Abstract
Background and aims
In acute pancreatitis (AP), patients with persistent organ failure [POF, duration of organ failure (OF) ≥48 h] and transient organ failure (TOF, duration of OF <48 h) have different outcomes. We have compared the clinical course and outcome of patients with severe AP (SAP) with TOF and POF in the first week of hospitalization as well as the impact of change in the OF score in the first week on patient outcome.
Methods
Consecutive patients with SAP were evaluated for OF and its dynamics during the first week of hospitalization. The modified multiple organ failure score (MOFS) was used to identify OF, grade its severity and monitor its progression. The clinical course and outcome of patients were studied.
Results
Of 114 patients, mean age 39.2 ± 13.7 years, 37 (32.5%) patients had no OF, 34 (29.8%) had TOF and 43(37.7%) had POF. Patients with POF had the higher infected necrosis, increased requirement for percutaneous drain placement, surgery and higher mortality as compared with those with TOF. The odds ratio for mortality with persistent and deteriorating OF was 26.2 [confidence interval (CI) 5.1–134.9] compared with only persistent OF.
Conclusion
The dynamics of OF in the first week of SAP predicts the clinical course and outcome. Persistent and deteriorating OF indicates a poor outcome.
Introduction
Acute pancreatitis (AP) has a variable clinical course: mild AP has a very low mortality (less than 1%),1,2 whereas mortality for severe AP (SAP) can be as high as 10% to 30% depending on the presence of organ failure (OF) or necrosis.3 AP evolves through two distinct stages: the first stage is related to the inflammatory cytokine storm and usually lasts for a week. During this phase, the severity of AP is related to systemic inflammatory response (SIRS) elicited by acinar cell injury. About half the deaths occur within the first week or two, usually as a result of multi-organ failure.4–6 The mortality peak in the second phase is related to OF secondary to sterile or infected necrosis, or complications from surgical intervention. After the second week of illness, patients succumb to pancreatic infection associated with multi-organ failure.7–10 Predicting the severity of pancreatitis early in the course of disease is critical to optimize treatment.11 The Atlanta criteria are a widely used clinically-based classification system for severity of AP which incorporates OF and local complications.12 However, it does not differentiate between (i) single and multiple OF, and (ii) grade (severity) and duration of OF. Multi-organ failure that is persistent should render a patient more susceptible to death.12
The modified multiple organ failure score (MOFS, Modified Bernard score) is a composite scoring system widely used to assess multiple organ failure.13 Buter et al. applied MOFS (with the modification, by excluding hepatic dysfunction; as rise in bilirubin may be confounding in gall stone pancreatitis) for the grading and monitoring of organ dysfunction in AP.14 This scoring system is helpful as a score of ≥2 can be approximated to a OF definition using Atlanta criteria. The parameters are specific, easily obtained and are generally independent of therapy.14 Johnson et al. showed that patients who have OF which persists for three or more days (persistent organ failure, POF) have a greater risk of a fatal outcome as compared with patients with OF that resolves within 48 h (transient organ failure, TOF).15 Buter et al. described a poor outcome of patients with deteriorating organ dysfunction in SAP.14 However, there are no data on OF which takes into account the dynamics of OF (severity, duration and progression) in the early phase of SAP, particularly in the first week of illness. We hypothesized that those patients who have deterioration of MOFS either as a result of an increase in the number of organs failing or progressive worsening of OF in the first week of illness would have a poorer outcome. The aim of this study was to compare the clinical course and outcome of patients with TOF and POF in the first week of admission among those with SAP and to describe the dynamic nature of OF.
Methods
In this prospective observational study conducted in the departments of Gastroenterology and General Surgery in Postgraduate Institute of Medical Education and Research, a tertiary care referral centre at Chandigarh, India, between January 2009 and March 2012, 114 consecutive patients with SAP were included as per the inclusion and exclusion criteria. Informed consent was taken from all the patients. The study was approved by the institute's ethics board and the Indian Council of Medical Research (ICMR) guidelines for conducting a research were followed. The diagnosis of AP was made by (any two of the three); (a) acute abdominal pain, (b) elevated serum amylase (more than thrice upper limit of normal range) and (c) typical appearance on ultrasound (USG) and/or contrast-enhanced computed tomography (CECT). SAP was defined by the presence of any of the following; (i) organ failure,14 (ii) local complications (necrosis, abscess and pseudocyst), (iii) computed tomography severity index (CTSI) >7, and (iv) APACHE II score >8. Onset of OF in the first week of hospitalization was defined as early onset OF and onset after the first week was considered as late onset OF. The inclusion criteria were: (i) the presence of SAP, (ii) patients more than 12 years of age, and (iii) patients presenting within 48 hours of onset of abdominal pain. The exclusion criteria were: (i) patients with known severe pre-existing co-morbid illness (chronic kidney disease, cirrhosis, chronic obstructive airway disease, bronchial asthma and congestive heart failure) and (ii) patients known to have chronic pancreatitis.
All patients underwent a detailed clinical examination and relevant investigations. CECT abdomen was carried out to grade the severity of pancreatitis by calculating the Balthazar score and CTSI after 72 h of onset of pain. The APACHE II score (at 24 h) and C-reactive protein (CRP) levels (at 48 h) were calculated. Patients were monitored for the presence and severity of OF (as per modified organ failure score, MOFS, Table 1) every day during the first week. Thus, we evaluated renal, pulmonary, cardiovascular, central nervous and coagulation system failure. Patients with MOFS of ≥ two in any organ systems were recorded as having OF on that day. Patients with OF lasting for < 48 hours were considered to have TOF and those with duration of OF lasting for ≥ 48 hours were considered to have POF. The MOFS difference (MOFS on Day 7 minus MOFS on day 1) was calculated as a measure of dynamic change in severity of OF.
Table 1.
Modified multiple organ failure score14
| Score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Cardiovascular system (SBP, mm of Mercury) | >90 | <90 | <90, fluid nonresponsive | <90, pH<7.3>7.2 | <90, pH<7.2 |
| Respiratory (FIO2/PO2) | >400 | 301–400 | 201–300 | 101–200 | <101 |
| Glasgow Coma Scale | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Platelet count (109/l) | >120 | 81–120 | 51–80 | 21–50 | <21 |
| Creatinine (mg/dl) | <1.5 | 1.5–1.9 | 2.0–3.4 | 3.5–4.9 | >4.9 |
FiO2, fraction of inspired oxygen; PO2, partial pressure of oxygen; SBP, systolic blood pressure.
All patients were managed according to standard recommendations16 which included intensive resuscitation, fluid and electrolyte monitoring, nutritional support (nasojejunal feeding or total parenteral nutrition) and supportive care. Infected pancreatic necrosis (IN) was diagnosed if image-guided fine needle aspiration (considered when persistent fever and/or leucocytosis was (were) present and after excluding other causes) or the operative specimen showed isolates on bacterial culture and/or Gram staining. Patients with symptomatic fluid collection underwent radiologically-guided percutaneous catheter drainage (PCD) using a 10-Fr catheter (with upgrading of size when there was thick residual collection) as per the technique described earlier.17 Surgery in the form of necrosectomy and closed lesser sac drainage was offered on evidence of IN not responding to conservative management, worsening of OF in spite of medical management, an uncontrolled haemorrhage or local complications. The clinical course of these patients was followed until the completion of their hospital stay. Parameters evaluated for the hospital course and outcome included the occurrence of IN, blood stream infection, the need for PCD insertion, need for surgery, total hospital stay and mortality. The hospital course and mortality was compared between groups with and without OF.
Statistical analysis
The data was analysed using SPSS software (version 17; SPSS Inc., Chicago, IL, USA). For normally distributed data, continuous variables were compared using the Student's t-test. For more than two groups one-way anova was used. Quantitative data were described as a mean and standard deviation with 95% confidence intervals. Categorical data were shown as proportions. A comparison of the TOF and POF groups was carried out for various categorical variables using the chi-square test association to find out any statistical association between these. Spearman's correlation coefficients were also calculated between different quantitative variables. Univariate and multivariate analysis were used for the identification of predictors of mortality.
Results
Demographic data
We enrolled 114 patients with SAP in our study (Table 2) with a mean age of 39.2 ± 13.7 years, (male to female ratio; 2.1:1). Alcohol (n = 57, 50.0%) was the most common cause followed by gall stones (n = 35, 30.7%) and others, 19.3% [8 cases of idiopathic pancreatitis, 7 cases of traumatic pancreatitis, 6 cases of post-endoscopic retrograde cholangiopancreatography pancreatitis and 1 case of hyperparathyroidism (and hypercalcemia) associated pancreatitis]. The organ systems assessed as per MOFS and the nature of OF is described in Table 3. As per the MOFS for defining and grading severity of organ failure, 37 (32.5%) patients had no OF, whereas 34 (29.1%) had TOF and 43 (37.7%) had POF (Table 4). Twenty-one patients had 2 organ failures and 16 patients had >2 organ failures.
Table 2.
Baseline characteristics of all patients on admission
| Age (years)a | 39.2 ± 13.7 |
| Gender (M/F) | 78/36 (68.4/31.6) |
| Aetiology (alcohol/gallstone/others) N (%) | 57/35/22 (50/30.7/19.3) |
| APACHE II (24 h)a | 8.3 ± 5.9 |
| CRP (mg/l) (48 h)a | 142.2 ± 61.5 |
| BMI (kg/m2)a | 23.2 ± 1.9 |
| MOFS differencea | 0.2 ± 1.3 |
| CTSIa | 7.6 ± 2.8 |
| No OF/transient OF/persistent OF, N (%) | 37/34/43 (32.5/29.8/37.7) |
BMI, Body Mass Index; CRP, C-reactive protein; CTSI, Computerized Tomography Severity Index; DBA, duration between onset of pain and admission; MOFS, Modified Organ Failure Score; N, number of patients; OF, organ failure.
Mean ±standard deviation.
Table 3.
Details of organ failure in the 1st week of hospitalization
| No organ failure N (%) | Transient organ failure N (%) | Persistent organ failure N (%) | |
|---|---|---|---|
| Renal | 45 (39.5) | 24 (21) | 45 (39.5) |
| Respiratory | 61 (53.5) | 15 (13.2) | 38 (33.3) |
| Cardiovascular | 88 (77.2) | 4 (3.5) | 22 (19.3) |
| Coagulation | 92 (80.7) | 1 (0.9) | 21 (18.4) |
| Central nervous system | 107 (93.9) | 0 | 7 (6.1) |
Table 4.
Comparison of characteristics of patients with different types of organ failure
| No organ failure | Transient organ failure | Persistent organ failure | P-value | |
|---|---|---|---|---|
| N (%) | 37 (32.5) | 34 (29.8) | 43 (37.7) | |
| Age in yearsa | 36.7 ± 13.9 | 38.9 ± 12.8 | 41.5 ± 14.2 | 0.30 |
| Gender (male/female) | 28/9 | 25/9 | 25/18 | 0.18 |
| Aetiology | 0.30 | |||
| Gall stone | 8 | 11 | 16 | |
| Alcohol | 18 | 8 | 11 | |
| Others | 11 | 5 | 6 | |
| APACHE II (at 24 h)a | 3.70 ± 1.4 | 6.79 ± 3.2 | 13.65 ± 5.9 | <0.001 |
| CRP (at 48 h) (mg/l)a | 143.6 ± 68.3 | 132.7 ± 51.4 | 148.6 ± 61.5 | 0.055 |
| BMI (kg/m2)a | 22.5 ± 2.1 | 22.8 ± 1.3 | 24.3 ± 1.8 | <0.001 |
| MOFS differencea | 0.03 ± 0.1 | 0.41 ± 0.6 | 0.93 ± 1.8 | <0.001 |
| Necrosis (Balthazar score)a | 2.3 ± 6.5 | 2.8 ± 7.0 | 3.8 ± 4.7 | <0.001 |
| CTSIa | 3.2 ± 1.5 | 4.6 ± 1.8 | 8.4 ± 1.7 | <0.001 |
| Blood stream infection (N) (%) | 6 (16.2) | 2 (5.9) | 23 (53.5) | <0.001 |
| Infected necrosis (N) (%) | 3 (8.1) | 1 (2.9) | 11 (25.6) | 0.008 |
| PCD insertion (N) (%) | 6 (16.2) | 7 (20.6) | 20 (46.5) | 0.005 |
| Surgery (N) (%) | 4 (10.8) | 2 (5.9) | 13 (30.2) | <0.001 |
| Hospitals stay in daysa | 10.7 ± 10.6 | 13.8 ± 10.7 | 21.5 ± 16.6 | 0.003 |
| ICU stay in daysa | 4.3 ± 3.2 | 3.2 ± 1.2 | 12.8 ± 5.7 | 0.004 |
| Ventilator requirement, (N) (%) | 4 (10.8) | 0 | 17 (39.5) | <0.001 |
| Mortality (N) (%) | 3 (8.1) | 0 | 18 (41.9) | <0.001 |
BMI, Body Mass Index; CRP, C-reactive protein; CTSI, Computerized Tomography Severity Index; DBA, Duration between onset of pain and admission; MOFS, Modified Organ Failure Score; N, number of patients; OF, organ failure; PCD, percutaneous catheter drainage.
Mean ±standard deviation.
Markers of disease severity
The markers of disease severity were different between the different organ failure groups. The mean APACHE II scores were significantly different between the groups (anova P < 0.001) and between each other on post-hoc analysis. The serum CRP levels were comparable between the groups (anova P = 0.055) but were significantly different between the TOF and POF groups (post-hoc, P = 0.048). There was a significant difference in body mass index (BMI) between the groups (anova P < 0.001). However, post-hoc analysis showed no difference between patients with no OF and those with TOF (P = 0.814). The difference between patients with no OF compared with POF (P < 0.001) and those with TOF compared with POF (P = 0.001) were significant. The radiological markers of severity, Balthazar score (P < 0.001) and CTSI (P < 0.001) were significantly different between the groups. Similarly, the MOFS difference was significantly different between the groups; no OF versus TOF (P = 0.03), no OF versus POF (P = 0.001) and TOF versus POF (P = 0.001).
Infections during the course of illness
The occurrence of blood stream infections were significantly higher in patients with POF when compared with those with TOF (χ2 P < 0.001) and similarly an occurrence of IN also followed the same trend (χ2 P = 0.001). The predominant isolate in blood was Escherichia coli and from infected necrosis it was Enterococcus species. The antibiotic sensitivity pattern showed that most bacteria were sensitive to quinolones and third generation cephalosporins, ceftriaxone and ceftazidime.
Requirement for interventions
Patients with POF had a higher requirement for PCD insertion (χ2 P = 0.01) and surgery (χ2 P = 0.007) as compared with patients with TOF. Specific indications for surgery (n = 19) were IN and multiple organ dysfunction syndrome in 12, failure of conservative management in 5, pseudoaneurysmal bleed in 1 and emphysematous pancreatitis in 1 patient.
Outcome
ICU admission was required for 2 patients in the TOF group and 20 patients in the POF group (χ2 P = 0.01). The mean duration of ICU stay for patients in the POF group was 12.8 ± 5.7 days and 3.2 ± 1.2 days in the TOF group (χ2 P = 0.004). The total duration of hospital stay was significantly more in patients with more severe OF (anova P = 0.003). Overall mortality was 18.4%. Mortality was 40% for patients with POF and nil for the TOF group (χ2 P < 0.001). The specific cause of death was MODS in 17 patients, post-operative bleed in 1, emphysematous pancreatitis in 1 and unrelated in 2 (intracranial haemorrhage and acute myocardial infarction).
Predictors of mortality
Univariate analysis showed APACHE II scores, serum CRP levels, BMI, CTSI, deterioration of organ failure (MOFS difference), OF and IN as predictors of mortality. On multivariate analysis, the MOFS difference [odds ratio (OR) = 7.5; confidence interval (CI) = 2.3 to 27.3, P = 0.001] APACHE II scores (OR = 12.6, CI = 3.7 to 34.4, P = 0.001) and infected necrosis (OR = 9.4, CI = 6.3 to 13.9, P = 0.001) were independent predictors of mortality. There was a significant difference between MOFS of patients who died (mean = 1.93 ± 1.81) and those who survived (mean = −0.28 ± 0.67), P < 0.001.
Stratified risk analysis of patients with persistent OF showed that those with persistent and deteriorating OF (83.3% of total deaths) had significantly higher mortality as compared with those with persistent OF alone (16.7% of total deaths). 3/24 (12.5%) patients with POF and 15/19 (78.9%) patients with persistent and deteriorating OF died, P < 0.001 (Table 5, Figure 1). The risk estimate for mortality was 26.2 times (CI 5.1–134.9) when persistent OF showed a deteriorating trend in the first week. Thus, a double hit in the course of SAP with persistent and deteriorating OF predicts an extremely poor outcome.
Table 5.
Mortality in different subgroups of patients according to type of organ failure
| Type of organ failure | Morality |
|---|---|
| Early onset transient organ failure | 0% |
| Early onset persistent organ failure | 41.9% |
| Only persistent organ failure | 12.5% |
| Persistent and deteriorating organ failure | 78.9% |
| Late onset organ failure | 33% |
Figure 1.
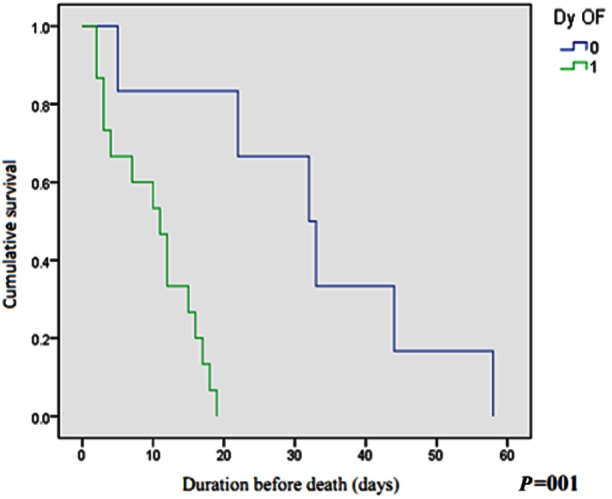
The hazard of mortality in the two groups based on progression of organ failure (Kaplan–Meier curve) Time of event (days) No change or improving MOFS (0) 5, 22, 32, 33, 44, 58 Worsening MOFS (1) 2, 2, 3, 3, 4, 7, 10, 11, 12, 15, 16, 17, 18, 19 Dy OF, dynamic change in organ failure.
Late onset organ failure
Nine patients in the group with no organ failure developed late onset OF, all of whom had sepsis and three had infected necrosis. Of the nine patients who developed late onset OF, six required PCD insertion, four required surgery and three (33.3%) succumbed to illness.
Discussion
In this study, we found that the patients with POF had higher APACHE II scores, higher CRP levels, higher radiological indices of severity, more frequent blood stream infection, IN, a requirement of PCD, surgery and mortality when compared with those with TOF. Deteriorating organ failure in the first week of hospital stay conferred a much higher risk of mortality, over and above the risk associated with POF.
There is increasing evidence that OF is not an all or none process as defined in the Atlanta criteria,12 and the time of onset, and duration and progression of OF influence the outcome. Johnson et al. studied 290 patients with predicted SAP of which early OF was present in 174 (60%) patients and OF was persistent in 103 patients.15 Mortality was 35% in the persistent and 1.4% in the transient OF group. No patients without OF died. Lytras et al.18 studied 234 patients with AP of which 64 patients had predicted SAP. They identified 33 patients with early onset OF (OF < 5 days from onset of symptoms). The early onset OF group was subdivided into two groups; TOF group (24 patients,73%) and POF group (9 patients,33%). There was no mortality in the group without OF. The mortality was 8.3% in the group with TOF and 77.7% in the group with POF, P < 0.001. Buter et al., in a study of 121 patients with predicted SAP,14 found that the mortality was (2/68) 3% in patients without organ dysfunction, 0 in patients with resolving organ dysfunction and (11/20) 55% in patients with deteriorating organ dysfunction, P = 0.001. They found deteriorating organ function as an independent predictor of mortality in concordance with our findings. Buter et al. did not analyse the duration of OF and its impact on outcome. These studies had already established the importance of POF in predicting a poor outcome. However, except for the study by Buter et al. other studies have not taken into account the dynamicity of OF (overall progression of OF in the first week of hospitalization for AP). There are no studies so far that have examined the impact of both POF and deterioration of OF on mortality in the same cohort of patients.
Sharma et al. described three subgroups of fulminant, subfulminant and late onset SAP, depending upon the time of onset of OF. Their study showed that the early onset of OF is associated with higher mortality.19 The mortality in patients with late onset OF was 33%, similar to the present observation (30%).
Dynamics of early organ failure
In this study, we looked objectively into the duration of OF, its severity and the plotted trajectory of OF over the first week of hospitalization. We calculated the MOFS score on each of the first 7 days of hospitalization and correlated the outcome with change in the MOFS score (MOFS in day 7 minus MOFS in day 1). A positive MOFS difference thus indicated deterioration in overall severity of OF. This could be because of multiple organ systems failing and/or progressive worsening of severity of OF. We found that there was a significant difference between MOFS difference between patients who succumbed to illness and those who survived. Patients who had deteriorating MOFS had significantly higher mortality as compared with those who had no change or improvement of MOFS. Subgroup analysis of mortality in patients with persistent OF showed that patients with persistent and deteriorating OF as compared with persistent OF alone had significantly higher mortality. In total, 12.5% patients with persistent OF alone and 78.9% patients with persistent and deteriorating OF died. The risk estimate for mortality is 26.2 times (CI 5.1–134.9) when persistent OF shows a deteriorating trend in the first week. Thus a double hit in the early course of SAP with persistent and deteriorating OF results in extremely severe acute pancreatitis with a very poor prognosis; confirming our hypothesis.
The strength of this study lies in the number of patients studied with SAP and uniformity in the study group as only patients presenting within 48 h of onset of symptoms were considered. As compared with previous studies on the duration of OF and deterioration of OF, we looked into the course of disease with respect to duration of OF, severity and progression. However, the limitation of this study is that we did not calculate MOFS beyond first week. Continued monitoring of MOFS beyond the first week could have helped in identifying the onset and course of late onset OF. Further multicentre studies involving larger numbers of patients are required to validate our findings. At present the information helps only in prognostication; however, a multifactorial scoring system incorporating the time of onset, duration and dynamic changes in OF in the early phase of acute pancreatitis can be developed. Future studies should evaluate the therapeutic advantage of prioritizing patients with persistent and deteriorating OF for intensive care.
In conclusion, our study clearly shows that OF in the first week of SAP is a dynamic process. Monitoring of the dynamics of disease in the first week of SAP with modified organ failure score (MOFS) helps in identifying patients who are likely to have a complicated course and we recommend monitoring MOFS in patients with SAP. The patients with persistent OF have a poor overall outcome and the mortality is highest in the subgroup of patients with persistent OF that is deteriorating in the first week of disease. This sub group of patients requires more aggressive management.
Author contributions
Rakesh Kochhar: concept, design, manuscript writing and final approval of manuscript; Ragesh Babu Thandassery: design, data collection, data analysis and manuscript writing; Thakur Deen Yadav: concept and surgical intervention; Usha Dutta: data analysis; Sreekanth Appasani: data collection, data analysis; Kartar Singh: design and final approval of manuscript.
Conflicts of interest
None declared.
References
- 1.Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, et al. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448–1453. doi: 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Triester SL, Kowdley KV. Prognostic factors in acute pancreatitis. J Clin Gastroenterol. 2002;34:167–176. doi: 10.1097/00004836-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Dervenis C, Johnson CD, Bassi C, Bradley E, Imrie CW, McMahon MJ, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int J Pancreatol. 1999;25:195–210. doi: 10.1007/BF02925968. [DOI] [PubMed] [Google Scholar]
- 4.Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis? Int J Pancreatol. 2000;28:91–95. doi: 10.1385/IJGC:28:2:091. [DOI] [PubMed] [Google Scholar]
- 5.Renner IG, Savage WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;40:1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- 6.McKay CJ, Buter A. Natural history of organ failure in acute pancreatitis. Pancreatology. 2003;3:111–114. doi: 10.1159/000070078. [DOI] [PubMed] [Google Scholar]
- 7.United Kingdom guidelines for the management of acute pancreatitis. British Society of Gastroenterology. Gut. 1998;42(Suppl. 2):S1–13. doi: 10.1136/gut.42.2008.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 9.Buchler MW, Gloor B, Muller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollen TL, van Santvoort HC, Besselink MG, van Leeuwen MS, Horvath KD, Freeny PC. The Atlanta Classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 11.Rau BM. Predicting the severity of pancreatitis early in the course of disease is critical for optimizing treatment. Curr Gastroenterol Rep. 2007;9:107–115. doi: 10.1007/s11894-007-0004-5. [DOI] [PubMed] [Google Scholar]
- 12.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 13.Bernard GR, Doig G, Hudson LD. Quantification of organ failure for clinical trials and clinical practice. Am J Respir Crit Care Med. 1995;151:A323. [Google Scholar]
- 14.Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302. doi: 10.1046/j.0007-1323.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsmark CE, Baillie J AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022–2044. doi: 10.1053/j.gastro.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 17.Wig JD, Kishore GSB, Kochhar R, Yadav TD, Kudari AK, Doley RP. Correlates of organ failure in severe acute pancreatitis. J Pancreas (Online) 2009;10:271–275. [PubMed] [Google Scholar]
- 18.Lytras D, Manes K, Triantopoulou C, Paraskeva C, Delis S, Avgerinos C. Persistent early organ failure: defining the high-risk group of patients with severe acute pancreatitis? Pancreas. 2008;36:249–254. doi: 10.1097/MPA.0b013e31815acb2c. [DOI] [PubMed] [Google Scholar]
- 19.Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am J Gastroenterol. 2007;102:1–8. doi: 10.1111/j.1572-0241.2007.01446.x. [DOI] [PubMed] [Google Scholar]


