% | 83% |
| African-American | 10% | 5% | 4% |
| Hispanic | 20% | 18% | 13% |
| Asian | 3% | 0% | 0% |
P < 0.05 versus patients with BMI of <25 kg/m2.
BMI, body mass index; SD, standard deviation.
Table 2.
Aetiology of hepatocellular carcinoma in patients undergoing orthotopic liver transplantation
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | BMI ≥ 30 kg/m2 | |
|---|---|---|---|
| Hepatitis B | 5% | 2% | 0% |
| Hepatitis C | 46% | 46% | 36% |
| Laennec's cirrhosis | 5% | 10% | 11% |
| Hepatitis C + Laennec's cirrhosis | 37% | 27% | 30% |
| Non-alcoholic steatohepatitis | 0% | 5% | 9% |
| Cryptogenic steatohepatitis | 7% | 4% | 4% |
| Other | 0% | 8% | 10% |
BMI, body mass index.
Table 3.
Serum biochemical parameters in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma
| Serum biochemistries | BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | BMI ≥ 30 kg/m2 |
|---|---|---|---|
| Serum bilirubin, mg/dl, mean ± SD | 4 ± 7 | 5 ± 8 | 7 ± 10 |
| Serum creatinine, mg/dl, mean ± SD | 1.0 ± 1.1 | 1.0 ± 0.1 | 1.0 ± 0.5 |
| α-fetoprotein level, U/l, mean ± SD | 41 ± 54 | 167 ± 381 | 162 ± 281 |
BMI, body mass index; SD, standard deviation.
Figure 1.
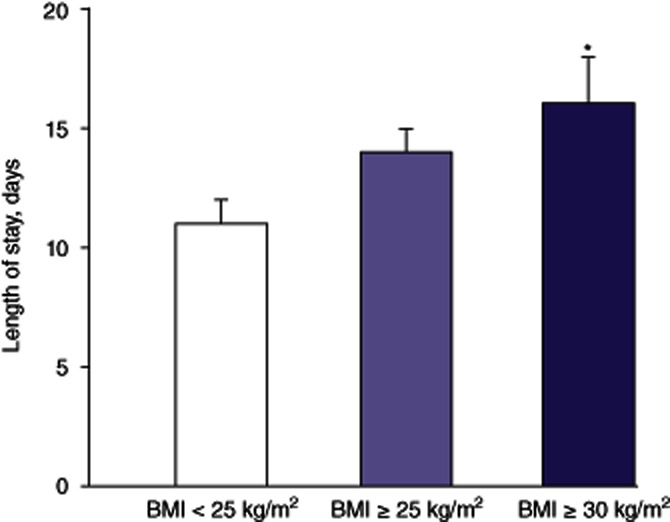
Length of stay in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma, according to body mass index (BMI). *P < 0.05 versus BMI < 25 kg/m2
Table 4.
Incidences of Clavien–Dindo complications in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | BMI ≥ 30 kg/m2 | |
|---|---|---|---|
| Grade I | 5% | 4% | 2% |
| Grade II | 21% | 9% | 4% |
| Grade III | 18% | 38%a | 45%a |
| Grade IV | 18% | 15% | 21% |
| Grade V | 3% | 5% | 4% |
P < 0.05 versus patients with BMI of <25 kg/m2.
BMI, body mass index.
Figure 2.

Incidences of complications (Clavien–Dindo Grades III–V) in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma, according to body mass index (BMI). *P < 0.05 versus BMI < 25 kg/m2
Figure 3.
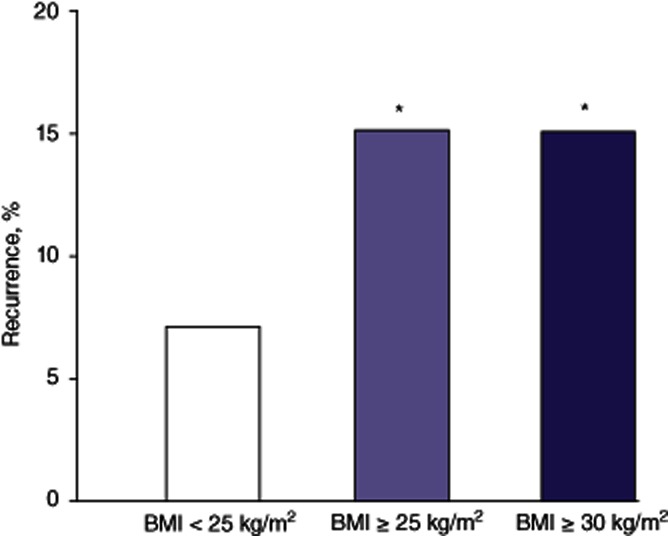
Incidences of recurrence in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma, according to body mass index (BMI). *P < 0.05 versus BMI < 25 kg/m2
Figure 4.

Months to recurrence in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma, according to body mass index (BMI). *P < 0.05 versus BMI < 25 kg/m2
Figure 5.

Survival in patients undergoing orthotopic liver transplantation for hepatocellular carcinoma. BMI, body mass index
Discussion
Obesity has been shown to be associated with increased morbidity and mortality following major abdominal procedures.10,11 The present group has previously shown that visceral fat infiltration is associated with inferior oncologic outcomes following pancreatoduodenectomy for pancreatic adenocarcinoma.8,9 However, the utility of BMI as a surrogate marker for obesity in determining oncologic outcomes following OLT for HCC has not been reported in the literature. This study demonstrates that obesity is a predictor of poorer oncologic outcomes. Specifically, these data show that a BMI of ≥25 kg/m2 and, particularly, a BMI of ≥30 kg/m2, is associated with an increased incidence of life-threatening complications and that obesity is associated with an increased LoS. Furthermore, the study demonstrates that the incidence of HCC recurrence is doubled in overweight and obese patients. Importantly, for those with a BMI of ≥25 kg/m2, the time to recurrence of HCC after OLT is also decreased significantly. Therefore, the present authors believe that the negative impact of recipient obesity on OLT for HCC results in increased morbidity and an inferior oncologic outcome; recipient BMI should be taken into consideration in future policies for the allocation of livers to patients with HCC.
Recipient demographics are an important predictor of outcomes following liver transplant. In the present study, recipient age and sex were similar among the three groups of patients. Nair et al. recently demonstrated that African-American and Asian ethnicities are independent predictors of decreased graft survival and patient survival.13 Patient race did not differ across the groups in the present study, although the proportion of Hispanic patients treated at this centre is larger than the national average. However, Hispanic race is not associated with poorer outcomes following liver transplantation.13 As expected the most common aetiology for liver failure was hepatitis C. This trend is similar to nationwide trends in the aetiology of liver failure in transplant recipients.6 The three groups of patients in the present study did not differ with respect to aetiology of HCC. Interestingly, obese patients were found to have an increased incidence of non-alcoholic steatohepatitis (NASH) compared with non-obese patients, but this trend did not achieve statistical significance. However, larger studies looking at nearly 18 000 patients have demonstrated that severe (BMI ≥ 35 kg/m2) and morbid (BMI ≥ 40 kg/m2) obesity are particularly associated with statistically significantly increased incidences of cryptogenic steatohepatitis and NASH.13 The present data support this finding, but the sample size in the present study was too small to allow the adequate classification of patients into severely obese and morbidly obese groups.
Biochemical recipient characteristics that have been shown to predict survival include serum bilirubin and serum creatinine. Thuluvath et al. demonstrated that increased bilirubin and serum creatinine are independent pre-transplant predictors of decreased survival at both the 1-month and 1-year time-points after OLT.14 The present study demonstrated no differences among the three groups in either of these pre-transplant parameters. Furthermore, no statistically significant differences in preoperative α-fetoprotein levels were found among the three groups.
Both cold and warm ischaemia times are important parameters that determine graft and patient survival. No differences in cold ischaemia time were noted across the three groups. More importantly, no differences were noted in warm ischaemia times. This would suggest that transplanting a liver into an obese individual is a technically feasible operation and is not associated with prolonged warm ischaemia time. This belief is supported by Nair et al., who demonstrated in a group of 121 patients that intraoperative complications and intraoperative transfusion requirements were similar in obese and non-obese patients.15 Therefore, concerns based on the technical difficulty of the operation should not deter OLT in obese patients.
Morbidity following major abdominal surgery has been found to be increased in patients with increasing BMI.10 This theory has been supported in the liver transplant literature. Keeffe et al. 16 and Sawyer et al. 17 independently demonstrated that morbidity increases in obese patients following OLT. Sawyer et al.17 noted an increase in wound infection rates to 20% in obese patients compared with 4% in non-obese patients and an increased incidence of multi-system organ failure in obese (15%) compared with non-obese (2%) recipients. However, Braunfeld et al. reported that obese patients did not have an increased incidence of postoperative complications or LoS.18 However, their study included a cohort of only 40 obese patients.18 A larger single-institution study reported by Schaeffer et al., evaluating BMI-related outcomes in 167 liver transplants, noted that obesity increased the incidence of Clavien–Dindo Grade III complications, including wound dehiscences and ventral hernias.19 The present data support the theory that obesity results in an increased incidence of postoperative complications. The current study shows that complications which result in surgical, radiologic or endoscopic interventions (Clavien–Dindo Grade III) were increased in patients who were overweight or obese. Furthermore, the percentage of life-threatening complications was increased in both the overweight and obese groups in comparison with the non-obese group. Although postoperative complications after negative-margin (R0) resections for intra-abdominal malignancies have historically been related to increased cancer recurrence postoperatively, neither single-institution studies nor systematic reviews have identified this as a significant risk factor for recurrence after OLT for HCC.20,21 This is possibly because unlike other contexts following surgery for intraoperative malignancies in which a Clavien–Dindo Grade III complication would result in an increased recurrence by an immunosuppressive mechanism, post-OLT patients are already immunosuppressed.
The incidence of primary graft non-function has been reported to be increased in morbid obesity.6 The present data on outcomes in overweight and obese patients do not substantiate this finding. The incidence of primary graft non-function in non-obese patients did not differ statistically from that in the overweight and obese patient groups. Importantly, Nair et al. also demonstrated that the increase in primary graft non-function did not reach statistical significance in obese patients (BMI 30–39 kg/m2). Furthermore, as expected, these increased complications resulted in an increased LoS in obese patients. This is also supported by previous findings reported in the literature: the Johns Hopkins group noted that increasing BMI was associated with an increased LoS and increased hospital costs.15 This is important because it represents an increased resource utilization by overweight and obese recipients of liver transplants.
Early postoperative mortality was infrequent (4%) in the present study and was not affected by increasing BMI. Specifically, 67% (n = 5) of patient deaths occurred as a result of bacterial sepsis in the immediate postoperative period. The remaining 33% (n = 2) of patient deaths occurred secondary to cardiovascular events and one patient suffered an intraoperative cardiac arrest. This is supported by Braunfeld et al., who noted no difference in early mortality between non-obese and obese patients.18 However, both the present study and that reported by Braunfeld et al.18 included a limited number of patients in the obese group and therefore these data should be interpreted with caution. As noted previously, major abdominal procedures have been shown to result in increased mortality in patients who are obese.10,11 Further studies in larger cohorts of patients are required to identify whether early postoperative mortality is indeed unchanged in patients undergoing OLT in the setting of obesity.
Since the institution of the Milan Criteria in 1996,22 with the demonstration of an acceptable recurrence-free survival in patients with HCC undergoing OLT, enthusiasm for liver transplantation in patients with HCC has increased. In a large UNOS study looking at 985 patients with HCC, Yao et al. noted a recurrence rate of only 7.6%.3 The present study noted a similar recurrence rate of 7% in the non-obese group. However, it also reports a novel finding of the doubling of recurrence rates in both overweight and obese patients following OLT for HCC. Importantly, this increase was statistically significant. Furthermore, time to recurrence was significantly decreased in both these groups compared with the non-obese patient group. With respect to patterns of recurrence, Marsh et al. demonstrated that up to 35% of recurrences occur within the transplanted liver itself.23 The present study demonstrates a similar percentage (42%) of recurrences occurring in the liver following OLT for HCC. Patterns of recurrence were not influenced by BMI.
Factors influencing recurrence rates include tumour pathology and the provision of LRT prior to liver transplantation.24 With respect to tumour pathology, specifically tumour size and vascular invasion have been shown to increase the incidence of recurrence of HCC. There were no differences in either tumour size or vascular involvement among the groups in the present study. Iwatsuki and colleagues demonstrated that lymphatic involvement is a significant negative predictor of survival following OLT for HCC.25 No differences were noted in lymphatic involvement between the non-obese and overweight or obese groups in the current study. Furthermore, with respect to LRT, Yao et al. suggested that patients who received preoperative LRT have a potential survival advantage.24 No differences according to the administration of preoperative LRT emerged among patient groups in the present study. This lack of differences in parameters that are known to influence oncologic outcomes further consolidates the potential role of increased adiposity in promoting recurrence.
The effects of fatty infiltration of the liver and its progression to NASH and subsequently HCC are well described in the literature. However, it is important to note that the majority of recurrences following OLT for HCC are extrahepatic, as shown in both the present study and that by Marsh et al.23 These recurrences are influenced by the systemic milieu of the patient. Obesity is believed to result in a proinflammatory and protumorogenic milieu. This effect is mediated through a dysfunctional production of adipose-derived adipokines (leptin and adiponectin), cytokines and chemokines, which are collectively referred to as adipocytokines.26,27 Leptin, which is elevated in obese humans, has been shown to increase proliferation and suppress apoptosis in colorectal and pancreatic cancer cell lines.28 In addition, leptin increases cell invasion and the induction of leptin receptors can upregulate expression of angiogenic factors, including vascular endothelial growth factor.29,30 However, even more significant is evidence demonstrating a direct effect of leptin on HCC. Both short and long isoforms of leptin receptors have been observed in HCC cells at greater concentrations than in other tissues, suggesting that leptin may be involved in HCC.31 Furthermore, Saxena et al. demonstrated that leptin promotes HCC growth, invasiveness and migration and implicated the JAK/STAT pathway as a critical mediator of leptin action.31 These findings were independently confirmed by Chen et al.32 Ribatti's group showed a correlation with leptin and increased angiogenesis in HCC.33 Further, delineation of the mechanistic pathway of leptin's effects on HCC were described by Stefanou et al.,34 who showed that leptin could affect HCC progression and invasion through its interaction with cytokines and matrix metalloproteinases (MMPs) in the tumorigenic microenvironment. The second most predominant adipokine, adiponectin, is downregulated in the obese state. The significance of this adipokine is underscored by the fact that adiponectin in vitro inhibits leptin-induced cell proliferation, migration and invasiveness of HCC cells.35 Therefore, the decrease in adiponectin coupled with the increase in leptin, in the setting of obesity, synergistically enhances HCC proliferation, migration and invasiveness. Therefore, the presence of an obesity-induced protumorogenic systemic milieu may result in increased tumour growth and migration of occult microscopic extrahepatic metastases present at the time of liver transplantation. This may account for the earlier occurrence and increased incidence of recurrence of HCC in the setting of obesity following OLT.
Increasing BMI has been shown to result in decreased survival in both lung and renal transplant recipients.36,37 However, the effects of obesity on survival following OLT have been contentious. Several single-institution reports have noted that BMI of ≥30 kg/m2 is not associated with a decrease in survival.16–18 However, two large recent UNOS studies suggested otherwise. One study looking at 18 172 patients demonstrated decreased survival in severely obese (BMI ≥ 35 kg/m2) and morbidly obese (BMI ≥ 40 kg/m2) patients.6 Furthermore, the second study, which looked at outcomes in 38 876 patients, noted that BMI of ≥35 kg/m2 is a significant predictor of negative outcomes at both the 1-month and 1-year time-points following liver transplant.14 The present data demonstrate a trend towards decreased survival in both overweight and obese patients. However, the small sample size in the present study prevented the elucidation of differences in survival between patients who were severely (BMI ≥ 35 kg/m2) and morbidly (BMI ≥ 40 kg/m2) obese. Therefore, the present study's lack of statistical significance with respect to survival may be secondary to a type II error caused by the sample size in comparison with those of the studies looking at UNOS data. Further cohort studies with larger sample sizes are necessary to further evaluate survival in HCC in the setting of obesity.
In summary, in patients with HCC undergoing OLT, obesity results in an increased LoS and a higher risk for life-threatening complications. Furthermore, it portends an earlier recurrence of HCC; this represents a novel finding not previously reported. Therefore, it is concluded that BMI represents an important prognostic marker for obesity and portends an increased risk for complications and a poorer oncologic outcome following OLT for HCC. Further studies corroborating these data are needed to determine whether recipient BMI should be taken into consideration in future policies for the allocation of livers to patients with HCC.
Conflicts of interest
None declared.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao FY, Bass NM, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: lessons from the first year under the Model of End-stage Liver Disease (MELD) organ allocation policy. Liver Transpl. 2004;10:621–630. doi: 10.1002/lt.20159. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 6.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing OLT in the United States. Hepatology. 2000;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer Working Group on Evaluation of Cancer-Preventive Strategies. Weight Control and Physical Activity. Lyon: IARC Press; 2002. [Google Scholar]
- 8.Mathur A, Hernandez J, Shaheen F, Shroff M, Dahal S, Morton C, et al. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB. 2011;13:404–410. doi: 10.1111/j.1477-2574.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208:989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. discussion 994–996. [DOI] [PubMed] [Google Scholar]
- 10.Flancbaum L, Choban PS. Surgical implications of obesity. Annu Rev Med. 1998;49:15–34. doi: 10.1146/annurev.med.49.1.215. [DOI] [PubMed] [Google Scholar]
- 11.Choban PS, Flancbaum L. The impact of obesity on surgical outcome: a review. J Am Coll Surg. 1997;185:593–603. doi: 10.1016/s1072-7515(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of OLT: a cohort study. Lancet. 2002;26:287–293. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 14.Thuluvath PJ, Yoo HY, Thompson RE. A model to predict survival at one month, one year, and five years after liver transplantation based on pre-transplant clinical characteristics. Liver Transpl. 2003;9:527–532. doi: 10.1053/jlts.2003.50089. [DOI] [PubMed] [Google Scholar]
- 15.Nair S, Cohen DB, Cohen MP, Tan H, Maley W, Thuluvath PJ. Postoperative morbidity, mortality, costs, and longterm survival in severely obese patients undergoing OLT. Am J Gastroenterol. 2001;96:842–845. doi: 10.1111/j.1572-0241.2001.03629.x. [DOI] [PubMed] [Google Scholar]
- 16.Keeffe EB, Gettys C, Esquivel CO. Liver transplantation in patients with severe obesity. Transplantation. 1994;57:309–311. [PubMed] [Google Scholar]
- 17.Sawyer RG, Pelletier SJ, Pruett TL. Increased early morbidity and mortality with acceptable longterm function in severely obese patients undergoing liver transplantation. Clin Transplant. 1999;13:126–130. doi: 10.1034/j.1399-0012.1999.130111.x. [DOI] [PubMed] [Google Scholar]
- 18.Braunfeld MY, Chan S, Pregler J, Neelakanta G, Sopher MJ, Busuttil RW, et al. Liver transplantation in obese patients. J Clin Anesth. 1996;8:585–590. doi: 10.1016/s0952-8180(96)00142-0. [DOI] [PubMed] [Google Scholar]
- 19.Schaeffer DF, Yoshida EM, Buczkowski AK, Chung SW, Steinbrecher UP, Erb SE, et al. Surgical morbidity in severely obese liver transplant recipients – a single Canadian centre experience. Ann Hepatol. 2009;8:38–40. [PubMed] [Google Scholar]
- 20.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143:182–188. doi: 10.1001/archsurg.2007.39. [DOI] [PubMed] [Google Scholar]
- 22.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 23.Marsh JW, Dvorchik I, Subotin M, Balan V, Rakela J, Popechitelev EP, et al. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after OLT: a pilot study. Hepatology. 1997;26:444–450. doi: 10.1002/hep.510260227. [DOI] [PubMed] [Google Scholar]
- 24.Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R, Jr, Kerlan R, et al. The impact of preoperative locoregional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5:795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 25.Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–228. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83 (Suppl.):461–465. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 27.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 29.Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide3-kinase-, rho, and rac-dependent signalling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 31.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signalling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, Guo IC. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by upregulating cyclin D1 and downregulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14:513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- 33.Ribatti D, Belloni AS, Nico B, Di Comite M, Crivellato E, Vacca A. Leptin–leptin receptor are involved in angiogenesis in human hepatocellular carcinoma. Peptides. 2008;29:1596–1602. doi: 10.1016/j.peptides.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10:442. doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS. The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant. 2010;29:1026–1033. doi: 10.1016/j.healun.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, et al. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–363. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]


