Abstract
Background
The aim of this study was to review a series of consecutive percutaneous cholecystostomies (PC) to analyse the clinical outcomes.
Methods
All patients who underwent a PC between 2000 and 2010 were reviewed retrospectively for indications, complications, and short- and long-term outcomes.
Results
Fifty-three patients underwent a PC with a median age was 74 years (range 14–93). 92.4% (n = 49) of patients were American Society of Anesthesiologists (ASA) III and IV. 82% (43/53) had ultrasound-guided drainage whereas 18% (10/53) had computed tomography (CT)-guided drainage. 71.6% (n = 38) of PC's employed a transhepatic route and 28.4% (n = 15) transabdominal route. 13% (7/53) of patients developed complications including bile leaks (n = 5), haemorrhage (n = 1) and a duodenal fistula (n = 1). All bile leaks were noted with transabdominal access (5 versus 0, P = 0.001). 18/53 of patients underwent a cholecystectomy of 4/18 was done on the index admission. 6/18 cholecystectomies (33%) underwent a laparoscopic cholecystectomy and the remaining required conversion to an open cholecystectomy (67%). 13/53 (22%) patients were readmitted with recurrent cholecystitis during follow-up of which 7 (54%) had a repeated PC. 12/53 patients died on the index admission. The overall 1-year mortality was 37.7% (20/53).
Conclusions
Only a small fraction of patients undergoing a PC proceed to a cholecystectomy with a high risk of conversion to an open procedure. A quarter of patients presented with recurrent cholecystitis during follow-up. The mortality rate is high during the index admission from sepsis and within the 1 year of follow-up from other causes.
Introduction
Acute cholecystitis is a common cause of acute surgical admissions and an early cholecystectomy at the index admission is a widely accepted method of treatment.1 A laparoscopic cholecystectomy for acute cholecystitis in selected patients is associated with a good outcome with minimal morbidity.2 However, the morbidity and mortality rates are significantly higher in high-risk groups to as high a 14–46%.3 A percutaneous cholecystectomy (PC) is a commonly used procedure to treat acute cholecystitis in patients with significant co-morbid disease,4 either as a bridging procedure in patients with severe sepsis5 followed by an interval cholecystectomy or a definitive procedure in patients who are unlikely to be fit for further surgery.6–8 This study aims to define the indications, complications and clinical outcomes of a PC in the treatment of acute cholecystitis at two university hospitals.
Methods
All consecutive patients who underwent a PC at two university hospitals, Ninewells Hospital, Dundee, UK and Auckland City Hospital (ACH), Auckland, New Zealand between January 2000 and October 2010 were reviewed from a prospectively maintained patient database.
The data collected included demographics, indications, American Society of Anesthesiologists (ASA) grading, duration of symptoms, results of laboratory and radiological investigations and the interval between admission and PC. The clinical outcomes included the duration of hospital stay, duration of PC catheter retention, presence of common bile duct stones, index cholecystectomy, morbidity and mortality. In addition, data on readmissions with recurrent cholecystitis requiring a repeat PC and 1-year mortality were obtained.
Definitions
Acute cholecystitis was defined as right upper quadrant pain associated with pyrexia, leucocytosis>11 × 109/ml, and ultrasound/computed tomography (CT) evidence of acute inflammation. The severity grading of acute cholecystitis was made according to the Tokyo Guidelines.9
Technique of percutaneous cholecystostomy
The PC was performed by consultant interventional radiologists under CT or ultrasound guidance. The later was used in conjunction with fluoroscopic screening to more accurately guide placement. A similar technique was used in both centres. The choice of modality varied according to the personal preference of the operator. A transhepatic approach was the preferred option as it reduces the risk of bile leak. However, a transperitoneal approach was employed when the gall bladder was grossly distended and adherent to the abdominal wall, or when an unfavourable anatomy renders transhepatic access very difficult.
Ultrasound or CT was used to guide the site of local anaesthetic and the subsequent cholecystostomy. Access was achieved using the Seldinger technique, in which a fine bore needle is initially directed into the gallbladder. If using CT guidance, the position of the needle, wires and catheter were identified on sequential focused CT scans. Contrast was not usually required. However, for fluoroscopic screening, contrast was instilled via the needle to ensure accurate placement of the needle tip within the gallbladder lumen. Using a series of dilators and wires, the tract was dilated to site a pigtail catheter (Flexima; Boston Scientific, Natick, MA, USA). The size of the catheter depended on the viscosity of the gallbladder contents but an 8-F was typical. The gallbladder contents were aspirated and sent for cultures, after which the catheter was left on free drainage.
The PC catheters were left in situ for a minimum period of 6 weeks prior to removal. Prior to removal of the catheter, all patients underwent a cholangiogram through the cholecystostomy to ensure flow of contrast into the common bile duct (CBD) and also to exclude CBD obstruction from stones. In the presence of CBD stones, an endoscopic retrograde cholangiopancreatography (ERCP) was performed to clear the CBD prior to removal of the catheter.
Statistical analysis
Statistical analysis was performed using SPSS software version 17 (SPSS, Chicago, IL, USA). Continuous data were described using median and range. Fisher's exact tests were used to analyse categorical data. A two-tailed P-value less than 0.05 was considered statistically significant.
Results
A total of 53 patients were identified with a median age of 74 years (range, 14–93). The demographics, ASA grading, laboratory parameters and radiological investigations are summarized in Table 1. Thirty-three patients (63%) had calculus cholecystitis, whereas 20 (37%) had acalculous cholecystitis. Seventy-seven per cent (41/53) of the patients had pericholecystic fluid confirming inflammation. Based on the Tokyo guidelines,9 40 patients were graded as grade 2 (moderate cholecystitis) and 13 patients as grade 3 (severe cholecystitis) with no patients with grade 1 cholecystitis (mild cholecystitis). The imaging modalities and the technique of PC (transhepatic versus transabdominal) are summarized in Table 1.
Table 1.
Patient demographics and pre-operative investigations
| Age – median (range): | 74 years (14–93 years) |
| Gender (male/female): | 1.5:1 |
| ASA grade – number of patients (%): | |
| Grade I | 1 (1.9%)) |
| Grade II | 3 (5.7%) |
| Grade III | 18 (33.9%) |
| Grade IV | 31 (58.5%) |
| Median duration symptoms(range): | 1 (1–35) day(s) |
| Median duration from admission to PC (range): 3 (1–15) day(s) | |
| Laboratory investigations – median (range): | |
| WCC | 14.33 (3.3−27.2) × 109/l |
| CRP | 188.35 (5–489) mg/l |
| Bilirubin | 20.35 (3–75 mg/dl |
| ALP | 180.07 (53–1160) u/l |
| ALT | 52.22 (7–402) u/l |
| Radiological investigations – number of patients (%) | |
| Ultrasonography (US) | 18 (33.9%) |
| CT scan | 15 (28.3%) |
| US+CT scan | 20 (37.7%) |
| PC technique | |
| Transhepatic: | 38 (71.6%) |
| Transabdominal: | 15 (28.4%) |
| Imaging modality for PC | |
| Ultrasound guided PC | 43 (82%) |
| CT guided PC | 10 (18%) |
| Microbiology | |
| Sterile | 19 (35.8%) |
| Escherichia coli | 18 |
| Klebsiella pneumoniae | 7 |
| Mixed growth | 5 |
| Enterococcus | 3 |
| Streptococcus milleri | 1 |
PC, percutaneous cholecystostomy; ASA, American Society of Anesthesiologists; WCC, white cell count; CRP, C-reactive protein; ALP, alkaline phosphatase ALT, alanine aminotransferase.
Clinical outcomes
Twelve patients required intensive care unit (ICU) admission for organ failure and sepsis for optimization after PC. The median ICU stay was 6 days (range 4–11). The median hospital stay was 15.5 days (range 7–120). Seven patients (13%) developed procedure-related complications including bile leaks (n = 5), haemorrhage (n = 1) and a duodenal fistula (n = 1). All bile leaks were noted with transabdominal access (5, transabdominal versus 0, transhepatic, P = 0.001). Four bile leaks were managed with percutaneous radiological drainage of the collection. One patient underwent a laparotomy wash out and a cholecystectomy to control the leak. One patient, who was ASA grade IV, developed a significant haemorrhage from the gallbladder bed after the PC; however, declined any blood product transfusion and died from hypotensive shock. One choledochoduodenal fistula was noted on follow-up cholangiogram which was managed conservatively.
Six patients with deranged liver function tests were found to have CBD stones on magnetic resonance cholangiopancreatography (MRCP) and underwent an ERCP and retrieval of the stones. Amongst 13 patients who were admitted with grade 3 cholecystitis, 12 died on the same admission.
The median time to removal of the PC catheter was 43 days (range: 28–114). The patients who underwent an index cholecystectomy had the drain removed at surgery. There was one instance of catheter dislodgement resulting in bile leak requiring an emergency laparotomy and a cholecystectomy and the patient subsequently died from sepsis and multiorgan failure. One patient had the catheter left for 114 days as he was lost to follow-up until he represented to the hospital with an unrelated medical problem at which point it was removed. There were no instances of the need to leave the PC catheter in situ because of obstruction at the cystic duct. The median overall follow-up was 910 days (range 53 days–9.3 years).
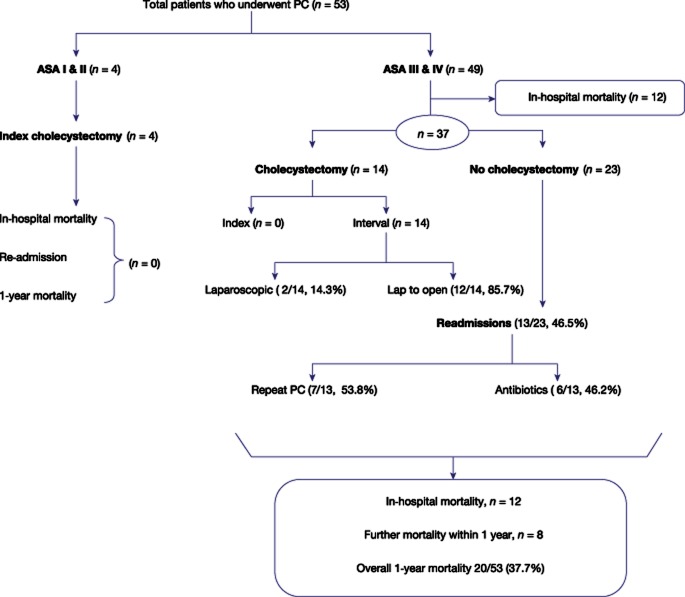
Surgical treatment
Thirty-four per cent (18/53) of patients eventually had a cholecystectomy. The surgical outcomes are summarized in Fig. 1. Amongst the 18 patients who underwent surgery, 4 patients were ASA I and II in whom a PC was planned as a bridging procedure. In the remaining patients, a PC was used as a palliation for control of sepsis; however, 14 patients became suitable for a cholecystectomy after improvement in their overall condition. The overall mortality during follow-up was 45% (n = 24/53). Amongst these, 12/24 (50%) died on the index admission from sepsis (n = 11) and haemorrhage (n = 1); and a further 8/24 (33.3%) died within the first year and the remaining > 1 year (n = 4). The cause of death in the 12 patients during the follow-up were pneumonia (n = 3), a myocardial infarction (n = 2), stroke (n = 2), an intracranial bleed (n = 1), pancreatic cancer (n = 1), a ruptured abdominal aortic aneurysm (n = 1), lung cancer (n = 1) and recurrent sarcoma (n = 1).
Figure 1.
Overall outcomes after a percutaneous cholecystostomy (PC)
Readmission
During follow-up, 13/53 (22%) of patients were readmitted with recurrence of symptoms. The median time to representation was 151 days (63–510). Seven patients with readmission required a repeat PC and the remaining patients were managed with antibiotic therapy with no further episodes during follow-up (Fig. 1). All of these patients had the PC catheter removed at 6 weeks. 6/7 (92%) patients with readmission on PC catheter cholangiogram at 6 weeks were noted to have a single large stone occluding the cystic duct and 1/7 patient had multiple stones with an associated CBD stone requiring ERCP and clearance of the duct. In all these patients, the PC catheter was removed after the drain output was minimal and non-bilious. No further readmissions were noted during follow up. There were no procedure-related complications.
Calculus versus acalculous cholecystitis
A subgroup analysis was performed comparing calculus and acalculous cholecystitis regarding the need for high dependency care and readmissions. 5/20 patients in the acalculous group required HDU/ICU admission compared with 7/33 (P = 0.74). There were 4 (4/20, 20%) readmissions in the acalculous group compared with 8 (8/33, 24%) in the calculus group (P = 1.00).
Discussion
The incidence of gallstone disease increases with the age.10 The management of acute cholecystitis in the elderly and critically ill patients can be challenging as a result of high mortality and morbidity.11 PC can be an emergency lifesaving procedure in these patients to resolve the acute phase with minimal intervention. Once the patient recovers adequately, further management can be either surgery or conservative management depending on the patients' co-morbid disease. In the present study, the indications for PC were two-fold. First, as a bridging procedure in low-risk patients (ASA grade I and II) who were severely septic, thus allowing them to recover before a cholecystectomy could be performed.12 Second, as a definitive procedure in high-risk patients (ASA grade III and IV). In the present study, only a small fraction of patients were suitable for definitive surgery either on the index admission or at a later stage with a high conversion rate to an open procedure. In the present series, PC was used as a bridging procedure in a 14-year-old patient with severe cholecystitis and sepsis (ASA I) and three patients with ASA II who were considered high risk for general anaesthesia owing to associated pneumonia.
The procedure-related morbidity for PC is between 8–44% (Table 2). The majority of complications are related to failure to place the catheter, catheter displacement, bile duct injury and intracholecystic haemorrhage.13 In the literature, both the transabdominal and transhepatic route have been described; however, the transhepatic route appears to be the more favoured route.12,14,15 In the present series, the most common procedural-related morbidity was bile leak, requiring radiological intervention and the placement of an abdominal drain to control the leak and this was more common with the transabdominal route. Based on this, we currently reserve the transabdominal route when the gall bladder is grossly distended and adherent to the abdominal wall, or when unfavourable anatomy renders transhepatic access very difficult.
Table 2.
Mortality and morbidity after a percutaneous cholecystostomy for acute cholecystitis in publications from 1995–2011
| Author (Year) | Patient group | Number | 30-day mortality (%) | Morbidity/complications(%) | Outcome: cholecystectomy – number (%) |
|---|---|---|---|---|---|
| Vingan (1995)16 | Various | 34 | 7 (21%) | 2 (6%) | – |
| Allmendinger (1995)17 | Pregnant | 2 | – | – | – |
| Lo (1995)18 | Various | 48 | 14 (24%)b | 7 (12%) | – |
| Melin (1995)19 | Various | 22 | 9 (41%)a | 4 (18%) | – |
| Avrahami (1995)20 | Various | 10 | (0%) | 1 (10%) | – |
| Sheridan (1995)21 | Burns patients | 5 | 1 (20%) | (0%) | – |
| Hultman (1996)22 | ICU patients | 33 | 17 (52%)a | 2 (6%) | – |
| Famulari (1996)23 | Various | 26 | – | 1 (4%) | – |
| Patterson (1996)24 | Various | 50 | 9 (18%) | 2 (4%) | – |
| Van Overhagen (1996)25 | Various | 33 | 2 (6%) | 6 (18%) | – |
| Hamy (1997)26 | Various | 41 | 5 (12%)a | 1 (2%) | – |
| Boggi (1999)27 | Various | 11 | (0%) | 2 (18%) | – |
| Chang (2000)28 | Veterans | 24 | 6 (25%) | 4 (17%) | – |
| Li (2004)14 | Various | 25 | 5 (20%) | 3 (12%) | 8 (32%) |
| Chok (2010)15 | Various | 23 | 5 (22%)c | 1 (4%) | 8 (35%) |
| Yun (2010)12 | Various | 44 | 6 (14%)c | – | 31 (70%) |
| Gumus (2011)7 | Haemodialysis | 14 | 3 (21%) | – | – |
| Melloul (2011)29 | ICU patients | 23 | 3 (13%) | 2 (8.7%) | – |
| Kim (2011)30 | Various | 63 | 11 (17%)c | 6 (10%) | – |
| present studyd | Various | 53 | ?? (18%)d | 7 | 18 (33%) |
Mortality defined as mortality until hospital discharge.
Mortality defined as mortality within 60 days after tube placement.
Mortality defined as mortality until the end of the follow-up period.
Current study: mortality defined as mortality until hospital discharge.
The majority of the patients undergoing PC are generally unfit for a cholecystectomy at initial presentation. Yun et al. reviewed a series of 44 patients of which 34 patients underwent PC as a bridging procedure and a subsequent cholecystectomy and the rest as palliation for control of sepsis.12 On the contrary, other studies have used PC as the definitive treatment for high-risk patients unsuitable for surgery.7,29 In the present study, 18 patients (33%) underwent a cholecystectomy. Four patients underwent PC as a bridging procedure and underwent a cholecystectomy on the index admission. Although in the remaining 14 patients the PC was employed initially as a definitive treatment, during follow-up they were deemed fit for a cholecystectomy and underwent surgery albeit with a higher conversion rate.
The timing of removal of the PC catheter is a contentious issue. The average time period reported in the literature is 4–6 weeks7,12 although some authors elected to remove the catheters on the index admission after resolution of the sepsis.29 Our policy was to leave the drain for 6 weeks to ensure resolution of inflammation and to prevent bile leak after removal and a patent cystic and bile duct after transcatheter cholangiography. The readmission rate with recurrent cholecystitis in the present study was 22%. This is slightly higher than the readmission rates described in the literature which range from 8% to 10%.14,29 More than half the patients who were readmitted in the present series required a repeat PC insertion. Single large stones occluding the drainage of the cystic duct appeared to the most common cause. Although no further interventions were needed apart from PC intervention, several other interventional modalities were described in the literature such as percutaneous cystic duct stent insertion31 and fluoroscopy guided gallstone removal30 to prevent recurrent attacks.
A third of patients in the current series presented with acalculus cholecystitis. Although acalculus cholecystitis is historically more common in patients in severe comorbid disease,32 there was no significant difference noted in the need for high-dependency care and readmissions between calculus and acalculus cholecystitis in the present series. In addition, although there is suggestion of gallbladder wall ischaemia and necrosis in patients with acalculus cholecystitis necessitating an open cholecystostomy,32 more recent evidence and the data from the present series suggests a PC is safe with good post procedure outcomes and with advancing radiological techniques is increasingly becoming the technique of choice over an open procedure (Table 2). This is further reflected in the paucity of publications related to an open cholecystostomy in the recent past.
In conclusion, the present series has shown that a PC is a viable option for patients with acute cholecystitis when an immediate cholecystectomy is found to be unsafe. The procedure-related morbidity is acceptable and a third of patients subsequently undergo a cholecystectomy albeit with a higher conversion rate. A third of patients are readmitted with recurrent symptoms which are manageable with a repeat PC. The in-hospital mortality and the 1-year mortality were high reflecting the comorbid status of the population.
Conflicts of interest
None declared.
References
- 1.Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of day-case laparoscopic cholecystectomy. Br J Surg. 2008;95:161–168. doi: 10.1002/bjs.6105. [DOI] [PubMed] [Google Scholar]
- 2.Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–150. doi: 10.1002/bjs.6870. [DOI] [PubMed] [Google Scholar]
- 3.Houghton PW, Jenkinson LR, Donaldson LA. Cholecystectomy in the elderly: a prospective study. Br J Surg. 1985;72:220–222. doi: 10.1002/bjs.1800720327. [DOI] [PubMed] [Google Scholar]
- 4.Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330–333. [PubMed] [Google Scholar]
- 5.Paran H, Zissin R, Rosenberg E, Griton I, Kots E, Gutman M. Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg. 2006;4:101–105. doi: 10.1016/j.ijsu.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Leveau P, Andersson E, Carlgren I, Willner J, Andersson R. Percutaneous cholecystostomy: a bridge to surgery or definite management of acute cholecystitis in high-risk patients? Scand J Gastroenterol. 2008;43:593–596. doi: 10.1080/00365520701851673. [DOI] [PubMed] [Google Scholar]
- 7.Gumus B. Percutaneous cholecystostomy as a first-line therapy in chronic hemodialysis patients with acute cholecystitis with midterm follow-up. Cardiovasc Intervent Radiol. 2011;34:362–368. doi: 10.1007/s00270-010-0025-6. [DOI] [PubMed] [Google Scholar]
- 8.Spira RM, Nissan A, Zamir O, Cohen T, Fields SI, Freund HR. Percutaneous transhepatic cholecystostomy and delayed laparoscopic cholecystectomy in critically ill patients with acute calculus cholecystitis. Am J Surg. 2002;183:62–66. doi: 10.1016/s0002-9610(01)00849-2. [DOI] [PubMed] [Google Scholar]
- 9.Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majeski J. Laparoscopic cholecystectomy in geriatric patients. Am J Surg. 2004;187:747–750. doi: 10.1016/j.amjsurg.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Edlund G, Ljungdahl M. Acute cholecystitis in the elderly. Am J Surg. 1990;159:414–416. doi: 10.1016/s0002-9610(05)81285-1. discussion 6. [DOI] [PubMed] [Google Scholar]
- 12.Yun SS, Hwang DW, Kim SW, Park SH, Park SJ, Lee DS, et al. Better treatment strategies for patients with acute cholecystitis and American Society of Anesthesiologists classification 3 or greater. Yonsei Med J. 2010;51:540–545. doi: 10.3349/ymj.2010.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzellino G, de Manzoni G, Ricci F, Castaldini G, Guglielmi A, Cordiano C. Emergency cholecystostomy and subsequent cholecystectomy for acute gallstone cholecystitis in the elderly. Br J Surg. 1999;86:1521–1525. doi: 10.1046/j.1365-2168.1999.01284.x. [DOI] [PubMed] [Google Scholar]
- 14.Li JC, Lee DW, Lai CW, Li AC, Chu DW, Chan AC. Percutaneous cholecystostomy for the treatment of acute cholecystitis in the critically ill and elderly. Hong Kong Med J. 2004;10:389–393. [PubMed] [Google Scholar]
- 15.Chok KS, Chu FS, Cheung TT, Lam VW, Yuen WK, Ng KK, et al. Results of percutaneous transhepatic cholecystostomy for high surgical risk patients with acute cholecystitis. ANZ J Surg. 2010;80:280–283. doi: 10.1111/j.1445-2197.2009.05105.x. [DOI] [PubMed] [Google Scholar]
- 16.Vingan H. Don't forget cholecystostomy in the era of ERCP and laparoscopic cholecystectomy. Am J Gastroenterol. 1995;90:669–670. [PubMed] [Google Scholar]
- 17.Allmendinger N, Hallisey MJ, Ohki SK, John Straub J. Percutaneous cholecystostomy treatment of acute cholecystitis in pregnancy. Obstet Gynecol. 1995;86:653–654. doi: 10.1016/0029-7844(95)00087-8. [DOI] [PubMed] [Google Scholar]
- 18.Lo LD, Vogelzang RL, Braun MA, Nemcek AA., Jr Percutaneous cholecystostomy for the diagnosis and treatment of acute calculous and acalculous cholecystitis. J Vasc Interv Radiol. 1995;6:629–634. doi: 10.1016/s1051-0443(95)71150-2. [DOI] [PubMed] [Google Scholar]
- 19.Melin MM, Sarr MG, Bender CE, van Heerden JA. Percutaneous cholecystostomy: a valuable technique in high-risk patients with presumed acute cholecystitis. Br J Surg. 1995;82:1274–1277. doi: 10.1002/bjs.1800820939. [DOI] [PubMed] [Google Scholar]
- 20.Avrahami R, Badani E, Watemberg S, Nudelman I, Deutsch AA, Rabin E, et al. The role of percutaneous transhepatic cholecystostomy in the management of acute cholecystitis in high-risk patients. Int Surg. 1995;80:111–114. [PubMed] [Google Scholar]
- 21.Sheridan RL, Ryan CM, Lee MJ, Mueller PR, Tompkins RG. Percutaneous cholecystostomy in the critically ill burn patient. J Trauma. 1995;38:248–251. doi: 10.1097/00005373-199502000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Hultman CS, Herbst CA, McCall JM, Mauro MA. The efficacy of percutaneous cholecystostomy in critically ill patients. Am Surg. 1996;62:263–269. [PubMed] [Google Scholar]
- 23.Famulari C, Macri A, Galipo S, Terranova M, Freni O, Cuzzocrea D. The role of ultrasonographic percutaneous cholecystostomy in treatment of acute cholecystitis. Hepatogastroenterology. 1996;43:538–541. [PubMed] [Google Scholar]
- 24.Patterson EJ, McLoughlin RF, Mathieson JR, Cooperberg PL, MacFarlane JK. An alternative approach to acute cholecystitis. Percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Surg Endosc. 1996;10:1185–1188. doi: 10.1007/s004649900275. [DOI] [PubMed] [Google Scholar]
- 25.van Overhagen H, Meyers H, Tilanus HW, Jeekel J, Laméris JS. Percutaneous cholecystectomy for patients with acute cholecystitis and an increased surgical risk. Cardiovasc Intervent Radiol. 1996;19:72–76. doi: 10.1007/BF02563896. [DOI] [PubMed] [Google Scholar]
- 26.Hamy A, Visset J, Likholatnikov D, Lerat F, Gibaud H, Savigny B, et al. Percutaneous cholecystostomy for acute cholecystitis in critically ill patients. Surgery. 1997;121:398–401. doi: 10.1016/s0039-6060(97)90309-3. [DOI] [PubMed] [Google Scholar]
- 27.Boggi U, Di Candio G, Campatelli A, Oleggini M, Pietrabissa A, Filipponi F, et al. Percutaneous cholecystostomy for acute cholecystitis in critically ill patients. Hepatogastroenterology. 1999;46:121–125. [PubMed] [Google Scholar]
- 28.Chang L, Moonka R, Stelzner M. Percutaneous cholecystostomy for acute cholecystitis in veteran patients. Am J Surg. 2000;180:198–202. doi: 10.1016/s0002-9610(00)00476-1. [DOI] [PubMed] [Google Scholar]
- 29.Melloul E, Denys A, Demartines N, Calmes JM, Schäfer M. Percutaneous drainage versus emergency cholecystectomy for the treatment of acute cholecystitis in critically ill patients: does it matter? World J Surg. 2011;35:826–833. doi: 10.1007/s00268-011-0985-y. [DOI] [PubMed] [Google Scholar]
- 30.Kim YH, Kim YJ, Shin TB. Fluoroscopy-guided percutaneous gallstone removal using a 12-Fr sheath in high-risk surgical patients with acute cholecystitis. Korean J Radiol. 2011;12:210–215. doi: 10.3348/kjr.2011.12.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comin JM, Cade RJ, Little AF. Percutaneous cystic duct stent placement in the treatment of acute cholecystitis. J Med Imaging Radiat Oncol. 2010;54:457–461. doi: 10.1111/j.1754-9485.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- 32.Barie PS, Eachempati SR. Acute acalculous cholecystitis. Curr Gastroenterol Rep. 2003;5:302–309. doi: 10.1007/s11894-003-0067-x. [DOI] [PubMed] [Google Scholar]