Abstract
Human immunodeficiency virus type 1 (HIV-1)-associated dementia (HAD) is correlated with increased monocyte migration to the brain, and the incidence of HAD among otherwise asymptomatic subjects appears to be lower in India than in the United States and Europe (1 to 2% versus 15 to 30%). Because of the genetic differences between HIV-1 strains circulating in these regions, we sought to identify viral determinants associated with this difference. We targeted Tat protein for these studies in view of its association with monocyte chemotactic function. Analyses of Tat sequences representing nine subtypes revealed that at least six amino acid residues are differentially conserved in subtype C Tat (C-Tat). Of these, cysteine (at position 31) was highly (>99%) conserved in non-subtype C viruses and more than 90% of subtype C viruses encoded a serine. We hypothesized a compromised chemotactic function of C-Tat due to the disruption of CC motif and tested it with the wild type C-Tat (CS) and its two isogenic variants (CC and SC) derived by site-directed mutagenesis. We found that the CS natural variant was defective for monocyte chemotactic activity without a loss in the transactivation property. While the CC mutant is functionally competent for both the functions, in contrast, the SC mutant was defective in both. Therefore, the loss of the C-Tat chemotactic property may underlie the reduced incidence of HAD; although not presenting conclusive evidence, this study provides the first evidence for a potential epidemiologic phenomenon associated with biological differences in the subtype C viruses.
Human immunodeficiency virus type 1 (HIV-1) displays extraordinary genetic variation, leading to the classification of the viral strains into phylogenetically distinct groups and subtypes (25). Of the various subtypes of HIV-1, subtype C is linked to ∼50% of the infections globally (11) and is associated with rapidly growing epidemics in sub-Saharan Africa and parts of Asia, including India and China (13). In addition to genetic and demographic factors, biological properties unique to the subtype C viruses may also play a role in their exponential proliferation (12, 34). HIV-1 subtype C exhibits properties distinct from other subtypes at the molecular and biological levels, and it is presently not known whether these differences translate to differential pathogenic properties (20).
The prevalence of HIV-1-associated dementia (HAD) among otherwise asymptomatic subjects in United States and Europe has been estimated at 15 to 30% (18, 47). In contrast, Satishchandra et al. (40) and others (45) have documented an unusually low incidence (about 1 to 2%) of HAD in India. It should be noted that the low levels of HAD in economically developing societies like India are often attributed to underdiagnosis, shorter life expectancy, or other factors (19, 42). In an extensive follow-up of earlier studies, however, Satishchandra et al. (40) found six cases of HAD among 427 HIV-infected asymptomatic individuals (1.4%), indicating that the low incidence of HAD is not an artifact (A. Nalini, P. Satishchandra, M. Gourie-Devi, N. Khanna, V. Santosh, V. Ravi, A. Desai, A. Chandramukhi, T. C. Yasha, A. Mahadevan, T. Suresh, P. N. Jayakumar, and S. K. Shankar, Abstr. XVII World Congr. Neurol., London, United Kingdom, abstr. 971, 2001).
Since subtype C viruses predominate in India and other developing countries and are genetically distinct from the subtype B viruses that are prevalent in the United States and Europe, we sought to identify genetic and biologic differences between the viral subtypes as a basis for an explanation of the differences in the levels of prevalence of HAD. Among HIV-1 proteins, Tat exhibits strong monocyte chemotactic properties (2, 3); the increased migration of the activated monocytes to the brain is strongly correlated with HAD (23). Therefore, we evaluated structural and functional aspects of Tat that could influence monocyte chemotactic function and explain the low frequency of HAD. This study presents an analysis of Tat sequences for identification of the features that are differentially conserved in C-Tat and experimental evidence to show that one of differentially conserved amino acids attenuates the monocyte chemotactic functions of Tat without affecting its transactivation properties.
A total of 1,081 group M HIV-1 sequences encoding the first exon of tat were obtained from the Los Alamos sequence database (25). On the basis of the results of phylogenetic analysis, identical and near-identical sequences were removed to obtain a subset of 518 sequences (comprised of subtypes A [35 sequences], B [226], C [145], D [17], AE [23], F [27], G [33], H [5], and K [7]) that were subjected to analyses. Sequences were aligned using CLUSTAL_X (44) and manually edited. Evolutionary model parameters (fA = 0.3765, fC = 0.2972, fG = 0.1824, and fT = 0.1440; α = 0.7015; R matrix values, RA->C = 1.1099, RA->G = 2.9067, RC->T = 3.8450, RA->T = 0.6997, RC->G = 0.6703, and RG->T = 1.0000; the proportion of invariable sites was 0.0838 [corresponding to a TVM+G+I model]) were estimated using PAUP* 4.0b10 (43) and MODELTEST (35). A total of 146 subtype C Tat (C-Tat) sequences were separately analyzed using maximum likelihood methods to rule out the presence of a common ancestry among C-Tat sequences that did not encode S31 (model parameters for 153 sequences [including 146 C-Tat sequences], fA = 0.2851, fC = 0.2521, fG = 0.2194, and fT = 0.2434; α = 0.6867; R matrix values, RA->C = 4.4114, RA->G = 7.4767, RC->T = 9.1592, RA->T = 1.3915, RC->G = 1.2480, and RG->T = 1.0000; the proportion of invariable sites was 0.2228 [corresponding to a TVM+G+I model]). The heuristic search for a maximum-likelihood (ML) tree was done using PAUP* and a subtree-pruning-regrafting branch-swapping algorithm. The starting tree was obtained by neighbor joining; 26 ML trees with a log likelihood score of −5,168.4784 were obtained from 2,844,407 rearrangements. Multifurcations were edited by assigning low values, and the trees were edited and displayed. The presence of signature amino acid residues that characterize subtype C viruses was assessed using Vespa (24).
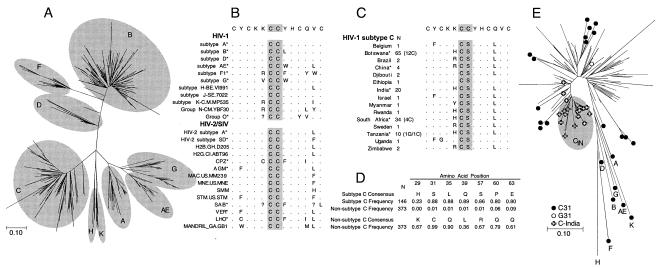
Phylogenetic analysis of the first exon of tat revealed monophyletic clusters of subtype C and other subtypes (Fig. 1A) consistent with the phylogenetic relationship observed for other regions of the viral genome (6, 28, 30, 41). Six amino acid positions were conserved in 70% or more of subtype C sequences (Fig. 1D). Among these, we selected C31S for a focused study in view of its presence within the highly conserved dicysteine motif and its impact on Tat biological functions (21, 36, 39). While >99% of non-subtype C viruses encode a cysteine at position 31 (Fig. 1B and D), about 90% of the sequences identified as belonging to subtype C in the database (http://hiv-web.lanl.gov) encoded a serine (regardless of the geographic origin of the subtype C viruses) (Fig. 1C and D). Of the 34 subtype C sequences that did not encode S31, 13 were potential recombinants. Analysis of 18 that clustered unambiguously with subtype C indicated the absence of a shared ancestry; thus, these appeared to have evolved independently (Fig. 1E). In consistency with previous reports identifying the sequences of subtype C viruses in India as being genetically distinct from other subtype C sequences (27, 33, 37, 41), Tat1 sequences from India clustered together.
FIG. 1.
Genetic features of HIV-1 Tat sequences. (A) Phylogenetic relationships among group M HIV-1 Tat exon 1 sequences, illustrating the monophyletic subtype-specific clustering of sequences. Subtypes corresponding to each of the clusters in the neighbor-joining phylogram are shown. (B) Dicysteine motif within the cysteine-rich domain (Tat1 amino acids 25 to 37) of HIV-1, HIV-2, and SIV. Sequences representing different subtypes of HIV-1, HIV-2, and SIV are shown; dots indicate identity with the sequence at the top, and the dicysteine motif is highlighted. Asterisks (*) indicate sequence data obtained from consensus sequences derived in this study; the other sequence data were obtained from the database. (C) Subtype C sequences from different countries, illustrating the level of conservation of the C31S substitution regardless of geographic origin. Similar findings for the uniform substitution of Q35L and the high level of heterogeneity at position 29 (as identified in panel D) are also evident. The number of sequences obtained from each country and used in these analyses and the number that carried C or G at position 31 are shown. (D) Signature amino acids present in 70% or more subtype C sequences. Corresponding amino acids for non-subtype C sequences as well as their frequencies are illustrated. Position 29 in C-Tat is shown because of the high level of diversity that appears to be unique to subtype C viruses. A total of 14 different amino acids are seen at this position. (E) Phylogenetic analysis consistent with independent evolution of subtype C sequences with C31. The ML phylogram was generated using 146 subtype C sequences and non-subtype C representative sequences. The sole subtype H sequence that encoded S31 (AF190128) was used.
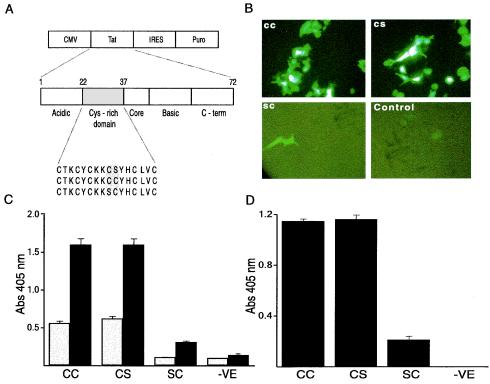
A cysteine-rich domain of Tat governs the transactivation functions of Tat (21). Work from several laboratories has established that of the seven positions with cysteines, C31 may be the only amino acid dispensable for the transactivation property of Tat (21, 36). We used green fluorescent protein (GFP) or secreted alkaline phosphatase (SEAP) as a reporter under the control of an HIV-1 long terminal repeat (LTR) to perform a series of transient transfection experiments to analyze transactivation properties of multiple isogenic C-Tat expression vectors (Fig. 2A). The first exon of C-Tat from an Indian clinical sample was cloned into a pIRESpuro vector (BD Biosciences Clontech, Palo Alto, Calif.). Mutations were introduced (using an overlap-PCR strategy) into the CS motif of Tat at positions 30 and 31 to generate CC and SC mutants (Fig. 2A). 293 cells were cotransfected with different Tat expression vectors and reporter plasmids expressing GFP or SEAP under the control of an LTR. All functional assays of Tat contained a cytomegalovirus-galactosidase expression vector as an internal standard. Tat vectors with C30 (CC and CS) induced high levels of GFP expression, while substituting serine at position 30 (SC) led to reduced GFP expression (Fig. 2B). Quantitative analysis of Tat transactivation with an SEAP reporter confirmed that cysteine at position 30, but not at position 31, is critical for Tat transactivation (Fig. 2C). To test the effect of C31S mutation on virus production, we transfected HLM-1 cells (which harbor an integrated virus defective for Tat) with vectors encoding CC, CS, and SC versions of C-Tat. Culture medium was harvested at different time points, and p24 levels were measured using a commercial kit (NEN Life Sciences, Boston, Mass.). CC- and CS-Tat produced comparable levels of p24, while the levels produced by SC were significantly lower (Fig. 2D). In summary, the results of experiments with isogenic variants of C-Tat suggested that cysteine at position 31 is not critical for LTR transactivation. Since C31 is conserved in nearly all HIV-1 non-subtype C viruses and HIV-2 and simian immunodeficiency virus (SIV) but not in subtype C viruses (Fig. 1B), it remains to be seen whether C31 is associated with a yet-to-be-identified important function.
FIG. 2.
Functional evaluation of Tat transactivation using isogenic variants of C-Tat. (A) Schematic diagram of the Tat mammalian expression vectors encoding the isogenic C-Tat proteins. Differences within the dicysteine motif of these vectors are highlighted. Cys, cysteine; CMV, cytomegalovirus; IRES, internal ribosome entry site; Puro, puromycin; C-term, carboxy terminus. (B) Transactivation of LTR-driven GFP expression by different Tat vectors in 293 cells. (C) Transactivation of LTR-driven SEAP expression by different Tat vectors in 293 cells. SEAP in the culture medium was quantified on day 1 (open bars) and day 3 (filled bars). (D) Rescue of the Tat-defective virus by isogenic C-Tat proteins. HLM-1 cells were transfected with different C-Tat variant expression vectors. Culture supernatants were collected on days 1, 3, 5, and 7 following transfection, and p24 levels in the culture supernatants were determined using a commercial kit. Results of experiments using samples from day 3 (when antigen levels peaked) are presented; similar results were observed for samples from other days. Abs, absorbance; −VE, parental vector.
Tat secreted from the infected cells (8, 10) is readily taken up by cells and can reach the nucleus and modulate the expression of a variety of cellular genes (32). Among the properties ascribed to extracellular Tat, a strong macrophage/monocyte-specific chemokine activity (2, 26, 46) is highly relevant to HAD (since infiltration of activated monocytes to the brain is believed to be a critical event) (14, 23, 31). A significant correlation between the degree of macrophage staining and the severity of dementia has been reported (15, 16). Tat (functioning as a chemokine by itself and/or indirectly via the stimulation of monocyte chemotactic protein 1 [MCP-1] secretion by astrocytes) has been implicated in the recruitment of monocytes to the brain (4, 5, 9, 46). Peptide mapping to delineate determinants of chemokine properties has identified the cysteine-rich and core domains of Tat as being responsible for this activity (1). Potent monocyte chemokines such as MCP-1, MIP-1, RANTES, and others possess a chemokine fold and a dicysteine motif essential for monocyte chemotaxis (38). Disruption of the dicysteine motif has been shown to inhibit the monocyte chemotactic activity of MCP-1 (22) and subtype B Tat (1) peptides.
To evaluate the significance of subtype C-Tat in monocyte chemotaxis, we used recombinantly expressed Tat proteins and a modified microchemotaxis system to perform cell migration assays (29). Human peripheral blood mononuclear cells were collected from anonymous blood donors and supplied by The New York City Blood Center. Monocytes were enriched by RossetteSep technology (StemCell Technologies, Vancouver, Canada). Monocyte migration was set up in a 48-well chamber with a polycarbonate membrane (5-μm pore size) coated with polyvinylpyrrolidone (Neuroprobe, Gaithersburg, Md.) for a gradient of different Tat proteins in a 2-h chemotaxis assay (1). Migrated cells were fixed and stained with Diff-Quick (Dade Behring, Newark, Del.), and a minimum of 10 representative fields for each test group was counted using ×20 magnification.
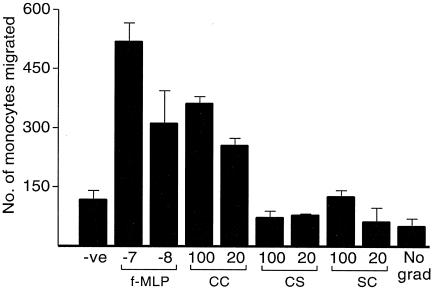
Monocytes migrated in a dose- and time-dependent manner with a gradient of CC-Tat. However, disruption of the dicysteine motif (CS or SC) in Tat resulted in a significant reduction in the migration of monocytes (Fig. 3). A monoclonal antibody against subtype B-Tat inhibited monocyte migration (data not shown). Identical results were obtained with different lots of human monocytes under different experimental conditions. These results suggest that C-Tat is inherently deficient in monocyte chemotaxis due to a C31S substitution. On the basis of our own results and those of the previous studies (2, 21), we propose that C31 in Tat derived from non-subtype C strains of HIV-1, HIV-2, and SIV is evolutionarily conserved to preserve the dicysteine motif necessary for its monocyte chemotactic function. Subtype C viruses appear to relinquish this function, possibly to gain an advantage of a different kind.
FIG. 3.
Monocyte migration induced by isogenic Tat proteins. f-MLP peptide was used as a positive control at 10−7 and 10−8 M concentrations. Tat proteins were used at concentrations of 100 and 20 ng/ml (12 and 2.4 nM, respectively) as indicated. No grad, wells with 100 ng of CC-Tat protein/ml in both the compartments. Differences in the numbers of monocytes that migrated with Tat-CC and Tat-CS were statistically significant (odds ratio = 4.95; confidence interval = 0.95 [3.4 to 7.2]; P < 0.0001 at 100 ng/ml).
This is the first report illustrating genetic and functional differences between the Tat proteins of subtype C and non-subtype C viruses and how this variation might impact the differential levels of incidence of HAD among HIV-infected individuals in India (40, 45) and (potentially) other countries where subtype C viruses predominate. However, it should be noted that HAD is expected to have a complex etiology and that other viral or host gene products and/or interactions may also play a role. For instance, polymorphism at the MCP-1 locus has been shown to be associated with the incidence of HAD in a clinical cohort (17).
Socioeconomic factors (such as underreporting and underdiagnosis of dementia and different diagnostic criteria applied for the identification of the demented subjects) have influenced the incidence of other forms of dementia (such as Alzheimer's disease) (7, 42). However, no such information is available on the incidence of HAD caused by strains other than HIV-1 subtype B.
In summary, we have identified a natural variation within C31S, the dicysteine motif of HIV-1 Tat, which is differentially conserved among subtype C viruses. We show that a C31S mutation attenuates monocyte chemotactic function without modulating transactivation property. Another characteristic of subtype C viruses is the absence of X4 viruses that use the chemokine receptor CXCR4. Given the chemokine properties of Tat, we speculate that a role for C31S mutation exists in the absence of evolution and outgrowth of X4 viruses.
Acknowledgments
U.R. acknowledges institutional financial support from JNCASR and the AIDS International Training and Research Program (NIH D43-TW01403) of the Albert Einstein College of Medicine (Program Director, Vinayaka Prasad). R.S. was supported by NIH grant AI041870. L.R. and N.B.S. are recipients of the C.S.I.R. fellowship of the government of India.
A number of reagents used in this study were obtained through the U.S. AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, and The Centralised Facility for AIDS Reagents, National Institute for Biological Standards and Control, UNAIDS. We thank Sunhee Lee and Chandrabhas Narayana for helpful discussions.
REFERENCES
- 1.Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. M. Noonan. 1998. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. [DOI] [PubMed] [Google Scholar]
- 2.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. Proudfoot, S. Alouani, T. N. Wells, G. Mariani, R. L. Rabin, J. M. Farber, and D. M. Noonan. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli, R., A. Barbero, S. Ferrini, P. Scapini, M. Cassatella, F. Bussolino, C. Tacchetti, D. M. Noonan, and A. Albini. 2000. Human immunodeficiency virus transactivator protein (Tat) stimulates chemotaxis, calcium mobilization, and activation of human polymorphonuclear leukocytes: implications for Tat-mediated pathogenesis. J. Infect. Dis. 182:1643-1651. [DOI] [PubMed] [Google Scholar]
- 4.Benelli, R., R. Mortarini, A. Anichini, D. Giunciuglio, D. M. Noonan, S. Montalti, C. Tacchetti, and A. Albini. 1998. Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS 12:261-268. [DOI] [PubMed] [Google Scholar]
- 5.Bonwetsch, R., S. Croul, M. W. Richardson, C. Lorenzana, L. D. Valle, A. E. Sverstiuk, S. Amini, S. Morgello, K. Khalili, and J. Rappaport. 1999. Role of HIV-1 Tat and CC chemokine MIP-1α in the pathogenesis of HIV associated central nervous system disorders. J. Neurovirol. 5:685-694. [DOI] [PubMed] [Google Scholar]
- 6.Cassol, S., B. G. Weniger, P. G. Babu, M. O. Salminen, X. Zheng, M. T. Htoon, A. Delaney, M. O'Shaughnessy, and C. Y. Ou. 1996. Detection of HIV type 1 env subtypes A, B, C, and E in Asia using dried blood spots: a new surveillance tool for molecular epidemiology. AIDS Res. Hum. Retrovir. 12:1435-1441. [DOI] [PubMed] [Google Scholar]
- 7.Chandra, V., M. Ganguli, G. Ratcliff, R. Pandav, S. Sharma, J. Gilby, S. Belle, C. Ryan, C. Baker, and E. Seaberg. 1994. Studies of the epidemiology of dementia: comparisons between developed and developing countries. Aging (Milan) 6:307-321. [DOI] [PubMed] [Google Scholar]
- 8.Chang, H. C., F. Samaniego, B. C. Nair, L. Buonaguro, and B. Ensoli. 1997. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421-1431. [DOI] [PubMed] [Google Scholar]
- 9.Conant, K., A. Garzino-Demo, A. Nath, J. C. McArthur, W. Halliday, C. Power, R. C. Gallo, and E. O. Major. 1998. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc. Natl. Acad. Sci. USA 95:3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esparza, J., and N. Bhamarapravati. 2000. Accelerating the development and future availability of HIV-1 vaccines: why, when, where, and how? Lancet 355:2061-2066. [DOI] [PubMed] [Google Scholar]
- 12.Essex, M. 1998. State of the HIV pandemic. J. Hum. Virol. 1:427-429. [PubMed] [Google Scholar]
- 13.Essex, M. 1999. Human immunodeficiency viruses in the developing world. Adv. Virus Res. 53:71-88. [DOI] [PubMed] [Google Scholar]
- 14.Gartner, S., and Y. Liu. 2002. Insights into the role of immune activation in HIV neuropathogenesis. J. Neurovirol. 8:69-75. [DOI] [PubMed] [Google Scholar]
- 15.Glass, J. D., H. Fedor, S. L. Wesselingh, and J. C. McArthur. 1995. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 38:755-762. [DOI] [PubMed] [Google Scholar]
- 16.Glass, J. D., S. L. Wesselingh, O. A. Selnes, and J. C. McArthur. 1993. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 43:2230-2237. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, E., B. H. Rovin, L. Sen, G. Cooke, R. Dhanda, S. Mummidi, H. Kulkarni, M. J. Bamshad, V. Telles, S. A. Anderson, E. A. Walter, K. T. Stephan, M. Deucher, A. Mangano, R. Bologna, S. S. Ahuja, M. J. Dolan, and S. K. Ahuja. 2002. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc. Natl. Acad. Sci. USA 99:13795-13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton, R. K., I. Grant, N. Butters, D. A. White, D. Kirson, J. H. Atkinson, J. A. McCutchan, M. J. Taylor, M. D. Kelly, R. J. Ellis, et al. 1995. The HNRC 500-neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1:231-251. [DOI] [PubMed] [Google Scholar]
- 19.Hira, S. K., G. J. Dore, and T. Sirisanthana. 1998. Clinical spectrum of HIV/AIDS in the Asia-Pacific region. AIDS 12(Suppl. B):S145-S154. [PubMed] [Google Scholar]
- 20.Hu, D. J., A. Buve, J. Baggs, G. van der Groen, and T. J. Dondero. 1999. What role does HIV-1 subtype play in transmission and pathogenesis? An epidemiological perspective. AIDS 13:873-881. [DOI] [PubMed] [Google Scholar]
- 21.Jeang, K. T., H. Xiao, and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 274:28837-28840. [DOI] [PubMed] [Google Scholar]
- 22.Kaji, M., M. Ikari, S. Hashiguchi, Y. Ito, R. Matsumoto, T. Yoshimura, J. Kuratsu, and K. Sugimura. 2001. Peptide mimics of monocyte chemoattractant protein-1 (MCP-1) with an antagonistic activity. J. Biochem. (Tokyo) 129:577-583. [DOI] [PubMed] [Google Scholar]
- 23.Kaul, M., G. A. Garden, and S. A. Lipton. 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988-994. [DOI] [PubMed] [Google Scholar]
- 24.Korber, B., and G. Myers. 1992. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res. Hum. Retrovir. 8:1549-1560. [DOI] [PubMed] [Google Scholar]
- 25.Kuiken, C. L., B. Foley, B. Hahn, B. Korber, F. McCutchan, P. A. Marx, J. W. Mellors, J. I. Mullins, J. Sodroski, and S. Wolinsky. 2002. Human retroviruses and AIDS: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 26.Lafrenie, R. M., L. M. Wahl, J. S. Epstein, I. K. Hewlett, K. M. Yamada, and S. Dhawan. 1996. HIV-1-Tat protein promotes chemotaxis and invasive behavior by monocytes. J. Immunol. 157:974-977. [PubMed] [Google Scholar]
- 27.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louwagie, J., F. E. McCutchan, M. Peeters, T. P. Brennan, E. Sanders-Buell, G. A. Eddy, G. van der Groen, K. Fransen, G. M. Gershy-Damet, and R. Deleys. 1993. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS 7:769-780. [DOI] [PubMed] [Google Scholar]
- 29.Martinet, Y., N. Martinet, J. M. Vignaud, and F. Plenat. 1994. Blood monocyte chemotaxis. J. Immunol. Methods. 174:209-214. [DOI] [PubMed] [Google Scholar]
- 30.Naghavi, M. H., M. O. Salminen, A. Sonnerborg, and A. Vahlne. 1999. DNA sequence of the long terminal repeat of human immunodeficiency virus type 1 subtype A through G. AIDS Res. Hum. Retrovir. 15:485-488. [DOI] [PubMed] [Google Scholar]
- 31.Nath, A. 2002. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 186(Suppl. 2):S193-S198. [DOI] [PubMed] [Google Scholar]
- 32.Noonan, D., and A. Albini. 2000. From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 48:229-250. [DOI] [PubMed] [Google Scholar]
- 33.Novitsky, V., U. R. Smith, P. Gilbert, M. F. McLane, P. Chigwedere, C. Williamson, T. Ndung'u, I. Klein, S. Y. Chang, T. Peter, I. Thior, B. T. Foley, S. Gaolekwe, N. Rybak, S. Gaseitsiwe, F. Vannberg, R. Marlink, T. H. Lee, and M. Essex. 2002. Human immunodeficiency virus type 1 subtype C molecular phylogeny: consensus sequence for an AIDS vaccine design? J. Virol. 76:5435-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peeters, M., and P. M. Sharp. 2000. Genetic diversity of HIV-1: the moving target. AIDS 14:S129-S140. [PubMed] [Google Scholar]
- 35.Posada, D., and K. A. Crandall. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817-818. [DOI] [PubMed] [Google Scholar]
- 36.Rice, A. P., and F. Carlotti. 1990. Mutational analysis of the conserved cysteine-rich region of the human immunodeficiency virus type 1 Tat protein. J. Virol. 64:1864-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodenburg, C. M., Y. Li, S. A. Trask, Y. Chen, J. Decker, D. L. Robertson, M. L. Kalish, G. M. Shaw, S. Allen, B. H. Hahn, and F. Gao. 2001. Near full-length clones and reference sequences for subtype C isolates of HIV type 1 from three different continents. AIDS Res. Hum. Retrovir. 17:161-168. [DOI] [PubMed] [Google Scholar]
- 38.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 39.Ruben, S., A. Perkins, R. Purcell, K. Joung, R. Sia, R. Burghoff, W. A. Haseltine, and C. A. Rosen. 1989. Structural and functional characterization of human immunodeficiency virus tat protein. J. Virol. 63:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satishchandra, P., A. Nalini, M. Gourie-Devi, N. Khanna, V. Santosh, V. Ravi, A. Desai, A. Chandramuki, P. N. Jayakumar, and S. K. Shankar. 2000. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96). Indian J. Med. Res. 111:14-23. [PubMed] [Google Scholar]
- 41.Shankarappa, R., R. Chatterjee, G. H. Learn, D. Neogi, M. Ding, P. Roy, A. Ghosh, L. Kingsley, L. Harrison, J. I. Mullins, and P. Gupta. 2001. Human immunodeficiency virus type 1 Env sequences from Calcutta in eastern India: identification of features that distinguish subtype C sequences in India from other subtype C sequences. J. Virol. 75:10479-10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suh, G. H., and A. Shah. 2001. A review of the epidemiological transition in dementia—cross-national comparisons of the indices related to Alzheimer's disease and vascular dementia. Acta Psychiatr. Scand. 104:4-11. [DOI] [PubMed] [Google Scholar]
- 43.Swofford, D. L. 2002. PAUP* 4.0: phylogenetic analysis using parsimony (* and other methods). Sinauer Associates, Inc., Sunderland, Mass.
- 44.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadia, R. S., S. N. Pujari, S. Kothari, M. Udhar, S. Kulkarni, S. Bhagat, and A. Nanivadekar. 2001. Neurological manifestations of HIV disease. J. Assoc. Physicians India 49:343-348. [PubMed] [Google Scholar]
- 46.Weiss, J. M., A. Nath, E. O. Major, and J. W. Berman. 1999. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163:2953-2959. [PubMed] [Google Scholar]
- 47.White, D. A., R. K. Heaton, and A. U. Monsch. 1995. Neuropsychological studies of asymptomatic human immunodeficiency virus-type 1 infected individuals. The HNRC Group. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1:304-315. [DOI] [PubMed] [Google Scholar]