Abstract
Cesarean scar pregnancies (CSPs) are a relatively rare form of ectopic pregnancy in which the embryo is implanted within the fibrous scar of a previous cesarean section. A greater number of cases of CSPs are currently being reported as the rates of cesarean section are increasing globally and as detection of scar pregnancy has improved with use of transvaginal ultrasound (TVUS) with color Doppler imaging. Delayed diagnosis and management of this potentially life-threatening condition may result in complications, predominantly uterine rupture and hemorrhage with significant potential maternal morbidity. Diagnosis of a cesarean scar pregnancy (CSP) requires a high index of clinical suspicion, as up to 40% of patients may be asymptomatic. TVUS has a reported sensitivity of 84.6% and has become the imaging examination of choice for diagnosis of a CSP. Magnetic resonance imaging (MRI) has been used in a small number of patients as an adjunct to TVUS. In the present report, MRI is highlighted as a problem-solving tool capable of more precisely identifying the relationship of a CSP to adjacent structures, thereby providing additional information critical to directing appropriate patient management and therapy.
Keywords: Cesarean scar pregnancy, magnetic resonance imaging, scar ectopic pregnancy, transvaginal ultrasound, uterine artery embolization
INTRODUCTION

Cesarean scar pregnancies (CSPs) are a relatively rare form of ectopic pregnancy. The first case was reported in the English medical literature by Larsen and Solomon in 1978.[1] Implantation of a pregnancy within the fibrous scar of a previous cesarean section is being reported more frequently, and the incidence is now higher than that of cervical ectopic pregnancies.[2] There has been a substantial increase in published cases of CSPs in the medical literature since its first reporting. While this may reflect a “true” increase in incidence given the rise in cesarean section rates over the years, it may also be attributed to improved detection of this condition, with routine use of transvaginal ultrasound (TVUS).[3] Early detection of a CSP is critical, as delayed diagnosis and management of this potentially life-threatening condition may result in complications, such as uterine rupture and hemorrhage with serious maternal morbidity and potential hysterectomy.
Diagnosis of such a condition should be made based on a high index of suspicion from clinical and imaging investigations combined with the patient's history and clinical manifestations. Improvements in ultrasound and magnetic resonance (MR) technology may enable earlier detection of a CSP, thus facilitating prompt intervention to avoid potential complications. To our knowledge, there is no standardized approach to treat this condition at present; however, various treatment options are available and should be tailored to meet the needs of each individual case. Further characterization of a CSP using magnetic resonance imaging (MRI) after initial ultrasonography may provide additional information useful for directing patient management and therapy (e.g. conservative versus surgical management).
We describe a case of an ectopic pregnancy implanted in the fibrous scar of a previous cesarean section, which led to vaginal bleeding. The patient was initially managed conservatively with systemic intramuscular methotrexate and bilateral uterine artery embolization, but ultimately required hysterectomy.
CASE REPORT
A 36-year-old woman (gravida 5, para 2) at approximately 8 weeks and 6 days gestation presented to her obstetrician complaining of heavy vaginal bleeding that had started 3 days prior. At the time of presentation, the vaginal bleeding had decreased to occasional spotting. The patient did not have abdominal pain or other constitutional symptoms. She also had no history of fibroids, endometriosis, or abnormal pap smears. She did report a medical history significant for beta thalassemia minor and a past surgical history of two prior cesarean sections for macrosomia. Serum beta-human chorionic gonadotropin (beta-hCG) level at the time of presentation measured 199,760 mIU/mL. A subsequent pelvic ultrasound showed a retroflexed gravid uterus with a single viable pregnancy in the lower uterine segment abutting the urinary bladder [Figure 1a–d]. Two- and 3-dimensional multiplanar views on the TVUS demonstrated a normal uterine fundus superior to the gestational sac. Fetal measurements were consistent with an estimated gestational age of 8 weeks and 5 days. Fetal cardiac activity measured 159 beats per minute (bpm). Given the position of the gestation in the lower uterine segment and the history of two prior cesarean sections, the findings on initial ultrasound examination raised the suspicion of a cesarean scar ectopic pregnancy with possible isthmic-cervical involvement [Figure 1a–d]. In addition, the proximity of the gestation to the urinary bladder raised the possibility of urinary bladder involvement; however, ultrasound examination could not exclude this possibility with certainty. The patient was referred to the hospital for admission, further evaluation with MRI, and medical management with the possibility of surgical intervention.
Figure 1.
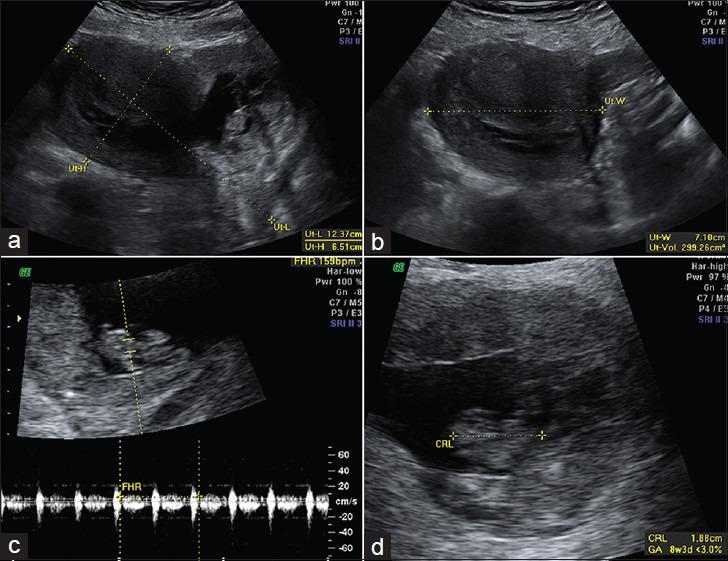
(a) Sagittal and (b) transverse images from a pelvic ultrasound show a gravid retroflexed uterus measuring 12.4 × 6.5 × 7.1 cm corresponding to a volume of 299.3 cc. (c, d) Additional transabdominal images show a single intrauterine gestation with positive fetal cardiac activity measuring 159 beats per minute and a crown-rump length measuring 2.1 cm corresponding to an estimated gestational age of 8 weeks and 5 days
MRI confirmed an intrauterine pregnancy bulging through the myometrium of the lower uterine segment with resultant mass effect on the superior right parasagittal aspect of the urinary bladder [Figure 2a–d] without direct invasion of the urinary bladder wall. The developing placenta was positioned inferiorly in this region with little or no surrounding myometrium. The findings on MRI were diagnostic of an ectopic pregnancy in the lower uterine segment, within the known cesarean scar, and confirmed the absence of urinary bladder involvement.
Figure 2.
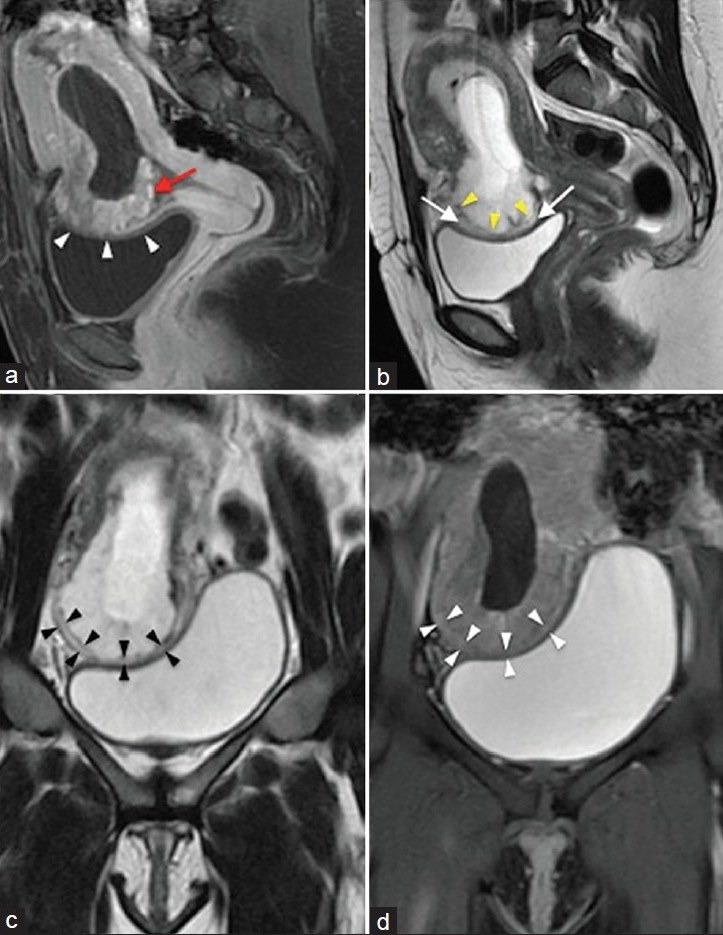
(a) Sagittal T1-weighted fat-saturated contrast-enhanced image of the uterus just off midline demonstrates an intrauterine gestational sac distending the endometrial cavity. The sac produces an outward bulge in the lower uterine segment at the site of cesarean section scar (white arrowheads) and is intimately related to the urinary bladder roof. Enhancing trophoblastic tissue/placenta (red arrow) is present at the bulging site. (b) Sagittal T2-weighted image of the uterus again shows the outward bulging gestational sac through the cesarean scar. Marked thinning of the myometrium is seen at the base/periphery of the bulging gestational sac. The central portion of the bulging gestation sac appears to be covered only by thin hypointense serosa (yellow arrowheads). Of note on this image is the preserved urinary bladder wall with a separating hyperintense fat plane (white arrows). (c) Coronal T2-weighted and (d) coronal T2-weighted fat-saturated images demonstrate the preserved hypointense urinary bladder wall at the level where the gestational sac bulging through the scar sits atop the urinary bladder. Note also the marked thinning of the overlying myometrium (black and white arrowheads)
Upon admission, conservative management was initiated. The patient underwent a successful bilateral uterine artery embolization via a right common femoral artery approach. The right uterine artery was embolized using five vials of 500-700 μm microspheres and stasis of blood flow was achieved. The left uterine artery was embolized using four vials of 500-700 μm microspheres and an additional vial of 700-900 μm microspheres with near stasis of flow. The patient also received systemic intramuscular methotrexate therapy (80 mg administered via the left gluteus muscle). Quantitative serum beta-hCG levels began to decrease, though fetal cardiac activity remained present and measured in the range of 160 bpm. A second dose of 80 mg intramuscular methotrexate was administered 3 days later with serum beta-hCG levels continuing to decline; however, follow-up serial ultrasound examinations continued to demonstrate fetal cardiac activity in the 160 bpm range. The patient was deemed to have failed initial conservative therapy and was given the option of an intra-amniotic injection of potassium chloride with methotrexate or a hysterectomy. The patient ultimately decided on surgical management and underwent a total abdominal hysterectomy with extensive lysis of adhesions. Intraoperative findings revealed the urinary bladder to be densely adherent to the lower uterine segment and the unruptured pregnancy, primarily on the right as demonstrated on the MRI. Pathologic evaluation of the hysterectomy specimen revealed a gravid leiomyomatous uterus with decidua in the uterine corpus as well as serosal adhesions to adipose tissue and skeletal muscle at the prior cesarean section scar. A portion of spongy, placental tissue measuring 5.0 × 4.0 × 2.7 cm was attached to the anterior lower uterine segment with extension to the upper endocervical canal. An embryo with four limb buds and ambiguous genitalia was present within the gestational sac [Figure 3a–c]. No cytotoxic effects related to the embolization or chemotherapy were reported.
Figure 3.
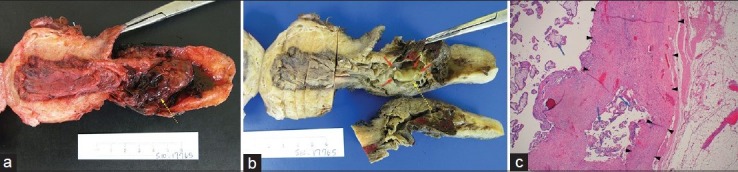
(a, b) Photographs of the gross specimen post-hysterectomy show a gestational sac with a single embryo (dashed yellow arrow) measuring 2.4 cm in length attached by a 2.6 cm long umbilical cord (not shown). Four limb buds (red arrows) are present with ambiguous genitalia. (c) Microscopic evaluation of the sample from the scarred portion of the lower uterine segment in the implantation site shows myometrium ranging from 0.1 cm to 1.8 cm in thickness (black arrowheads) and chorionic villi with trophoblasts (black arrows) invading into 1 mm of fibroadipose tissue, suggesting impending rupture. (Hematoxylin and eosin stain, ×20)
DISCUSSION
Once considered an extremely rare entity, cesarean scar pregnancies are becoming a more common occurrence with the increase in elective cesarean procedures and improvement in detection of CSPs with TVUS. While the cause and pathophysiology are still not well understood, to our knowledge, many theories regarding the mechanism of a CSP have been proposed. It has been hypothesized that poor vascularity in the anterior lower uterine segment impairs healing after cesarean procedures in some women, rendering this area vulnerable to formation of small dehiscent tracts or defects into which a trophoblast can implant.[3] Women who undergo multiple cesarean sections develop scars with larger surface areas, which may in turn increase the risk of a scar implantation. Two types of CSPs have been proposed by Vial et al.,: (i) implantation of the trophoblast on a prior cesarean scar with growth toward the uterine cavity and (ii) implantation deep in the scar defect with progression toward the bladder and abdominal cavity.[4] The former type may progress to a viable pregnancy with significant risk of hemorrhage, while the latter type is at greater risk of rupture.
Imaging and diagnosis
Diagnosis of a cesarean scar pregnancy requires a high index of clinical suspicion, as up to 40% of patients may be asymptomatic.[2] Alternatively, women may present with vaginal bleeding, as in our patient. Abdominal pain may not always be present.[2] Symptoms of severe acute abdominal pain with heavy vaginal bleeding should raise concerns for impending rupture while hemodynamic instability indicates rupture of a CSP.[3] Ultrasound is the first-line imaging modality for evaluation of a potential CSP, with the majority of CSPs diagnosed on the basis of TVUS. Transvaginal ultrasound has a reported sensitivity of 84.6%, and has become the imaging examination of choice for diagnosis.[2] Sonographic criteria initially proposed by Vial et al., have been adopted by others to aid in the diagnosis of a CSP: (i) A trophoblast located between the bladder and anterior uterine wall at the presumed site of the cesarean section scar, (ii) a gestational sac which is ovoid and regular in shape, as opposed to distorted and collapsed as can be seen in miscarriages, and (iii) a thin or discontinuous myometrium between the gestational sac and urinary bladder wall on sagittal images of the uterus through the amniotic sac. Additional sonographic findings may be helpful in avoiding incorrect diagnosis. For example, vascularity of the sac on color Doppler interrogation can aid in distinguishing a CSP from the avascular sac of an aborting pregnancy. Alternatively, a negative “sliding organ sign” when gentle pressure is applied to a sac seen at the level of the internal orifice of the uterus using the endovaginal probe can help differentiate a CSP from a spontaneous abortion in progress. Findings of high velocity (peak velocity >20 cm/sec) and low impedance (pulsatility index <1) waveforms on pulsed Doppler have been described with scar implantation.[5]
Since transvaginal ultrasound combined with color and pulsed Doppler evaluation is relatively reliable in diagnosing CSPs, most authors suggest limiting the use of MRI to cases in which TVUS findings are equivocal, or when the obstetrician requires additional information in preparation for surgery.[3] One drawback to evaluation of CSPs with MRI is the long acquisition time; thus, patients need be hemodynamically stable. Despite this limitation, MRI has been used previously in a small number of patients as an adjunct to ultrasound evaluation.[6] Though not essential for diagnosis, MRI can have an impact on patient management in rare or complicated forms of ectopic pregnancy, as with our patient. The decision to institute medical therapy versus surgery requires accurate characterization of a suspected ectopic pregnancy. The superior soft tissue differentiation and spatial resolution allows improved characterization of CSPs. Sagittal and axial T1- and T2-weighted sequences can depict the gestational sac implanted in the anterior lower uterine segment and more clearly define involvement of adjacent organs (e.g. urinary bladder). In addition, the multiplanar imaging capability of MR may be helpful in orienting the surgeon, should operative management be required.
The ultrasound findings in our patient were highly suspicious of a cesarean scar pregnancy; however, the low position of the gestational sac and its proximity to the urinary bladder presented a problem in light of the patient's desire for future fertility. While the ultrasound images did not clearly demonstrate urinary bladder involvement, the MR images were more conclusive and showed the intrauterine pregnancy bulging through the myometrium of the lower uterine segment, immediately adjacent to and exerting mass effect on the superior aspect of the urinary bladder without invasion of the bladder wall. These findings allowed the clinicians to safely proceed with conservative medical management. Though the patient ultimately required a hysterectomy, an attempt was made to preserve fertility according to the patient's wishes. In the case of our patient, additional evaluation with MRI played a role in the decision to institute conservative medical management and may have also helped with surgical planning.
Treatment options
Several options are available, both medical and surgical, for treatment of CSPs. While there is no standardized approach for the treatment of this condition to our knowledge, the general consensus is that cesarean scar pregnancies should not be managed expectantly due to the risk of uterine rupture and hemorrhage. Conservative medical management includes systemic or local administration of methotrexate, local injection of embryocides (e.g. potassium chloride, hyperosmolar glucose, or crystalline trichosanthin) into the gestational sac, or a combination of both.[3] The likelihood of treatment success with methotrexate is greatest when the serum beta-hCG level is less than 5,000 mIU/mL, during the first 6 weeks of pregnancy, or when the embryo exhibits no cardiac activity.[7] Of note, our patient had an admission serum beta-hCG level of 199,760 mIU/mL and the embryo did demonstrate cardiac activity. If conservative management is instituted, close follow-up of the pregnancy with TVUS is warranted. While serial TVUS with color Doppler evaluation has been shown to correlate well with serum beta-hCG levels, it has been suggested that the high-velocity, low-impedance flow associated with the gestation may not change despite intervention until the serum beta-hCG levels return to normal.[7] Patients who demonstrate such flow characteristics on follow-up TVUS despite progressively declining serum beta-hCG levels on follow-up may be at increased risk of catastrophic uterine rupture and hemorrhage, as was the case in our patient.
Surgical options include combined medical treatment with surgical sac aspiration, uterine curettage, hysteroscopic evacuation, laparoscopic removal, primary open hysterotomy, or hysterectomy. The patient's clinical symptoms and desire for future fertility, age and size of the gestation, and the clinician's experience dictates which treatment option is most appropriate. Patients who choose conservative medical management should be counseled regarding the possibility of requiring definitive surgical treatment in the event of failed medical therapy. In addition, it has been suggested that the risk of spontaneous uterine rupture is 17 times more likely in women with a prior cesarean section compared to those with an unscarred uterus.[7] This statistic, combined with the increased risk of uterine rupture after implantation of a pregnancy in the thin cesarean scar, should be taken into consideration when contemplating conservative versus surgical management.
An alternative to the more traditional treatment options presented is combined medical management with uterine artery embolization (UAE), which was the therapy initially instituted in our patient. This combined approach has been described previously.[8] UAE is minimally invasive, and may be an attractive option should patients present with vaginal bleeding yet maintain a desire to preserve fertility. One study of 66 patients found UAE combined with local methotrexate administration to be more effective in treating CSPs compared to traditional methods and had increased success rate, fewer complications, and a reduced risk for hysterectomy.[8] In our patient, UAE was combined with systemic methotrexate administration rather than local injection into the gestational sac. It has been suggested that absorption of systemic methotrexate by the conceptus may be limited due to poor vascularization of the fibrous cesarean scar.[9] This may have been a contributing factor in the failure of conservative therapy in our patient, who ultimately required treatment with hysterectomy.
CONCLUSION
Cesarean scar ectopic pregnancy remains a relatively rare entity; however, this potentially life-threatening condition is being reported more often as greater numbers of elective cesarean procedures are performed worldwide. TVUS remains the imaging modality of choice for diagnosis, though MRI may play a greater role in evaluating CSPs. The superior soft tissue characterization and anatomical information provided by MRI allows patients and clinicians to consider conservative management as initial therapy, especially with the increasing availability of minimally invasive UAE. Combined with conservative medical therapy, minimally invasive UAE makes preserving fertility in the face of a CSP more achievable. While there is no standardized approach at present, intervention must be instituted early to minimize complications and maternal morbidity regardless of which therapeutic option is decided upon. Treatment must be tailored to each individual case and requires careful discussion between the patient and clinician. In the end, the decision should be made based on the patient's clinical presentation, age of the gestation, desire for future fertility, and clinician's experience in managing this condition.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/16/109758
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus: An unusual case of postabortal haemorrhage.A case report. S Afr Med J. 1978;53:142–3. [PubMed] [Google Scholar]
- 2.Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: Etiology, diagnosis and management. Obstet Gynecol. 2006;107:1373–81. doi: 10.1097/01.AOG.0000218690.24494.ce. [DOI] [PubMed] [Google Scholar]
- 3.Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253–63. doi: 10.1111/j.1471-0528.2006.01237.x. [DOI] [PubMed] [Google Scholar]
- 4.Vial Y, Petignat P, Hohlfeld P. Pregnancy in a cesarean scar. Ultrasound Obstet Gynecol. 2000;16:592–3. doi: 10.1046/j.1469-0705.2000.00300-2.x. [DOI] [PubMed] [Google Scholar]
- 5.Jurkovic D, Jauniaux E, Kurjak A, Hustin J, Campbell S, Nicolaides KH. Transvaginal color Doppler assessment of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1991;77:365–9. [PubMed] [Google Scholar]
- 6.Shufaro Y, Nadjari M. Implantation of a gestation sac in a cesarean section scar. Fertil Steril. 2001;75:1217. doi: 10.1016/s0015-0282(01)01795-2. [DOI] [PubMed] [Google Scholar]
- 7.Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: Issues in management. Ultrasound Obstet Gynecol. 2004;23:247–53. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 8.Yang XY, Yu H, Li KM, Chu YX, Zheng A. Uterine artery embolisation combined with local methotrexate for treatment of caesarean scar pregnancy. BJOG. 2010;117:990–6. doi: 10.1111/j.1471-0528.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 9.Fylstra DL. Ectopic pregnancy within a cesarean scar: A review. Obstet Gynecol Surv. 2002;57:537–43. doi: 10.1097/00006254-200208000-00024. [DOI] [PubMed] [Google Scholar]


