Abstract
Non-dystrophic myotonias are rare diseases caused by mutations in skeletal muscle chloride and sodium ion channels with considerable phenotypic overlap between diseases. Few prospective studies have evaluated the sensitivity of symptoms and signs of myotonia in a large cohort of patients. We performed a prospective observational study of 95 participants with definite or clinically suspected non-dystrophic myotonia recruited from six sites in the USA, UK and Canada between March 2006 and March 2009. We used the common infrastructure and data elements provided by the NIH-funded Rare Disease Clinical Research Network. Outcomes included a standardized symptom interview and physical exam; the Short Form-36 and the Individualized Neuromuscular Quality of Life instruments; electrophysiological short and prolonged exercise tests; manual muscle testing; and a modified get-up-and-go test. Thirty-two participants had chloride channel mutations, 34 had sodium channel mutations, nine had myotonic dystrophy type 2, one had myotonic dystrophy type 1, and 17 had no identified mutation. Phenotype comparisons were restricted to those with sodium channel mutations, chloride channel mutations, and myotonic dystrophy type 2. Muscle stiffness was the most prominent symptom overall, seen in 66.7% to 100% of participants. In comparison with chloride channel mutations, participants with sodium mutations had an earlier age of onset of stiffness (5 years versus 10 years), frequent eye closure myotonia (73.5% versus 25%), more impairment on the Individualized Neuromuscular Quality of Life summary score (20.0 versus 9.44), and paradoxical eye closure myotonia (50% versus 0%). Handgrip myotonia was seen in three-quarters of participants, with warm up of myotonia in 75% chloride channel mutations, but also 35.3% of sodium channel mutations. The short exercise test showed ≥10% decrement in the compound muscle action potential amplitude in 59.3% of chloride channel participants compared with 27.6% of sodium channel participants, which increased post-cooling to 57.6% in sodium channel mutations. In evaluation of patients with clinical and electrical myotonia, despite considerable phenotypic overlap, the presence of eye closure myotonia, paradoxical myotonia, and an increase in short exercise test sensitivity post-cooling suggest sodium channel mutations. Outcomes designed to measure stiffness or the electrophysiological correlates of stiffness may prove useful for future clinical trials, regardless of underlying mutation, and include patient-reported stiffness, bedside manoeuvres to evaluate myotonia, muscle specific quality of life instruments and short exercise testing.
Keywords: non-dystrophic myotonia, SCN4A, CLCN1, myotonia, paramyotonia
Introduction
The non-dystrophic myotonias are a heterogeneous group of rare neuromuscular disorders (prevalence <1:100 000) caused by mutations in the skeletal muscle sodium (SCN4A) and chloride channels (CLCN1), and include the classic diseases myotonia congenita, paramyotonia congenita, hyperkalemic periodic paralysis with myotonia, and a diverse group of sodium channel myotonias (Emery, 1991; Ptacek et al., 1991; Hoffman and Wang, 1993; Lehmann-Horn and Rudel, 1996; Sun et al., 2001; Cannon, 2006; Fialho et al., 2007). Patients with CLCN1 mutations (myotonia congenita) have a muscular appearance, classic myotonia on exam (delayed relaxation after contraction), percussion myotonia, and warm up of myotonia with repeated activity (Streib, 1987). Inheritance is dominant or recessive with a more severe phenotype in the latter (Colding-Jorgensen, 2005; Fialho et al., 2007; Raja Rayan and Hanna, 2010). The SCN4A myotonias are a more diverse group of dominantly inherited disorders that run the spectrum from mild myotonia that does not interfere with daily activities, to severe muscle stiffness, or frank episodes of paralysis (Cannon, 2000). Patients with paramyotonia congenita demonstrate cold sensitivity, myotonia that ‘paradoxically’ worsens with repetitive activity, and episodic weakness (Ptacek et al., 1993; Miller et al., 2004; Cannon, 2006; Matthews et al., 2010). In contrast, patients with sodium channel myotonia, including the potassium aggravated myotonias, have variable cold-sensitivity and no episodic weakness (Trudell et al., 1987; Ptacek et al., 1992; Ricker et al., 1994; Orrell et al., 1998; Matthews et al., 2010). Patients with hyperkalemic periodic paralysis may have myotonia, but episodic weakness is usually the dominant feature. Although not life limiting, patients with non-dystrophic myotonia can experience significant lifetime morbidity due to stiffness and pain related to myotonia, and rarely, an infantile form of disease can be associated with respiratory distress (Lion-Francois et al., 2010). Myotonic dystrophy type 2 is caused by CCTG repeat expansion in the zinc finger protein 9 gene, and can present with a pure myotonic phenotype that can be difficult to clinically distinguish from non-dystrophic myotonia (Day et al., 2003; Meola and Moxley, 2004).
Electrodiagnostic studies in non-dystrophic myotonia have demonstrated characteristic mutation-related reductions in compound muscle action potential amplitude after short periods of exercise (Fournier et al., 2004; Tan et al., 2011). Patients with CLCN1 mutations can have a transient drop in compound muscle action potential amplitude that rapidly returns to baseline; compared with SCN4A mutations where there may be no change, or a delayed reduction in compound muscle action potential amplitude, facilitated by repetition or cold (Fournier et al., 2004).
Despite being one of the best characterized ion channel disorders, few prospective studies have compared the frequency of these classic findings in large groups of patients with genetically defined or clinically suspected non-dystrophic myotonia (Fialho et al., 2007; Matthews et al., 2008; Dupre et al., 2009; Trip et al., 2009). The frequency and sensitivity of symptoms and signs and their relationship to underlying mutation is an important clinical question with implications for diagnosis, management and therapeutic trial planning. The NIH-funded Rare Disease Clinical Research Network provided an unprecedented opportunity to study a large group of patients with non-dystrophic myotonia using a common infrastructure, data elements and centralized training. We have previously reported data on quantitative handgrip myotonia assessment and an interactive voice response diary of patient-reported symptoms in this population (Statland et al., 2011, 2012b). In this study we describe standardized patient-reported and quantitative measures of myotonia collected prospectively from a large international cohort of definite or clinically suspected non-dystrophic myotonia.
Materials and methods
We performed a prospective observational study from March 2006 to March 2009 as part of the NIH-funded Rare Disease Clinical Research Network’s Consortium for Clinical Investigation of Neurological Channelopathies (CINCH). Ninety-five subjects were recruited from six academic centres across the USA, Canada and UK. The evaluators were trained to perform all outcomes in a standardized manner at an investigator meeting. Informed consent was obtained from all study participants, and the protocol was approved by the relevant institutional review boards at all participating sites.
Subjects
Inclusion criteria were broad and were intended to mimic a clinician’s approach. Participants were eligible if they were ≥6 years of age, had clinical symptoms or signs of non-dystrophic myotonia, presence of myotonia on electromyography, and the absence of features suggestive of a diagnosis of myotonic dystrophy type 1 (ptosis, temporal wasting, mandibular weakness, premature cataracts, and evidence of multisystem defects). Participants with genetically confirmed myotonic dystrophy type 2 could be included if they had clinical and electrical myotonia in absence of typical systemic involvement. Participants were required to stop anti-myotonic medications 5 days before each evaluation. For patients with a prior history of taking medications associated with electrical myotonia (Niebroj-Dobosz and Kwiecinski, 1983; Sonoda et al., 1994; Rutkove et al., 1996), symptoms had to persist upon discontinuation. Patients were excluded if they had positive genetic testing for myotonic dystrophy type 1.
Outcomes
Baseline data included age, gender, self-reported race and ethnicity, medical history, and family history.
Clinical assessment
A standardized symptom questionnaire was administered, specifying details on four symptoms: stiffness, weakness, fatigue and pain. The questionnaire had the participant indicate the location, frequency, severity, alleviating factors and precipitating factors for symptoms in an evaluator-guided interview format. Severity was graded on a 1–9 scale, with 1 being minimal, and 9 the worst ever experienced. Participants also underwent a standardized physical examination/myotonia assessment. Each patient was tested for presence/absence of clinical myotonia (hand grip, lid lag, eye closure) and percussion myotonia over the thenar eminence and extensor digitorum communis muscle. Warm-up and paradoxical myotonia were assessed by asking the participant to perform five sequential 3 s manoeuvres (forced handgrip/eye closure followed by rapidly opening the fist or eyes; looking up then quickly returning gaze to neutral position). In addition time to open fists/eyes after forced closure were measured on a stopwatch.
Quality of life instruments
Participants completed the Short Form 36 Item Health Survey (SF-36). The SF-36 is a widely used generic questionnaire to assess patients’ self-reported health status across physical, mental, and social domains (Ware and Sherbourne, 1992). There are 36 items assessing eight domains (physical functioning, social functioning, role limitations due to physical, role limitations due to emotional, energy/vitality, mental health, body pain, general health perception) in addition to a physical composite score and a mental composite score. A higher score indicates better health, that is, a higher level of functioning or less pain. Participants also completed the muscle specific Individualized Neuromuscular Quality of Life (Vincent et al., 2007). The Individualized Neuromuscular Quality of Life has been validated for adults with various neuromuscular diseases including dystrophic and non-dystrophic myotonias (Vincent et al., 2007; Sansone et al., 2012) and consists of 45 items covering 10 domains: four assessing muscle symptoms (weakness, fatigue, pain, and muscle locking), five evaluating the impact of the muscle disease on areas of life (activities, independence, social, emotional and body image) and one assessing treatment effectiveness. The Individualized Neuromuscular Quality of Life summary score is a composite of the five domains designed to assess impact of disease on quality of life, and can be thought of as a per cent of overall detrimental impact on a patient’s life. Higher scores indicate a worse perception of quality of life.
Laboratory tests and genetic analysis
Creatine kinase, thyroid stimulating hormone, blood urea nitrogen, creatinine and K+ were tested at baseline. A separate consent was obtained for genetic testing. Samples were analysed at the University of Rochester and in the UK. Genetic testing was performed using a tiered method based on clinical impression following the baseline visit, first looking for previously published mutations and then sequencing the whole chloride channel gene and exons 22 and 24 of the sodium channel (Fialho et al., 2007; Matthews et al., 2008). Myotonic dystrophy genetic testing was not performed as part of this study. Participants were grouped into chloride channel mutations (CLCN1) and sodium channel mutations (SCN4A). Those with unidentified mutations were not included in the phenotypic comparisons. SCN4A participants were further broken down by specific mutation. CLCN1 participants were subdivided into recessive or dominant mode of inheritance based on the number of mutations and family history. There were some subjects who could not be further classified (n = 5). Investigators were asked to provide a clinical diagnosis after physical exam and electrodiagnostic testing for each participant that did not have a previous molecular confirmation.
Functional evaluation
The ‘get-up-and-go’ test is a commonly used functional measure to assess balance with published standardized norms (Mathias et al., 1986). We modified this test to capture changes in the timed walk related to either warm-up or paradoxical worsening of myotonia. In this protocol, participants were asked to get up and walk 30 ft after 10 min of rest in a chair. This was repeated four times at 30-s intervals, and time determined using a stopwatch.
Strength testing
Manual muscle testing was performed on the following muscles bilaterally: shoulder abductors, elbow flexors and extensors, wrist flexors and extensors, hip flexors, extensors and abductors, knee flexors and extensors, ankle plantar and dorsiflexors. We used a 13-point modified Medical Research Council scale, and individual muscle scores were averaged to create a composite manual muscle test score (Personius et al., 1994). Quantitative hand grip dynamometry was obtained using a force transducer connected to automatic capturing software (QMA system, Computer Source). Each hand grip recorded was the best of three maximal voluntary isometric contractions recorded in kg force.
Electrodiagnostic assessment
Procedures performed at each visit included a short exercise test, a short exercise test after cooling, and a prolonged exercise test using a standardized protocol with minor modifications (McManis et al., 1986; Fournier et al., 2004, 2006), as well as needle electromyography of proximal and distal muscles. The short and prolonged exercise tests record compound muscle action potentials before and after periods of exercise at specific time points. Post-exercise compound muscle action potential amplitudes were calculated as per cent change from the average pre-exercise baseline measurement. Abnormal decrement was determined as ≥10% compound muscle action potential reduction in the short exercise test and 20% in the prolonged exercise test, as previously reported (Fournier et al., 2004). Because the main electrodiagnostic distinction between various types of myotonic disorders is best determined by the short exercise test (Fournier et al., 2006; Tan et al., 2011), we initially assigned patterns as previously described by Fournier et al. (2004) (Supplementary Table 1) using the prolonged exercise test for additional determination of pattern when the short exercise test was normal. The electrodiagnostic patterns for all participants were determined by a single blinded evaluator. Electromyographic myotonia was graded on a 1+ to 3+ scale (Streib, 1987) in the following muscles: biceps, abductor digiti minimi, vastus lateralis, tibialis anterior, and thoracic paraspinal muscles.
Statistical analysis
Statistical comparisons were restricted to participants with CLCN1 mutations, SCN4A mutations, and myotonic dystrophy type 2. Standard statistical methods were used for all descriptive statistics including the calculation of the median and the first and third quartiles (i.e. interquartile range). Complete case analysis was employed throughout. The test for differences in distribution among the three mutation categories employed the Kruskal-Wallis test for factors that were either continuous data, or ordered data with more than seven levels (e.g. 0–9 severity score). The Pearson’s chi-square test without continuity correction was used for testing difference in frequencies among the mutation categories. All P-values presented are two-tailed. The box and whisker plot reflect the standard calculations of the median and the first (lower hinge) and third (upper hinge) quartiles. The whisker, measured from the median, is either, one and a half times the upper minus the lower hinge, or the most extreme raw value, whichever is less. Individual raw values beyond the whisker are indicated with a dot. Descriptive analysis and statistical tests were conducted using S+ 8.1 (TIBCO Spotfire) with the exception of the box and whisker plot that was constructed with Stata 11.1 (StataCorp).
Results
Demographics
Ninety-five participants were recruited between March 2006 and March 2009. Two participants dropped out before study visits. Of the 93 remaining participants, 32 had CLCN1 mutations, 34 had SCN4A mutations, and nine had myotonic dystrophy type 2 (Table 1). One participant had myotonic dystrophy type 1 (89 CTG repeats) and 17 others did not have an identified mutation, and were excluded from analysis. Gender was balanced with the exception of the CLCN1 group, which had a majority of males (75.0%). There was no difference in race or ethnicity based on underlying mutation, with participants largely Caucasian and non-Hispanic. Disability and unemployment due to non-dystrophic myotonia ranged from 3.1% to 23.5% between groups. Muscle stiffness was the most prominent symptom overall in non-dystrophic myotonia (54.7%), ranging from 75.0% for CLCN1 to 11.1% for myotonic dystrophy type 2. Weakness was the most prominent symptom in myotonic dystrophy type 2 (44.4%, P = 0.005).
Table 1.
Patient characteristics
| CLCN1 | SCN4A | DM2 | P-valuea | |
|---|---|---|---|---|
| n = 32 | n = 34 | n = 9 | ||
| Age, median (IQR) | 42 (29–52) | 46 (36.5–58) | 49 (44–62) | 0.17 |
| Gender, female, n (%) | 8 (25.0) | 18 (52.9) | 6 (66.7) | 0.022 |
| Ethnicity, Non-hispanic n(%) | 32 (100.0) | 34 (100.0) | 9 (100.0) | # |
| Race, White, n (%) | 27 (84.4)d | 33 (97.1) | 8 (88.9) | # |
| Disability due to disease, n (%)b | 2 (6.3) | 8 (23.5) | 1 (11.1) | # |
| Unemployed due to disease, n (%)c | 1 (3.1) | 5 (14.7) | 2 (22.2) | # |
| Most prominent symptom, n (%) | ||||
| Stiffness | 24 (75.0) | 16 (47.1) | 1 (11.1) | 0.0046 |
| Pain | 5 (15.6) | 5 (14.7) | 3 (33.3) | |
| Weakness | 0 (0.0) | 10 (29.4) | 4 (44.4) | |
| Fatigue | 3 (9.4) | 3 (8.8) | 1 (11.1) | |
IQR = interquartile range; DM2 = myotonic dystrophy type 2.
aSignificance level using the Pearson’s Chi-square test for a difference in the frequency among the mutation categories.
b% calculation includes not disabled.
c% calculation includes employed.
done subject missing the information was excluded from the calculations.
#Rarity of factor precluded the necessary conditions for computing a valid Pearson’s Chi-square test.
Clinical features
All CLCN1 participants reported muscle stiffness, with a median age of onset of 10 years, most common in the legs (90.6%) (Table 2). Interestingly, although 93.8% reported improvement of symptoms with exercise; 43.8% also reported worsening of stiffness with exercise (Supplementary Table 2). Cold was the most common trigger for symptoms (62.5%), and up to 25.0% of females reported worsening of stiffness either with pregnancy or during menstruation. On examination, 75% of participants had hand grip myotonia, showed evidence for warm-up of myotonia with repeated contractions, and had muscle hypertrophy (Table 2).
Table 2.
Clinical features
| Symptoms/Findings | CLCN1 n = 32 | SCN4A n = 34 | DM2 n = 9 | P-valuea |
|---|---|---|---|---|
| Stiffness | ||||
| n (%) | 32 (100.0) | 34 (100.0) | 6 (66.7) | # |
| Age of onset–median (IQR) | 10 (6–14.5)b | 5 (1–10) | 29 (22.3–36.5) | <0.001 |
| Average intensity, median (IQR)f | 5 (3.5–6)b | 5 (4–7)d | 4.5 (4–5.75) | 0.8 |
| Leg stiffness, n (%) | 29 (90.6) | 21 (61.8) | 2 (33.3) | 0.0028 |
| Face stiffness, n (%) | 2 (6.3) | 16 (47.1) | 0 (0.0) | <0.001 |
| Duration, mins, median (IQR) | 1 (0.25–2)d | 4.5 (1–13.5)c | 10 (2.69–13.8) | 0.019 |
| Pain with stiffness, n (%) | 17 (53.1) | 28 (82.4) | 6 (100.0) | 0.009 |
| Cold trigger, n (%)e | 20 (62.5) | 32 (94.1)b | 5 (83.3) | 0.0065 |
| Potassium trigger | 1 (3.1) | 0 (0.0)b | 0 (0.0) | # |
| Pregnancy trigger among females | 1 (16.7)c | 7 (43.8)c | 3 (60.0) | # |
| Menstrual cycle trigger among females | 2 (25.0) | 4 (23.5)b | 1 (25.0)b | # |
| Episodic weakness | ||||
| n (%) | 12 (37.5) | 26 (76.5) | 5 (55.6) | 0.006 |
| Cold trigger | 4 (33.3) | 22 (84.6) | 1 (20) | 0.001 |
| Potassium-rich diet | 1 (8.3) | 0 (0) | 0 (0) | # |
| Exam findings | ||||
| n (%) | 32 | 34 | 9 | |
| Grip myotonia | 24 (75) | 26 (76.5) | 3 (33.3) | 0.032 |
| Eye closure myotonia | 8 (25) | 25 (73.5) | 3 (33.3) | <0.001 |
| Warm-up phenomena | 24 (75) | 12 (35.3) | 2 (22.2) | 0.001 |
| Paradoxical grip myotonia | 1 (3.1) | 17 (50) | 1 (11.1) | <0.001 |
| Paradoxical eye closure myotonia | 0 (0.0) | 17 (50.0) | 0 (0.0) | <0.001 |
| Muscle hypertrophy | 24 (75.0) | 14 (41.2) | 0 (0.0) | <0.001 |
| Anti-myotonic treatment | ||||
| Mexiletine alone or in combination | 14 (43.8) | 9 (26.5) | 1 (11.1) | 0.12 |
| Other than mexiletine | 8 (25) | 9 (26.5) | 2 (22.2) | 0.97 |
| Strength testing | ||||
| MMT combined score–median (IQR) | 4.79 (4.31–4.97) | 4.73 (4.46–4.87) | 4.24 (4.23–4.51) | 0.031 |
| Grip strength, right–median (IQR) | 36.8 (19.6–49.5)b | 31.5 (24.3–40.5) | 24.9 (18.9–28.5) | 0.091 |
IQR = interquartile range; DM2 = myotonic dystrophy type 2; MMT = manual muscle testing.
aSignificance level using the appropriate test for a difference in the distribution among the mutation categories.
bOne subject missing the information was excluded from the calculations.
cTwo subjects missing the information were excluded from the calculations.
dThree subjects missing the information were excluded from the calculations.
eSubjects reporting “Not sure” were considered not having the event indicated.
fSeverity of symptom was recorded on a 9 point scale (1–9) with 0 representing no symptom present.
#Insufficient frequency of event to satisfy condition needed for statistical test.
Similar to CLCN1, all SCN4A participants also reported muscle stiffness, with a median age of onset of 5 years, most common in the legs (61.8%) and face (47.1%), and the majority (82.4%) reported pain with stiffness (Table 2). Cold was a trigger of stiffness in almost all participants (94.1%), and up to 43.8% of females reported worsening of stiffness either with pregnancy or during menstruation. Stiffness improved in 44.1% and worsened in 67.6% with exercise (Supplementary Table 2). Although 76.5% of SCN4A reported episodic weakness, most commonly triggered by cold, none reported an association with diet. On examination, 76.5% had handgrip myotonia, with a pattern of warm-up on repetition in 35.3% and a paradoxical worsening in 50%. Eye closure myotonia was seen in 73.5% participants (Table 2).
Participants with myotonic dystrophy type 2 reported stiffness less frequently (66.7%), with a median age of onset at 29 years, and all subjects reported pain with stiffness. Cold was the most common trigger, with 66.7% both improving and worsening with exercise. Only 33.3% of participants with myotonic dystrophy type 2 demonstrated handgrip myotonia, with warm-up in less than a quarter of the participants.
Comparing sensitivities of symptoms and signs by mutation category, there were significant differences in median age of symptom onset, with onset in the first decade for CLCN1 and SCN4A, and third decade in myotonic dystrophy type 2 (P < 0.001). Location of stiffness was also different based on underlying mutation: 90.6% of CLCN1 reported stiffness in the leg compared with 61.8% in SCN4A, and 33.3% in myotonic dystrophy type 2 (P = 0.003), whereas 47.1% of SCN4A reported stiffness in the face compared with 6.3% in CLCN1 (P < 0.001). Painful stiffness, cold sensitivity, and episodic weakness were seen more commonly in SCN4A and myotonic dystrophy type 2. On exam, eye closure myotonia was more common in SCN4A (73.5% compared with 25.0% for CLCN1 and 33.3% for myotonic dystrophy type 2, P < 0.001), and paradoxical eye closure myotonia was exclusive to SCN4A (50.0%, P < 0.001). Muscle hypertrophy was more common in CLCN1 (75.0%, compared with 41.2% in SCN4A, P < 0.001), and warm-up was more common but not exclusive to CLCN1 (75% compared with 35.3% in SCN4A, and 22.2% in myotonic dystrophy type 2, P = 0.001).
Electrodiagnostic data
Data were available for the short and prolonged exercise tests for 81 of the 93 participants enrolled in the study and 65 of 76 with identified mutations.
The median decrements on short exercise test were 16.2% and 4.5% at room temperature and 18.4% and 22.0% post-cooling, in CLCN1 and SCN4A, respectively (Table 3). The sensitivity of the short exercise test for ≥10% decrement was 59.3% in CLCN1 and 27.6% in SCN4A at room temperature. This sensitivity increased to 66.7% in CLCN1 and 57.6% in SCN4A post-cooling. Based on Fournier patterns, CLCN1 mutations were predominantly pattern II (48%) followed by patterns III (37%), I (11%) and IV (4%); SCN4A mutations as a group were predominantly pattern III (57%) followed by patterns I (32%), IV (7%) and V (4%); all myotonic dystrophy type 2 participants were pattern III.
Table 3.
Electrodiagnostic data
| Electrophysiological testing | CLCN1, n = 32 | SCN4A, n = 34 | DM2, n = 9 | P-valuea |
|---|---|---|---|---|
| CMAP amplitude decrement (percent change) on SET at room temperature–median score†⋄ | 16.2 (2–46)e | 4.48 (−1.36–10.3)e | 1.22 (−0.894–3.39)b | 0.057 |
| Number of patients with ≥10% decrement on SET at room temperature (%) | 16 (59.3)e | 8 (27.6)e | 0 (0)b | |
| CMAP amplitude decrement (percent change) on SET post cooling–median score†⋄ | 18.4 (8.08–36.4)e | 22.0 (4.76–49)b | 1.45 (-7.15–3.14)b | 0.004 |
| Number of patients with ≥10% decrement on SET post cooling (%) | 18 (66.7)e | 19 (57.6)b | 0 (0)b | |
| CMAP amplitude decrement (% change) PET Median score† | 13.1 (−0.97–30.5)d | 31.1 (13–54.9)c | 15.9 (9.77–26.5)b | 0.021 |
| Fournier pattern I–V distribution: number of patient (%) | I 3 (11), II 13 (48), III 10 (37), IV 1 (4)e | I 9 (32), III 16 (57), IV 2 (7), V 1 (4)f | III 8 (100)b | |
| Electrical myotonia grade median score (IQR) | ||||
| Tibialis anterior | 3 (1.75–3) | 3 (2–3)b | 1 (1–2) | 0.001 |
| Thoracic paraspinals | 3 (1.75–3) | 3 (2–3)c | 1 (0–1) | 0.001 |
| Biceps | 3 (2–3) | 3 (2–3)b | 0 (0–1) | 0.001 |
†IQR = interquartile range; ⋄0 represents normal SET; DM2 = myotonic dystrophy type 2; PET = prolonged exercise test; SET = short exercise test; CMAP = compound muscle action potential.
aSignificance level using the appropriate test for a difference in the distribution among the mutation categories.
bOne subject missing the information was excluded from the calculations.
cThree subjects missing the information were excluded from the calculations.
dFour subjects missing the information were excluded from the calculations.
eFive subjects missing the information were excluded from the calculations.
fSix subjects missing the information were excluded from the calculations.
Median electrical myotonia was grade 3+ in the tibialis anterior and thoracic paraspinal muscles in the SCN4A and CLCN1 participants compared with 1+ in particiapants with myotonic dystrophy type 2 (P < 0.001) (Table 3).
Functional evaluation
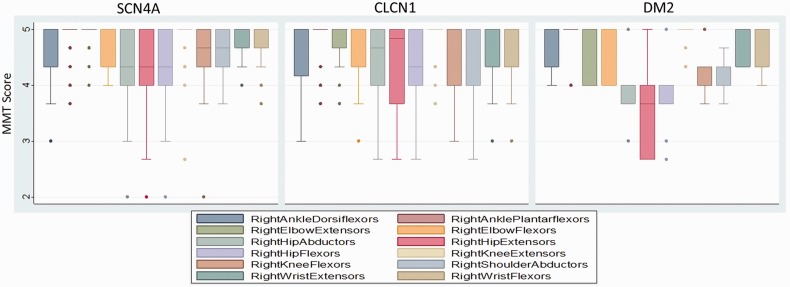
Weakness was modest in all participants (Table 2), with myotonic dystrophy type 2 the weakest overall (combined manual muscle test score 4.24 compared with 4.79 for CLCN1, and 4.73 for SCN4A; P = 0.03). The pattern of weakness was proximal involving the hip and shoulder muscles in all three mutation categories (Fig. 1). In both SCN4A and CLCN1 participants the ankle dorsiflexors were weaker than plantar flexors, and elbow and knee extensors were weaker than flexors.
Figure 1.
Manual muscle testing (MMT) for SCN4A (n = 32), CLCN1 (n = 34), and myotonic dystrophy type 2 (DM2) (n = 9). Box and whisker plots show distribution of manual muscle testing scores for each muscle. The box represents 50% of population; dark line is the median; whiskers are adjacent upper/lower values; and dots represent outliers.
On the get-up-and-go test, all three groups showed an improvement in the time taken to perform the test with each consecutive timed walk. However, the CLCN1 and myotonic dystrophy type 2 participants had a greater reduction in time from Test 1 to Test 4 (−0.98 s and −0.90 s, respectively) compared with SCN4A (−0.30 s) (P = 0.012).
Quality of life
The overall Individualized Neuromuscular Quality of Life score revealed that quality of life in the SCN4A and myotonic dystrophy type 2 participants was impacted to a significantly greater degree than the CLCN1 group (P = 0.017); with activities and independence life domains having the greatest influence (Table 4). The SF-36 physical composite score and mental composite score were not different for the three cohorts (P ≥ 0.10) with only role limitations for physical reasons affecting the SCN4A and myotonic dystrophy type 2 cohorts to a greater extent (P = 0.043).
Table 4.
Quality of life data
| QOL | CLCN1 | SCN4A | DM2 | P-Value |
|---|---|---|---|---|
| Weakness INQoL | 47.4 (36.8–60.5) [13] | 63.2 (36.8–84.2) [1] | 52.6 (47.4–63.2) | 0.46 |
| Muscle locking INQoL | 47.4 (32.9–63.2) [2] | 63.2 (44.7–73.7) [3] | 63.2 (47.4–63.2) [4] | 0.073 |
| Pain INQoL | 34.2 (17.1–56.6) [10] | 50 (26.3–65.8) [4] | 50 (30.3–68.4) [1] | 0.35 |
| Fatigue INQoL | 42.1 (26.3–65.8) [9] | 55.3 (25–80.3) [10] | 68.4 (42.1–84.2) | 0.18 |
| Activities INQoL | 32.4 (22.2–49.8) [2] | 44.4 (27.8–88.9) [1] | 50 (38.9–75) | 0.044 |
| Independence INQoL | 2.78 (0–26.4) [1] | 22.2 (11.1–47.2) [1] | 30.6 (25–41.7) | 0.0023 |
| Social Relations INQoL | 11.1 (4.17–24.5) [1] | 25 (6.48–50) [1] | 27.8 (13.9–35.2) | 0.064 |
| Emotions INQoL | 25 (5.56–37.5) [1] | 38.9 (16.7–63.9) [1] | 36.1 (27.8–55.6) | 0.013 |
| Body Image INQoL | 27.8 (1.39–50) [1] | 16.7 (0–63.9) [1] | 41.7 (22.2–52.8) | 0.47 |
| QOL INQoL | 9.44 (5–20) [1] | 20 (10–28.3) [1] | 20.6 (17.8–22.8) | 0.017 |
| Perceived treatment effects INQoL | 33.3 (0–62.5) [1] | 0 (0–8.33) [1] | 0 (0–33.3) | 0.037 |
| Expected treatment effects INQoL | 0 (0–54.2) [1] | 0 (0–16.7) [1] | 0 (0–8.33) | 0.076 |
| Physical function SF36 | 44.4 (34.9–47.6) [1] | 45.5 (28.6–50.2) [4] | 29.7 (25.5–42.3) | 0.1 |
| Role physical SF-36 | 49.5 (42.2–56.9) [1] | 39.7 (27.5–52) [5] | 37.3 (29.9–44.6) | 0.043 |
| Bodily pain SF-36 | 51.1 (41.6–55.4) [1] | 46.1 (29.2–55.4) [5] | 46.1 (33.4–46.1) | 0.31 |
| General health SF-36 | 52.9 (38.6–56.7) [1] | 48.2 (33.9–55.3) [5] | 37.7 (31.5–47.2) | 0.083 |
| Vitality SF36 | 45.8 (42.7–55.2) [1] | 45.8 (36.5–52.1) [5] | 36.5 (36.5–45.8) | 0.22 |
| Social function–SF36 | 51.4 (45.9–56.8) [1] | 45.9 (29.6–56.8) [5] | 45.9 (35–56.8) | 0.18 |
| Role emotional SF36 | 55.9 (50–55.9) [1] | 52 (32.6–55.9) [5] | 44.2 (36.4–55.9) | 0.19 |
| Mental Health SF-36 | 52.8 (48.6–58.5) [1] | 50 (41.6–55.6) [5] | 44.4 (41.6–52.8) | 0.17 |
| Physical Component–SF-36 | 46.3 (38.8–49.8) [1] | 45.7 (29.2–51.9) [5] | 32.8 (30.9–41.9) | 0.096 |
| Mental Component–SF-36 | 55.2 (49.4–57.9) [1] | 50.3 (40.5–55.8) [5] | 43.2 (41–59) | 0.17 |
Values in square brackets represents the number of subjects with a missing value for the indicated table cell. INQoL = Individualized Neuromuscular Quality of Life.
Treatment for stiffness
Of all mutation confirmed participants with non-dystrophic myotonia, 40 (60.6%) were taking an anti-myotonic medication. Of these, 23 took mexiletine alone or in combination with another drug (Table 2).
Genetic features
SCN4A mutations
Thr1313Met mutations were most common (32%) followed by Arg1448His (21%) and Gly1306Ala (15%) mutations (Table 5). Cold sensitivity for stiffness was present in most participants. Paradoxical myotonia of grip and eye closure was most common in Thr1313Met (91%) and Arg1448His (71%). Gly1306Ala participants showed a mix of paradoxical myotonia (60%) and warm-up (40%) on exam. Ser804Phe has been reported with warm-up, but we observed paradoxical myotonia in our participant. Most of the Thr1313Met participants showed a modified Fournier pattern I (89%), but interestingly all of our Arg1448His participants showed a Fournier pattern III. The type III pattern was also the most frequent pattern seen in the remaining sodium mutations. In participants without previous genetic confirmation, the investigators accurately diagnosed sodium channel category after completion of clinical exam and electrodiagnostic testing in 11 of 16 (68.75%) patients.
Table 5.
Genotype–phenotype of sodium channel cohort (n = 34)
| Phenotype | Thr1313Met | Arg1448His | Gly1306Ala | Met1592Val | Val1589Met | Val1293Ile |
|---|---|---|---|---|---|---|
| n | 11 (32%) | 7 (21%) | 5 (15%) | 3 (9%) | 2 (6%) | 2 (6%) |
| Cold trigger stiffness | 11 (100%) | 7 (100%) | 5 (100%) | 2 (67%) | 2 (100%) | 2 (100%) |
| Episodic weakness | 8 (73%) | 7 (100%) | 3 (60%) | 3 (100%) | 1 (50%) | 1 (50%) |
| Cold trigger weakness | 8 (73%) | 7 (100%) | 2 (40%) | 3 (100%) | 0 | 0 |
| Paramyotoniaa | 10 (91%) | 5 (71%) | 3 (60%) | 0 | 0 | 0 |
| Warm-up | 1 (9%) | 2 (29%) | 2 (40%) | 2 (67%) | 2 (100%) | 2 (100%) |
| Fournier pattern I-V n (%) | I 8 (89), III 1 (11)c | III 5 (100)c | I 1 (20), III 3 (60), IV 1 (20) | III 1 (50), V 1 (50)b | III 2 (100) | III 1 (50), IV 1 (50) |
| Phenotype in our cohort | PMCd | PMCd/SCM (PAM) | SCM (PAM)/PMC | Cannot define | SCM (PAM) | SCM (PAM) |
| Reported phenotype | PMCR1 | PMC; HyperPP/PMCR1 | PAM/PMCR1 | HyperPPR2; HyperPP/PMCR3 | PAMR4, PMCR1 | PAMR5 |
Data are expressed as n/total(%).
aCombined grip and eye closure paramyotonia.
bOne subject missing information excluded from the calculations.
cTwo subjects missing information excluded from the calculations.
dOne patient had warm up of grip, eye closure myotonia and eye closure paramyotonia.
R1 = Matthews et al., 2008; R2 = Rojas et al., 1991; R3 = Kelly et al., 1997; R4 = Heine et al., 1993; R5 = Koch et al., 1995.
HyperPP = hyperkalemic periodic paralysis; PAM = potassium aggravated myotonias; PMC = paramyotonia congenital; SCM = sodium channel myotonia.
Two novel SCN4A mutations were identified: Val717Ala and Ser1434Pro. Stiffness was the most prominent symptom and both participants had cold-triggered episodic weakness. Exam findings were variable with paramyotonia in Ser1434Pro and warm-up in Val717Ala. EDX patterns conformed to Fournier pattern III.
CLCN1 mutations
Participants with recessive CLCN1 mutations (n = 15) demonstrated earlier age of onset at 6 years versus 12 years for dominant mutations (n = 12) (P = 0.04) (Table 6). There were no differences in location or severity of stiffness in both groups. Fournier pattern II was seen most frequently in patients with recessive mutations (64%) compared with dominant (27%). Interestingly Fournier pattern type I was seen in 14% of participants with recessive mutations and in 9% dominant mutations. In participants without previous genetic confirmation, the investigators accurately diagnosed CLCN category after completion of clinical exam and electrodiagnostic testing in 25 of 29 (86.20%).
Table 6.
Genotype–phenotype of CLCN1 cohort
| Phenotype | Autosomal dominant | Autosomal recessive | P-value |
|---|---|---|---|
| n = 12 | n = 15 | ||
| Age of onset of stiffness (median, IQR) | 12 (7–15.8) | 6 (4.25–11.8)a | 0.04 |
| Leg stiffness | 12 (100) | 13 (86.7) | 0.57 |
| Average severity of stiffness | 5 (3.5–6) | 5 (3.5–6.5) | 0.94 |
| Average duration of stiffness | 2 (0.383–2.5)a | 1.5 (0.313–2)a | 0.96 |
| Cold trigger for stiffness | 6 (50) | 10 (66.7) | 0.63 |
| Creatine kinase level IU/l | 145 (111–348)c | 244.5 (162–430)b | 0.2 |
| Muscle hypertrophy | 7 (58.3) | 13 (86.7) | 0.22 |
| Fournier pattern I-V n (%) | I 1 (9), II 3 (27), III 6 (55), IV 1 (9)a | I 2 (14), II 9 (64), III 3 (22)a |
IQR = interquartile range.
aOne subject missing the information was excluded from the calculations.
bThree subjects missing the information was excluded from the calculations.
cFive subjects missing the information was excluded from the calculations.
One novel CLCN1 mutation was detected: c.1-59C>A homozygous mutation. The participant had whole chloride channel gene sequencing and this mutation was not seen in normal or ethnically matched controls. Clinical features in this participant were typical of other CLCN1 participants, with stiffness, grip myotonia, warm-up, and absence of paramyotonia with Fournier pattern II demonstrated on the short exercise test.
Discussion
Our international study represents one of the largest prospectively collected cohorts of participants with genetically defined or clinically suspected non-dystrophic myotonia. Although many typical features can help distinguish between sodium and chloride channel mutations in non-dystrophic myotonia, we found considerable phenotypic overlap, which has implications both for diagnosis and management, as well as for future therapeutic trial planning. Consistent with previous reports we found stiffness was the most prominent symptom with onset in the first decade, a preponderance of leg stiffness in CLCN1 and facial stiffness in SCN4A, and a higher frequency of eye closure myotonia and paradoxical myotonia in SCN4A. Warm-up of myotonia was seen in one-third of SCN4A participants, and there was significant variability in electrodiagnostic patterns on short exercise testing. This presents a diagnostic dilemma for clinicians evaluating patients with suspected non-dystrophic myotonia, and for researchers planning clinical trials. However, common themes in this cohort suggest possible solutions: although the sensitivity was low, paradoxical eye closure myotonia was unique to SCN4A; and stiffness was the most prominent and frequent symptom, regardless of underlying mutation, making outcomes designed to assess stiffness and the muscular manifestations of stiffness attractive targets for future clinical trials.
Before this study, the largest observational cohort studied 62 participants with genetically confirmed non-dystrophic myotonia using standardized interviews, and found transient weakness and leg muscle myotonia was more common in CLCN1, and paradoxical myotonia and eyelid myotonia more common in SCN4A. Our results support their findings, and expand the observation that the warm-up phenomenon was observed in both SCN4A and CLCN1 mutations (Trip et al., 2009). A Canadian study evaluating 50 genetically confirmed participants with non-dystrophic myotonia also found considerable phenotypic overlap, with warm-up of myotonia in 79% of SCN4A participants (Dupre et al., 2009). The most distinguishing characteristic they found was tongue myotonia in recessive CLCN1 subjects, which was not assessed here.
Environmental factors reported to increase myotonia include pregnancy, dietary potassium, temperature changes, hunger, fatigue, and emotional stress, and some of these have traditionally been thought to help distinguish the different mutations (Orrell et al., 1998; Lacomis et al., 1999; Fialho et al., 2008; Basu et al., 2009). Typically, cold sensitivity has been associated with SCN4A mutations (Orrell et al., 1998; Wu et al., 2001; Miller et al., 2004); however, cold sensitivity was also seen in more than half of our CLCN1 participants, consistent with findings from a large CLCN1 cohort (Fialho et al., 2007). Although traditionally exercise is reported to improve myotonia in CLCN1 mutations, and worsen myotonia in SCN4A mutations, both CLCN1 and SCN4A participants reported that exercise both improved and worsened stiffness. Pregnancy and menstruation enhanced stiffness for both CLCN1 and SCN4A participants. Although the mechanism of this is unclear, it could perhaps be due to a hormonal influence on skeletal muscle ion channels. A previous study suggested that in patients with non-dystrophic myotonia with chloride sensitive mutations, pregnancy-induced lowering of serum chloride reduces the chloride conductance sufficiently to aggravate myotonia (Colding-Jorgensen, 2005).
Electrodiagnostic testing with established sensitivities and cut-offs have been recommended to guide genetic testing in non-dystrophic myotonia and are attractive quantitative targets for clinical trial outcomes. Fournier et al. (2004) reported on five electrodiagnostic patterns in skeletal muscle channelopathies using the short and prolonged exercise tests with some correlation to certain mutations. Further studies determined the short exercise test to be the more useful and practical test for guiding choice of molecular diagnostic testing in myotonic disorders (Fournier et al., 2006; Tan et al., 2011). In our study, among all mutation-confirmed participants, although pattern I was predominantly seen in participants with SCN4A mutations (67% in Thr1313Met and 8% in Gly1306Ala), it was not exclusive to paramyotonia congenita as previously reported (Fournier et al., 2004), with the remaining 25% belonging to participants with both dominant and recessive CLCN1 mutations. In addition, five of our participants with the SCN4A Arg1448His mutation presented with pattern III rather than pattern I, unlike prior reports (Fournier et al., 2004), thus reducing the sensitivity of pattern I for diagnosing paramyotonia congenita. Fournier pattern II was specific to CLCN1 (23% dominant and 69% recessive) and not seen in any with SCN4A mutations, consistent with Tan et al. (2011) and contrary to Fournier et al. (2006). The participants with myotonic dystrophy type 2 presented with Fournier pattern III, rather than pattern II as previously noted (Fournier et al., 2006). Pattern III was ubiquitous and seen in both recessive and dominant CLCN1 mutations and all sodium channel mutations studied, and indeed was the most common pattern encountered at room temperature. Of interest one of the three participants with M1592V mutation (commonly associated with hyperkalemic periodic paralysis or paramyotonia congenita) presented with Fournier pattern V (assigned to hypokalemic periodic paralysis) and another with pattern III rather than the expected pattern IV. Our findings suggest that abnormalities in electrophysiological exercise testing may guide selection of mutational analysis for non-dystrophic myotonia; however it is not sensitive or specific enough to make a definitive diagnosis in most patients.
This is the only multinational study to utilize standard validated quality of life questionnaires in large number of participants with non-dystrophic myotonia. Overall the quality of life of the SCN4A participants was more affected compared with individuals with CLCN1. Participants with SCN4A reported a higher prevalence of pain with stiffness, episodic weakness, longer duration of stiffness, and had a higher Individualized Neuromuscular Quality of Life summary score. The SCN4A cohort symptom impact scores are more similar to those found in individuals with limb girdle and facioscapulohumeral muscular dystrophies, although the life domains were less affected in the SCN4A group (Rose et al., 2012) which is not unexpected, given the non-progressive nature of non-dystrophic myotonia when compared to the dystrophies. A previous study suggested the perception of quality of life in skeletal muscle channelopathies was similar to myotonic dystrophy, and therefore non-dystrophic myotonia should not be considered benign (Sansone et al., 2012). The overall SF-36 scores reported did not discriminate between CLCN1 and SCN4A and in our cohort were more similar to the Dutch non-dystrophic myotonia group (Trip et al., 2009). The SF-36 and Individualized Neuromuscular Quality of Life scores reported here were dissimilar to the Italian cohort (Sansone et al., 2012) suggesting the possibility of other influences, for example environmental or cultural.
The impact of non-dystrophic myotonia on quality of life perhaps can be appreciated more when considering that over half of the participants with non-dystrophic myotonia (61%) in our cohort were prescribed anti-myotonic medications. Approximately half used mexiletine alone or in combination, and the benefit of treatment in the CLCN1 cohort was significant, revealed in the perceived treatment effects item of the Individualized Neuromuscular Quality of Life. In a recent randomized controlled trial in non-dystrophic myotonia, mexiletine was found to significantly reduce stiffness, in addition to improving electrophysiological parameters and quality of life measures (Statland et al., 2012a).
Although differences can be seen between the broader categories of SCN4A and CLCN1, this study is limited in its power to distinguish clinical or quantitative differences between specific mutations. Functional expression studies were not performed on the three novel mutations reported here (two SCN4A, one CLCN1). Other limitations include the possibility of differences in patient-interpretation of questions in our standardized questionnaire, and a possible bias toward participants more likely to enrol in an observational study, which may appeal to participants who are more functionally independent, and less severely affected. In patients with dominant mutations and affected family members, symptoms may be recognized earlier which would influence the reported age of onset of stiffness. Seventeen participants did not have identified mutations using our standard genetic screening technique. Some may turn out to have myotonic dystrophy type 2 on routine commercial testing (nine tested negative for myotonic dystrophy type 2). And although full SCN4A sequencing or multiplex ligation-dependent probe amplification of CLCN1 gene might identify other known SCN4A mutations, deletions or duplications in CLCN1 (Raja Rayan et al., 2012), or novel mutations, other studies have also shown that a small percentage of patients remain genetically undiagnosed despite full analysis of CLCN1 and SCN4A (Trip et al., 2008). Despite this, our study represents one of the largest, geographically distributed non-dystrophic myotonia cohorts in the literature, and the frequencies of signs and symptoms are likely representative of the broader non-dystrophic myotonia population. Genetic testing is the gold standard for definitive diagnosis and in future, DNA chip technology might replace time consuming electrodiagnostic studies in the initial evaluation of patients with non-dystrophic myotonia. However, cost is a limitation and an argument could be made that from a clinical standpoint, available treatment strategies are similar for all non-dystrophic myotonia subtypes. However, it is important to emphasize knowledge of the specific mutation does have utility: (i) to distinguish non-dystrophic myotonia from myotonic dystrophy, which can be life-limiting and requires surveillance for multi-system involvement; (ii) the potential for future mutation-specific treatments; (iii) mutation-specific characteristics might help understand disease pathophysiology better; and (iv) accurate genetic counselling can be offered to affected individuals
Despite the diagnostic challenges in non-dystrophic myotonia, there are common themes observed in our non-dystrophic myotonia cohort that provide important information for the design of future treatment trials: (i) standardized assessments can be performed at multiple centres internationally to enable recruitment of larger numbers of participants than previously felt feasible; (ii) patient-reported stiffness and handgrip myotonia are common regardless of underlying mutation and are attractive outcomes for future clinical trials; (iii) grip myotonia, eye closure myotonia, and warm-up/paramyotonia in hand grip and eye closure occur frequently, are easy to perform, and are useful bedside tests in evaluating non-dystrophic myotonia; (iv) the short exercise test may be a useful quantitative measure for early phase testing and proof of concept studies; and (v) the muscle disease specific questionnaire Individualized Neuromuscular Quality of Life is a more relevant instrument for determining symptom impact on quality of life in non-dystrophic myotonia compared with the generic SF-36.
Supplementary Material
Acknowledgements
The authors acknowledge the study participants and their families for their commitment and generosity—they are the impetus for this international collaboration.
Appendix 1
The Consortium of Clinical Investigation of Neurologic Channelopathies (CINCH):
Steering Committee: Principal Investigator: Richard J Barohn, MD (Kansas City, KS); Site Principal Investigators: Anthony A. Amato, MD (Boston, MA), Robert C Griggs, MD (Rochester, NY), Stephen C Cannon, MD, PhD (Dallas, TX), Michael Hanna, MD (London, UK), Shannon Venance, MD, PhD (London, ON); Chief Statistician: Brian N Bundy, PhD (Tampa, FL); Study Coordinator at Lead Site: Laura Herbelin, BS (Kansas City, KS); CINCH Lead Research Coordinator: Kimberly Hart (Rochester, NY); and CINCH Program Manager: Barbara Herr, MS (Rochester, NY). Investigators/CINCH Trainees: Louis Ptacek (San Francisco, CA), Yunxia Wang, MD (Kansas City, KS), David Saperstein, MD (Kansas City, KS), Mazen Dimachkie, MD (Kansas City, KS), Jeffrey Statland, MD (Rochester, NY), Doreen Fiahlo, MD (London, UK), Emma Matthews, MD (London, UK), Dipa Raja Rayan, MD (London, UK), Mohammad Salajegheh, MD (Co-Principal investigator, Boston, MA), Ronan Walsh, MD (Boston, MA), Jaya Trivedi, MD (Co-Principal Investigator, Dallas, TX), Angelika Hahn, MD (Co-Principal Investigator, London, ON), James Cleland, MD (Rochester, NY), Paul Twydell, DO (Rochester, NY). Data Management and Coordinating Center Principal Investigator: Jeffrey Krischer, PhD (Tampa, FL). Clinical Evaluators: Laura Herbelin, BS (Kansas City, KS), Merideth Donlan PT, (Boston, MA), Rhonda McLin, PTA(Dallas, TX), Shree Pandya, PT, MS, Katy Eichinger, PT, DPT, NCS (Rochester NY), Karen Findlater, PT (London, ON), Liz Dewar PT (London UK). Clinical Coordinators: Laura Herbelin, BS (Kansas City, KS), Kristen Roe (Boston, MA), Nina Gorham (Dallas, TX), Christine Piechowicz (London, ON), Kimberly Hart (Rochester, NY). Genetics Team: Ming Qi (Rochester, NY), Kuang-Hsiang Chuang (Rochester, NY), Andrea Haworth (London, UK), Richa Sud (London, UK), and Sam McCall (London, UK). Participating Members of the Data Management and Coordinating Center: Joseph Gomes (Tampa, FL), Holly Ruhlig, MS, ARNP, CCRC (Tampa, FL), Bonnie Patterson, BA CCRP (Tampa, FL), David Cuthbertson, MS (Tampa, FL), Rachel Richesson, PhD, MPH (Tampa, FL), and Jennifer Lloyd (Tampa, FL).
Funding
This work was supported by the National Institute of Health [5 U54 NS059 065-0552 (NINDS/ORD)]. This work was also supported by the UT Southwestern Medical Centre [CTSA Grant UL1TR000451 NCATS/NIH]; University of Kansas Medical Centre [CTSA Grant ULI RR 033179 NCRR/NIH]; an MRC Centre grant [G0800674], NHS National Specialist Commissioning, and the UCLH Biomedical Research Centre London; the University of Rochester [CTSA Grant UL1 RR 024160 NCRR/NIH]; R37 AR42703/NIAMS [S.C.]; and Muscular Dystrophy Association Clinical Research Training Grant [J.S.].
Supplementary material
Supplementary material is available at Brain online.
References
- Basu A, Nishanth P, Ifaturoti O. Pregnancy in women with myotonia congenita. Int J Gynaecol Obstet. 2009;106:62–3. doi: 10.1016/j.ijgo.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Spectrum of sodium channel disturbances in the nondystrophic myotonias and periodic paralyses. Kidney Int. 2000;57:772–9. doi: 10.1046/j.1523-1755.2000.00914.x. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci. 2006;29:387–415. doi: 10.1146/annurev.neuro.29.051605.112815. [DOI] [PubMed] [Google Scholar]
- Colding-Jorgensen E. Phenotypic variability in myotonia congenita. Muscle Nerve. 2005;32:19–34. doi: 10.1002/mus.20295. [DOI] [PubMed] [Google Scholar]
- Day JW, Ricker K, Jacobsen JF, Rasmussen LJ, Dick KA, Kress W, et al. Myotonic dystrophy type 2: molecular, diagnostic and clinical spectrum. Neurology. 2003;60:657–64. doi: 10.1212/01.wnl.0000054481.84978.f9. [DOI] [PubMed] [Google Scholar]
- Dupre N, Chrestian N, Bouchard JP, Rossignol E, Brunet D, Sternberg D, et al. Clinical, electrophysiologic, and genetic study of non-dystrophic myotonia in French-Canadians. Neuromuscul Disord. 2009;19:330–4. doi: 10.1016/j.nmd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Emery AE. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- Fialho D, Kullmann DM, Hanna MG, Schorge S. Non-genomic effects of sex hormones on CLC-1 may contribute to gender differences in myotonia congenita. Neuromuscul Disord. 2008;18:869–72. doi: 10.1016/j.nmd.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Fialho D, Schorge S, Pucovska U, Davies NP, Labrum R, Haworth A, et al. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain. 2007;130(Pt 12):3265–74. doi: 10.1093/brain/awm248. [DOI] [PubMed] [Google Scholar]
- Fournier E, Arzel M, Sternberg D, Vicart S, Laforet P, Eymard B, et al. Electromyography guides toward subgroups of mutations in muscle channelopathies. Ann Neurol. 2004;56:650–61. doi: 10.1002/ana.20241. [DOI] [PubMed] [Google Scholar]
- Fournier E, Viala K, Gervais H, Sternberg D, Arzel-Hezode M, Laforet P, et al. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006;60:356–65. doi: 10.1002/ana.20905. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Wang J. Duchenne-Becker muscular dystrophy and the nondystrophic myotonias. Paradigms for loss of function and change of function of gene products. Arch Neurol. 1993;50:1227–37. doi: 10.1001/archneur.1993.00540110101010. [DOI] [PubMed] [Google Scholar]
- Lacomis D, Gonzales JT, Giuliani MJ. Fluctuating clinical myotonia and weakness from Thomsen's disease occurring only during pregnancies. Clin Neurol Neurosurg. 1999;101:133–6. doi: 10.1016/s0303-8467(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F, Rudel R. Channelopathies: the nondystrophic myotonias and periodic paralyses. Semin Pediatr Neurol. 1996;3:122–39. doi: 10.1016/s1071-9091(96)80041-6. [DOI] [PubMed] [Google Scholar]
- Lion-Francois L, Mignot C, Vicart S, Manel V, Sternberg D, Landrieu P, et al. Severe neonatal episodic laryngospasm due to de novo SCN4A mutations: a new treatable disorder. Neurology. 2010;75:641–5. doi: 10.1212/WNL.0b013e3181ed9e96. [DOI] [PubMed] [Google Scholar]
- Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387–9. [PubMed] [Google Scholar]
- Matthews E, Fialho D, Tan SV, Venance SL, Cannon SC, Sternberg D, et al. The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain. 2010;133(Pt 1):9–22. doi: 10.1093/brain/awp294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E, Tan SV, Fialho D, Sweeney MG, Sud R, Haworth A, et al. What causes paramyotonia in the United Kingdom? Common and new SCN4A mutations revealed. Neurology. 2008;70:50–3. doi: 10.1212/01.wnl.0000287069.21162.94. [DOI] [PubMed] [Google Scholar]
- McManis PG, Lambert EH, Daube JR. The exercise test in periodic paralysis. Muscle Nerve. 1986;9:704–10. doi: 10.1002/mus.880090805. [DOI] [PubMed] [Google Scholar]
- Meola G, Moxley RT., 3rd Myotonic dystrophy type 2 and related myotonic disorders. J Neurol. 2004;251:1173–82. doi: 10.1007/s00415-004-0590-1. [DOI] [PubMed] [Google Scholar]
- Miller TM, Dias da Silva MR, Miller HA, Kwiecinski H, Mendell JR, Tawil R, et al. Correlating phenotype and genotype in the periodic paralyses. Neurology. 2004;63:1647–55. doi: 10.1212/01.wnl.0000143383.91137.00. [DOI] [PubMed] [Google Scholar]
- Niebroj-Dobosz I, Kwiecinski H. Clofibrate-induced myotonia in rats. Acta Neurol Scand. 1983;67:222–8. doi: 10.1111/j.1600-0404.1983.tb04567.x. [DOI] [PubMed] [Google Scholar]
- Orrell RW, Jurkat-Rott K, Lehmann-Horn F, Lane RJ. Familial cramp due to potassium-aggravated myotonia. J Neurol Neurosurg Psychiatry. 1998;65:569–72. doi: 10.1136/jnnp.65.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Phys Ther. 1994;74:253–63. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, Johnson KJ, Griggs RC. Genetics and physiology of the myotonic muscle disorders. N Engl J Med. 1993;328:482–9. doi: 10.1056/NEJM199302183280707. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, Tawil R, Griggs RC, Storvick D, Leppert M. Linkage of atypical myotonia congenita to a sodium channel locus. Neurology. 1992;42:431–3. doi: 10.1212/wnl.42.2.431. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, Trimmer JS, Agnew WS, Roberts JW, Petajan JH, Leppert M. Paramyotonia congenita and hyperkalemic periodic paralysis map to the same sodium-channel gene locus. Am J Hum Genet. 1991;49:851–4. [PMC free article] [PubMed] [Google Scholar]
- Raja Rayan DL, Hanna MG. Skeletal muscle channelopathies: nondystrophic myotonias and periodic paralysis. Curr Opin Neurol. 2010;23:466–76. doi: 10.1097/WCO.0b013e32833cc97e. [DOI] [PubMed] [Google Scholar]
- Raja Rayan DL, Haworth A, Sud R, Matthews E, Fialho D, Burge J, et al. A new explanation for recessive myotonia congenita: exon deletions and duplications in CLCN1. Neurology. 2012;78:1953–8. doi: 10.1212/WNL.0b013e318259e19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker K, Moxley RT, 3rd, Heine R, Lehmann-Horn F. Myotonia fluctuans. A third type of muscle sodium channel disease. Arch Neurol. 1994;51:1095–102. doi: 10.1001/archneur.1994.00540230033009. [DOI] [PubMed] [Google Scholar]
- Rose MR, Sadjadi R, Weinman J, Akhtar T, Pandya S, Kissel JT, et al. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve. 2012;46:351–9. doi: 10.1002/mus.23320. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, De Girolami U, Preston DC, Freeman R, Nardin RA, Gouras GK, et al. Myotonia in colchicine myoneuropathy. Muscle Nerve. 1996;19:870–5. doi: 10.1002/(SICI)1097-4598(199607)19:7<870::AID-MUS9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sansone VA, Ricci C, Montanari M, Apolone G, Rose M, Meola G. Measuring quality of life impairment in skeletal muscle channelopathies. Eur J Neurol. 2012;19:1470–6. doi: 10.1111/j.1468-1331.2012.03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Gotow T, Kuriyama M, Nakahara K, Arimura K, Osame M. Electrical myotonia of rabbit skeletal muscles by HMG-CoA reductase inhibitors. Muscle Nerve. 1994;17:891–7. doi: 10.1002/mus.880170808. [DOI] [PubMed] [Google Scholar]
- Statland JM, Bundy BN, Wang Y, Rayan DR, Trivedi JR, Sansone VA, et al. Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA. 2012a;308:1357–65. doi: 10.1001/jama.2012.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statland JM, Bundy BN, Wang Y, Trivedi JR, Raja Rayan D, Herbelin L, et al. A quantitative measure of handgrip myotonia in non-dystrophic myotonia. Muscle Nerve. 2012b;46:482–9. doi: 10.1002/mus.23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statland JM, Wang Y, Richesson R, Bundy B, Herbelin L, Gomes J, et al. An interactive voice response diary for patients with non-dystrophic myotonia. Muscle Nerve. 2011;44:30–5. doi: 10.1002/mus.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streib EW. AAEE minimonograph #27: differential diagnosis of myotonic syndromes. Muscle Nerve. 1987;10:603–15. doi: 10.1002/mus.880100704. [DOI] [PubMed] [Google Scholar]
- Sun C, Tranebjaerg L, Torbergsen T, Holmgren G, Van Ghelue M. Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia. Eur J Hum Genet. 2001;9:903–9. doi: 10.1038/sj.ejhg.5200736. [DOI] [PubMed] [Google Scholar]
- Tan SV, Matthews E, Barber M, Burge JA, Rajakulendran S, Fialho D, et al. Refined exercise testing can aid DNA-based diagnosis in muscle channelopathies. Ann Neurol. 2011;69:328–40. doi: 10.1002/ana.22238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trip J, Drost G, Ginjaar HB, Nieman FH, van der Kooi AJ, de Visser M, et al. Redefining the clinical phenotypes of non-dystrophic myotonic syndromes. J Neurol Neurosurg Psychiatry. 2009;80:647–52. doi: 10.1136/jnnp.2008.162396. [DOI] [PubMed] [Google Scholar]
- Trip J, Drost G, Verbove DJ, van der Kooi AJ, Kuks JB, Notermans NC, et al. In tandem analysis of CLCN1 and SCN4A greatly enhances mutation detection in families with non-dystrophic myotonia. Eur J Hum Genet. 2008;16:921–9. doi: 10.1038/ejhg.2008.39. [DOI] [PubMed] [Google Scholar]
- Trudell RG, Kaiser KK, Griggs RC. Acetazolamide-responsive myotonia congenita. Neurology. 1987;37:488–91. doi: 10.1212/wnl.37.3.488. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007;68:1051–7. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Wu FF, Takahashi MP, Pegoraro E, Angelini C, Colleselli P, Cannon SC, et al. A new mutation in a family with cold-aggravated myotonia disrupts Na(+) channel inactivation. Neurology. 2001;56:878–84. doi: 10.1212/wnl.56.7.878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.