Abstract
Previous findings suggested that the human cerebellum is involved in the acquisition but not the long-term storage of motor associations. The finding of preserved retention in cerebellar patients was fundamentally different from animal studies which show that both acquisition and retention depends on the integrity of the cerebellum. The present study investigated whether retention had been preserved because critical regions of the cerebellum were spared. Visual threat eye-blink responses, that is, the anticipatory closure of the eyes to visual threats, have previously been found to be naturally acquired conditioned responses. Because acquisition is known to take place in very early childhood, visual threat eye-blink responses can be used to test retention in patients with adult onset cerebellar disease. Visual threat eye-blink responses were tested in 19 adult patients with cerebellar degeneration, 27 adult patients with focal cerebellar lesions due to stroke, 24 age-matched control subjects, and 31 younger control subjects. High-resolution structural magnetic resonance images were acquired in patients to perform lesion–symptom mapping. Voxel-based morphometry was performed in patients with cerebellar degeneration, and voxel-based lesion–symptom mapping in patients with focal disease. Visual threat eye-blink responses were found to be significantly reduced in patients with cerebellar degeneration. Visual threat eye-blink responses were also reduced in patients with focal disease, but to a lesser extent. Visual threat eye-blink responses declined with age. In patients with cerebellar degeneration the degree of cerebellar atrophy was positively correlated with the reduction of conditioned responses. Voxel-based morphometry showed that two main regions within the superior and inferior parts of the posterior cerebellar cortex contributed to expression of visual threat eye-blink responses bilaterally. Involvement of the more inferior parts of the posterior lobe was further supported by voxel-based lesion symptom mapping in focal cerebellar patients. The present findings show that the human cerebellar cortex is involved in long-term storage of learned responses.
Keywords: ataxia, cerebellum, conditioning, human brain mapping, learning
Introduction
One well-known function of the cerebellum is its contribution to motor learning (Bastian, 2011; Gao et al., 2012; see Thach, 1998 for reviews). The cerebellum plays an important role in the acquisition of new motor skills, motor adaptation and associative motor learning (Doyon et al., 2003; Gerwig et al., 2003; Donchin et al., 2012). Cerebellar learning has been studied in greatest detail in classical conditioning of the eye-blink reflex (Bracha, 2004; De Zeeuw and Yeo, 2005; Freeman and Steinmetz, 2011 for reviews). For this simple form of implicit learning it is commonly assumed that findings in animals can equally be applied to humans (Woodruff-Pak, 1997). Importantly, cerebellar lesions in humans are followed by profound disorders in the acquisition of the classically conditioned eye-blink response similar to findings in other mammals (Daum et al., 1993; Gerwig et al., 2003, 2010). Animal data show that the cerebellum is not only critically involved in the acquisition, but also in the storage of the learned response (Attwell et al. 2002; Kellett et al., 2010). In humans, however, a previous report demonstrated that cerebellar lesions affect acquisition but not retention of conditioned eye-blink responses that had been learned naturally before the insult (Bracha et al., 1997). Based on this, it was concluded that cerebellar substrates that are necessary for conditioned eye-blink response acquisition are not required for response retention. This study also proposed that engrained conditioned eye-blink responses are likely stored in extra-cerebellar components of eye-blink circuits.
Retention of classically conditioned eye-blink responses is difficult to test in patients with cerebellar lesions, because the ability to acquire new associations is impaired. This is different from animal studies, where lesions can be performed after successful acquisition has taken place. For that reason, Bracha et al. (1997) examined the reflex eye-blink to visual threat or menace (visual threat eye-blink responses). A suddenly approaching object results in anticipatory closure of the eyes. The visual threat eye-blink response shows the typical characteristic of conditioned responses (Bracha et al., 1997) and is thought to be acquired naturally in early childhood (Mac Keith, 1969; Liu and Ronthal, 1992). Bracha et al. (1997) found preserved visual threat eye-blink responses in patients with cerebellar lesions obtained in adulthood. Preserved conditioned eye-blinks were also observed in a comparable paradigm of anticipatory eye-blink responses triggered by kinaesthetic stimuli from the subject’s arm moving toward the subject’s face (kinaesthetic threat response; Bracha et al., 2000). At the same time, patients were unable to acquire the classically conditioned eye-blink response.
Given the many parallels in cerebellar contribution to acquisition of conditioned responses in humans and animals, findings of preserved retention are surprisingly different from observations in animals where cerebellar lesions completely and permanently abolish the learned conditioned response (Thompson, 1986; Christian and Thompson, 2003). The well-rehearsed nature of visual threat eye-blink responses could be a factor when comparing with animal experiments in which conditioned eye-blink responses were relatively recently acquired. However, other possibilities for the observation of preserved visual threat eye-blink responses in cerebellar patients need to be ruled out. The original visual threat eye-blink response study examined a small group of patients with focal lesions. Because of the limited size of these lesions, it is possible that visual threat eye-blink responses exhibited by these patients were under control of the healthy remainder of the cerebellum. Importantly, disparate effects on acquisition and retention could result from a differential sensitivity to the extent of cerebellar damage. Perhaps learning occurs in the cerebellum, and then after partial damage, acquisition is affected but retention is preserved. As the extent of the damage increases, both processes may be affected.
To test this possibility, we examined a larger group of patients with chronic progressive cerebellar degeneration. We expected that if conditioned eye-blink response expression in humans is cerebellum-dependent, visual threat eye-blink responses should be suppressed in individuals in which the cerebellar injury encompassed the eye-blink conditioning substrates more completely. Alternatively, if visual threat eye-blink responses are stored in extra-cerebellar circuits, then their expression should not be affected by these lesions. For a direct comparison with previous findings, an additional group of patients with focal lesions due to stroke was included. High-resolution structural MRIs were acquired and more recently developed methods of lesion–symptom mapping were performed.
Materials and methods
Study population
The first patient group consisted of nineteen adult patients (seven female, 12 male; mean age 55.3 ± 11.3 years; range 34–74 years) with pure cerebellar degeneration. All patients had disorders known to primarily affect the cerebellar cortex (Timmann et al., 2009). The second patient group consisted of 27 patients (six female, 21 male; mean age 52.3 ± 11.1 years; range 32–76 years) with focal lesions of the cerebellum due to stroke. Lesions were unilateral in all patients except one. Eleven patients suffered from stroke within the territory of the superior cerebellar artery, 14 from stroke within the territory of the posterior inferior cerebellar artery, one from cerebellar haemorrhage, and one from stroke within the posterior inferior cerebellar artery and superior cerebellar artery territory. Patients’ characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| ID | Age (years) | Gender | Disease | Disease duration | Total ICARS (max. 100) | Stand and Gait (max. 34) | Kinetic Function (max. 52) | Dysarthria (max. 8) | Oculomotor function (max. 6) |
|---|---|---|---|---|---|---|---|---|---|
| Cerebellar degeneration | |||||||||
| cer-deg-1 | 34 | F | SAOA | 7 years | 51 | 25 | 18 | 2 | 6 |
| cer-deg-2 | 44 | M | SAOA | 6 years | 12 | 7.5 | 2.5 | 2 | 0 |
| cer-deg-3 | 45 | M | SAOA | 15 years | 27.5 | 10 | 12.5 | 4 | 1 |
| cer-deg-4 | 46 | F | ADCA III | 28 years | 26.5 | 8 | 11 | 2.5 | 5 |
| cer-deg-5 | 48 | F | ADCA III | 8 years | 12.5 | 3 | 5 | 3.5 | 1 |
| cer-deg-6 | 49 | M | SAOA | 13 years | 41 | 8 | 26 | 2 | 5 |
| cer-deg-7 | 49 | M | ADCA III | 10 years | 44 | 21 | 15 | 4 | 4 |
| cer-deg-8 | 49 | M | ADCA III | 9 years | 27.5 | 10.5 | 9 | 2 | 6 |
| cer-deg-9 | 52 | F | SCA14 | 13 years | 23 | 9 | 12 | 1 | 1 |
| cer-deg-10 | 52 | M | Cerebellitis | 9 years | 50 | 25 | 16 | 4 | 5 |
| cer-deg-11 | 54 | M | SAOA | 19 years | 51 | 25 | 17 | 4 | 5 |
| cer-deg-12 | 56 | F | SCA 6 | 7 years | 26.5 | 7 | 14.5 | 0 | 5 |
| cer-deg-13 | 58 | F | ADCA III | 18 years | 24 | 1 | 21 | 2 | 0 |
| cer-deg-14 | 62 | M | SAOA | 13 years | 25.5 | 10.5 | 9 | 1 | 5 |
| cer-deg-15 | 62 | M | SAOA | 8 years | 22.5 | 7 | 13.5 | 2 | 0 |
| cer-deg-16 | 72 | M | SAOA | 6 years | 24 | 7.5 | 9.5 | 1 | 6 |
| cer-deg-17 | 72 | M | SCA6 | 16 years | 63 | 29.5 | 24 | 4.5 | 5 |
| cer-deg-18 | 73 | M | SCA6 | 12 years | 40.5 | 15.5 | 15 | 4 | 6 |
| cer-deg-19 | 74 | F | SCA6 | 7 years | 39.5 | 15 | 16.5 | 3 | 5 |
| Cerebellar stroke | |||||||||
| cer-foc-1 | 32 | F | PICA left | 3.1 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-2 | 33 | F | PICA right | 9 days | 4 | 4 | 0 | 0 | 0 |
| cer-foc-3 | 36 | M | PICA left | 1.6 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-4 | 41 | M | SCA left, PICA right | 8.6 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-5 | 43 | M | SCA right | 2.3 years | 2 | 1 | 1 | 0 | 0 |
| cer-foc-6 | 44 | F | SCA right | 26 days | 1 | 1 | 0 | 0 | 0 |
| cer-foc-7 | 47 | F | PICA right | 10.1 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-8 | 48 | M | SCA right | 1.3 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-9 | 49 | M | PICA left | 8 months | 0 | 0 | 0 | 0 | 0 |
| cer-foc-10 | 50 | M | PICA right | 8.6 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-11 | 51 | M | PICA left | 6 months | 4 | 1 | 3 | 0 | 0 |
| cer-foc-12 | 52 | M | PICA left | 9.4 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-13 | 54 | M | PICA left | 1.9 years | 4 | 4 | 0 | 0 | 0 |
| cer-foc-14 | 55 | M | SCA left | 2.9 years | 3.5 | 1.5 | 2 | 0 | 0 |
| cer-foc-15 | 55 | M | PICA left | 36 days | 0.5 | 0.5 | 0 | 0 | 0 |
| cer-foc-16 | 56 | M | SCA left | 7.2 years | 4 | 1 | 3 | 0 | 0 |
| cer-foc-17 | 56 | M | PICA right | 11.9 years | 1 | 1 | 0 | 0 | 0 |
| cer-foc-18 | 56 | F | PICA left | 1 year | 2 | 1 | 1 | 0 | 0 |
| cer-foc-19 | 57 | M | SCA left | 11.9 years | 0 | 0 | 0 | 0 | 0 |
| cer-foc-20 | 60 | M | SCA right | 6 years | 4 | 1.5 | 2.5 | 0 | 0 |
| cer-foc-21 | 60 | M | SCA left | 28 days | 2.5 | 2.5 | 0 | 0 | 0 |
| cer-foc-22 | 62 | F | PICA right | 56 days | 2.5 | 1 | 1.5 | 0 | 0 |
| cer-foc-23 | 76 | M | SCA right | 9.7 years | 2 | 0 | 2 | 0 | 0 |
| cer-foc-24 | 49 | M | Haemorrhage right | 180 days | 34 | 15 | 11.5 | 1.5 | 6 |
| cer-foc-25 | 75 | M | PICA left | 13 days | 6.5 | 4 | 2.5 | 0 | 0 |
| cer-foc-26 | 44 | M | SCA right | 23 days | 13.5 | 5.5 | 7 | 1 | 0 |
| cer-foc-27 | 72 | M | SCA right | 9 days | 2 | 1 | 0.5 | 0.5 | 0 |
SCA6 = spinocerebellar ataxia type 6; SCA14 = spinocerebellar ataxia type 14; SAOA = sporadic adult onset ataxia; ADCA III = autosomal dominant ataxia type III (a pure cerebellar disorder with autosomal dominant inheritance and inconclusive genetic testing); SCA = superior cerebellar artery; PICA = posterior inferior cerebellar artery; ICARS = International Cooperative Ataxia Rating Scale (Trouillas et al., 1997); total ICARS (maximum total score) and ICARS subscores (maximum subscore) are shown; kinetic function = upper and lower limb ataxia; M = male; F = female.
Twenty-four age- and sex-matched healthy subjects (nine female, 15 male; mean age 52.2 ± 10.1 years; range 33–74 years), without evidence of neurological deficits based on history and neurological examination served as controls. To assess age-related effects, an additional group of 31 young healthy subjects was included (16 female, 15 male; mean age 23.4 ± 2.3 years; range 21–30 years).
All patients were examined by an experienced neurologist (D.T.). Ataxia was rated using the International Cooperative Ataxia Rating Scale (ICARS; Trouillas et al., 1997). None of the patients had signs of extracerebellar involvement except brisk patellar reflexes and mild signs of pallhypesthesia at medial malleolus in a fraction of patients with cerebellar degeneration. All subjects gave written informed consent prior to participation. The study was approved by the local Ethics Committee of the University Clinic Essen.
Visual threat eye-blink response paradigm
Experimental set-up was based on the visual threat eye-blink response paradigm initially introduced by Bracha et al. (1997). In brief, eye closure is measured while a ball is moving towards and hitting the subject’s face. The visual stimulation of the ball moving toward the subject’s head is considered as the conditioned stimulus, and the impact of the ball as the unconditioned stimulus. The duration of the conditioned stimulus was ∼445 ms, and the duration of the unconditioned stimulus was ∼22 ms. Eye closure in a fixed time interval before ball hit were considered conditioned responses (conditioned eye-blink response), eye closure after the ball hit the unconditioned response.
During the experiment, subjects were seated comfortably at a table. The head was supported by a chin rest. Table and chin rest were height-adjustable. Height of the chin rest was adjusted in such a way that the ball would hit the midline of the forehead. A tennis ball (diameter 65 mm, mass 44 g) was attached to a 460 mm long rod. The rod was hold by a motor in front of the subject’s forehead (Fig. 1). Switching off the motor released the ball, which moved in free-fall towards the subject and hit the forehead of the subject. The motion of the ball accelerated from zero to a maximum of 1.34 m/s at the moment of impact (estimated kinetic energy 0.04 J). After each trial, the ball was moved back to its starting position in front of the subject’s forehead with the help of the motor. The experiment was controlled by a PC using a custom-written program in NI DIAdem (version 10.2, National Instruments, http://www.ni.com/diadem/). Subjects wore headphones. A continuous white noise of 56 dB SPL was applied bilaterally to mask environmental noise.
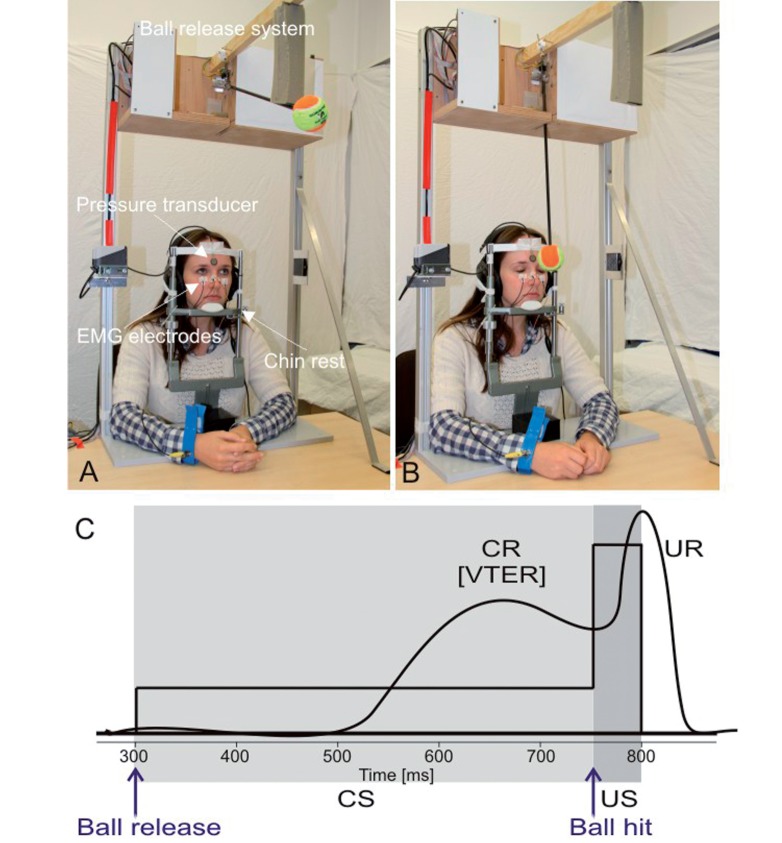
Figure 1.
Visual threat eye-blink response paradigm. (A) Picture of the experimental set-up before ball release. Ball is in its starting position. (B) Picture after ball release, but before ball hit on the forehead. Eyes are already closed representing the visual threat eye-blink response (VTER). (C) Schematic drawing of the paradigm. Conditioned stimulus (CS, ball moving towards the face) is indicated in light grey, unconditioned stimulus (US, ball hitting the forehead) in dark grey. For further details see text. CR = conditioned eye-blink response; UR = unconditioned response.
The exact duration of the conditioned stimulus varied between 430 and 456 ms (mean 444 ± 6 ms). Duration showed slight variations because of slight shifts of the subject’s head on the chin rest. The time of the ball’s impact was measured using a miniature pressure transducer (FSR 402 round force sensing resistor; Electrotrade GmBH) attached to the subject’s forehead during each trial.
Subjects were presented with 20 trials. The intertrial interval varied pseudorandomly between 15 s and 25 s. Surface electromyography (EMG) recordings were taken from orbicularis oculi muscles on both sides with electrodes fixed to the lower eyelid and to the nasion. Signals were fed to EMG amplifiers (sampling rate 1000 Hz, band pass filter frequency between 100 Hz and 2 kHz) and full wave rectified. Signals were recorded for 2000 ms beginning 300 ms before the onset of the conditioned stimulus and stored for off-line analysis.
Before the experiment subjects were informed that the ball would move forward and may hit their face. Subjects were instructed to look straight ahead and to avoid voluntary blinking.
Conditioned eye-blink responses were semi-automatically identified within the conditioned stimulus–unconditioned stimulus interval using custom-made software (Gerwig et al., 2010). Rectified EMG recordings were filtered using a series of non-linear Gaussian filters and further filtered offline (100 Hz). Response onset was defined where EMG activity reached 7.5% of the EMG maximum in each recording with a minimum duration of 30 ms and a minimum integral of 5 mV/ms. Trials were visually inspected and implausible identification of conditioned eye-blink responses was manually corrected. Trials with spontaneous blinks occurring before conditioned stimulus-onset were excluded from the analysis. Responses occurring within the 130 ms interval after conditioned stimulus-onset were considered as reflexive responses (i.e. alpha-responses) and not conditioned responses (Bracha et al., 1997). The percentage of conditioned eye-blink responses (and alpha-blinks) out of the trials without spontaneous blinks was calculated. Conditioned eye-blink response incidences were averaged across the right and left eyes.
The response latency and the response peak time were measured in both eyes in each trial. Conditioned eye-blink response onset latency and peak time were expressed as time following conditioned stimulus onset. Unconditioned response onset latency and peak time were expressed as time following unconditioned stimulus onset. Conditioned eye-blink response and unconditioned response timing parameters were averaged across trials, and the right and left eye. Because of individual differences in skin properties (e.g. skin thickness, thickness of underlying fatty layer) and muscle bulk, direct comparison of surface EMG amplitudes is not reliable. Normalization procedures are required, which have not been applied. Therefore, EMG amplitudes were not further considered.
Conditioned eye-blink response incidences, incidences of alpha blinks, and conditioned eye-blink response and unconditioned response timing parameters were compared between groups (degenerative patients, patients with focal lesions, matched control subjects, young control subjects) using Kruskal-Wallis H-tests. For post hoc comparisons Mann-Whitney U-tests were applied. Correlation analysis was performed to assess possible age effects, and relationship between conditioned eye-blink response incidence and cerebellar volume using Spearman’s rank correlation coefficients. Non-parametric tests were used because distributions of conditioned eye-blink response incidences were not normally distributed in the group of patients with cerebellar degeneration and the young control group based on histograms and Kolmogorov-Smirnov tests. In patients with unilateral focal lesions parameters were compared between the ipsi- and contralesional eyes using ANOVA with repeated measures. The null hypothesis rejection level for all tests was P < 0.05. Greenhouse–Geisser adjustments were applied where appropriate.
Lesion-symptom mapping in patients with cerebellar degeneration
Conventional volumetry and voxel-based morphometry were used to investigate possible positive correlations between the degree of atrophy in patients with cerebellar degeneration and a reduced number of visual threat responses. Conventional volumetry has the advantage that no spatial normalization of individual cerebella is required in order to perform group analysis. Voxel-based morphometry requires normalization, but allows for voxel-based lesion–symptom maps with no predefined anatomical regions (Timmann et al., 2009). The mean value of conditioned responses based on both eyes was used for correlation analysis.
High-resolution 3D T1-weighted MPRAGE scans were obtained for each patient with cerebellar degeneration and age-matched control subjects (176 sagittal slices, repetition time = 2300 ms, echo time = 2.26 ms, inversion = 900 ms, bandwidth 200 Hz/pixel, field of view phase = 93.8%, field of view = 256 × 240 mm2, matrix 256 × 240, prepolarized MRI GRAPPA R = 2, acquisistion time = 5 min 11 s, flip angle 9°, slice thickness 1 mm; voxel size of 1 × 1 × 1 mm3) using a 3 T MRI scanner (Siemens Magnetom Skyra) with a 20-channel head/neck coil. In addition, 3D-FLAIR and 2D T2-weighted sequences were acquired. MPRAGE, FLAIR and T2-weighted images were visually examined by one of the authors, an experienced neuroradiologist (S.G.). None of the cerebellar subjects revealed extracerebellar pathology.
Conventional volumetry
MPRAGE images were used to calculate the volume of the entire cerebellum, the volume of the cerebrum and the total intracranial volume (total intracranial volume). Volumetric analysis was performed semi-automatically by an experienced technician with the help of ECCET-software (http://eccet.de/). Details of analysis have been reported previously (Brandauer et al., 2008; Weier et al., 2012). In brief, the MPRAGE volumes were first processed with a Gaussian noise reduction filter. Secondly, the brainstem was semi-automatically segmented and separated from the cerebellar peduncles, which were included in the cerebellar volume. Next the cerebellum was semi-automatically marked and then segmented with a 3D filling algorithm that is able to differentiate between brain tissue and surrounding CSF. The total intracranial volume included brain and CSF volumes extending caudally to the foramen magnum. Total intracranial volume was manually traced on every 10th of the 176 sagittal slices of the filtered MPRAGE images. Segmented single slices were connected with an interpolation module to form a single 3D segment containing the CSF and cortex. For measurement of the whole brain volume all the grey matter and white matter voxels belonging to the cerebellum, cortex and brainstem were first automatically marked on the initially filtered magnetic resonance volumes and then segmented with the same 3D filling algorithm used to segment the cerebellum. Cerebral volume was calculated by subtracting the volume of the cerebellum from the whole volume. For all statistical comparisons volumes of the cerebellum and cerebrum were expressed as percentage of total intracranial volume (% total intracranial volume = targeted volume/total intracranial volume × 100).
Voxel-based morphometry
We implemented a version of the standard voxel-based morphometry method (Ashburner and Friston, 2005) using SUIT normalization to morph the individual’s cerebellum into the SUIT atlas space (http://www.icn.ucl.ac.uk/motorcontrol/imaging/suit.htm, Diedrichsen, 2006). All T1-weighted MRI scans were processed using the Spatially Unbiased Infratentorial (SUIT) toolbox, incorporated in SPM8 software package (Wellcome Department of Cognitive Neurology, London, UK). First, the cerebellum and brainstem were isolated from the MRI scans and exclusive cerebellar masks were created. The posterior fossa, which includes the cerebellum, was separated (cropped) from the rest of the brain. Next, a mask of the cerebellum was produced automatically. The algorithm to produce the mask is sometimes unable to differentiate between cerebellum and adjacent transverse sinus, bone marrow and parts of the overlaying occipital lobe (Diedrichsen, 2006). Therefore, masks need to be visually inspected and tissue not belonging to the cerebellum needs to be erased. This step was done using the program MRICroN (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Next, grey matter segmentation maps were generated, and a spatial normalization of the cerebellum to the SUIT template was performed. The resulting normalization parameters were used to reslice the grey matter segment into SUIT atlas space. A modulation of the grey matter segment was incorporated in order to compensate for volume changes during spatial normalization by multiplying the intensity value in each voxel with the Jacobian determinants. In order to preserve precision in the definition of cerebellar structures, a 4 mm default full-width at half-maximum Gaussian kernel was used for smoothing.
The processed images were analysed within the framework of the general linear model implemented in SPM8. The relationship between grey matter volume and (demeaned) conditioned eye-blink response incidence was investigated through a multiple regression analysis while controlling for age and total intracranial volume. A statistical height threshold of P < 0.001 (uncorrected for multiple comparisons; threshold t = 3.73) was set. An extent threshold of 50 contiguous voxels was included as partial correction. Anatomical localization of the cerebellar lobules was determined with the probabilistic MRI atlas of the human cerebellum (Diedrichsen et al., 2009).
Lesion-symptom mapping in patients with cerebellar stroke
T1-weighted MPRAGE, FLAIR and T2 scans were obtained in 18 stroke patients using a 3 T MRI scanner (Siemens Skyra; for parameters see ‘Cerebellar degeneration’ section). All patients had chronic disease (time since stroke > 6 months, Table 1). Eight patients presented with acute and subacute lesions (time since stroke < 6 months). In acute/subacute patients and one chronic patient (Patient cer-foc-24), the 3 T MRI scanner was not available at the time, and MRI scans were acquired on a 1.5 T MRI scanner (Siemens Avanto). Two of the eight patients declined study-related MRI scanning because of claustrophobia or tattoos (Patients cer-foc-2 and cer-foc-22). Diagnostic T1, T2 and FLAIR MRI scans were used instead. Extracerebellar pathology was excluded in all patients.
Cerebellar lesions of stroke patients were manually traced on axial, sagittal and coronal slices of the non-normalized MPRAGE data set and saved as regions of interest using MRIcroN software. FLAIR images were coregistered to the MPRAGE images. Regions of interest were adjusted based on lesion extent in FLAIR images where appropriate. Regions of interest were normalized using the spatially unbiased infra-tentorial template of the cerebellum (SUIT; Diedrichsen, 2006) using the SUIT toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) as outlined above. Normalization parameters were used to reslice the regions of interest from the individual participants into SUIT atlas space. In the two patients, who received diagnostic scans only, lesions were copied by drawing directly in the SUIT template. Diagnostic scans were not normalized because of coarse slice thickness (>3.3 mm). Superposition of individual lesions showed that lesions were equally distributed between superior and inferior parts of the cerebellum with maxima of lesion overlap in lobules V (n = 10), VIIb (n = 11) and VIIIa (n = 12).
Lesion-symptom mapping was performed with the help of MRIcroN. To allow inclusion of all 27 patients despite the wide range of time since lesion onset, decision was made to perform subtraction analysis as the main analysis tool for lesion–symptom mapping. Here, patients are considered either normal or abnormal based on a behavioural cut-off. Thus, no graduation of the behavioural abnormality is done. This is important in studies including patients with more acute disorders, because degree of abnormality may reflect the stage of recovery, but not differences in lesion localization (Rorden and Karnath, 2004).
The lesion maps for all left-sided lesions were flipped along the midline. Patients were divided in two subgroups depending on their behavioural performance on the ipsilesional side. In order to create a group of ‘unimpaired' and ‘impaired' patients, threshold was defined as conditioned eye-blink response incidences below the mean minus 1.5 standard deviations in the age-matched control group (<25%, see ‘Results’ section). Seven patients were considered impaired, 20 unimpaired. Subtraction analysis in MRIcroN subtracts for each lesioned voxel the percentage of unimpaired patients with a lesion in that voxel from the percentage of impaired patients with a lesion in that voxel (Karnath et al., 2002; Donchin et al., 2012). For example, in case 80% of the impaired patients and 40% of the unimpaired patients are lesioned for a voxel, then subtraction of the two numbers gives 40% consistency. Voxels that were at least 25% more likely to be lesioned in impaired patients were considered.
Because subtraction analysis is descriptive, the Liebermeister test was applied for statistical support. The Liebermeister test is a binomial test. Similarly to subtraction analysis, patients are grouped as normal or abnormal based on the behavioural cut-off (Rorden et al., 2007). Results are reported at a threshold of P < 0.05 false discovery rate (FDR) corrected for multiple comparisons. The Liebermeister test was performed using the non-parametric mapping software (NPM) as part of MRIcroN. Only voxels damaged in at least 10% of individuals (n = 3) were considered. Based on the findings of voxel-based morphometry analysis in patients with cerebellar degeneration a region of interest analysis was performed. As explicit mask the cerebellar areas were defined, which were found to be related to visual threat eye-blink response storage in voxel-based morphometry analysis at a liberal threshold of P < 0.05 uncorrected.
Results
Conditioned eye-blink response incidence comparing patient and control groups
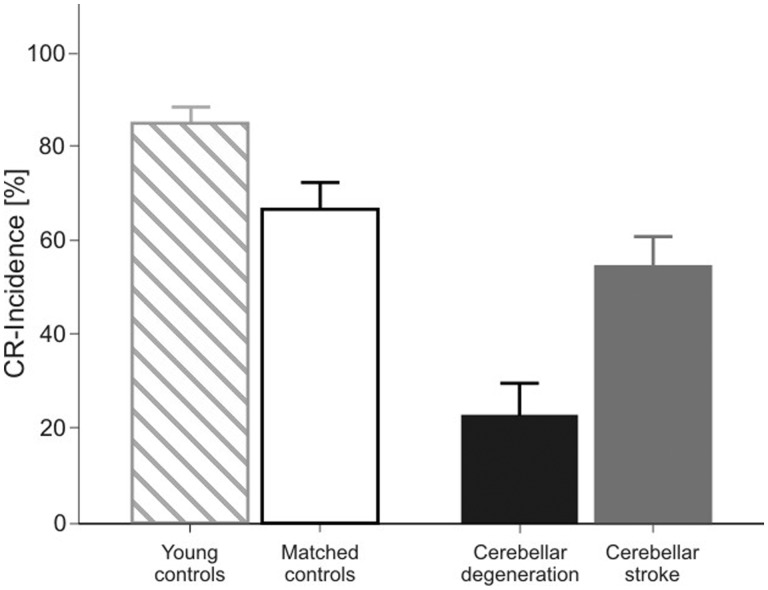
Figure 2 shows mean conditioned eye-blink response incidence in the four groups of subjects. Comparing the percentage of conditioned eye-blink responses between the group of patients with cerebellar degeneration and age-matched control subjects showed a marked reduction in patients (23.8% SD 31.1 versus 67.2% SD 28.4). Mean conditioned eye-blink response incidence was also reduced in focal-lesion patients (55.3% SD 31.8), but their deficit was small compared to patients with cerebellar degeneration. Compared with aged-matched control subjects, the younger control subjects showed a higher mean conditioned eye-blink response incidence (84.9% SD 18.8). Findings are further illustrated in Fig. 3 showing EMG traces from individual subjects. Whereas the younger-aged control showed conditioned eye-blink responses in all 20 trials, fewer conditioned eye-blink responses were produced by the age-matched control and stroke patient. Conditioned eye-blinks were absent in the patient with cerebellar degeneration.
Figure 2.
Mean conditioned eye-blink response (CR) incidence (plus standard error) of the visual threat eye-blink response for younger-aged controls (hatched columns), age-matched controls (open column), patients with cerebellar degeneration (black column) and patients with focal lesions due to stroke (grey column). Conditioned eye-blink response incidence represents the mean of both eyes.
Figure 3.
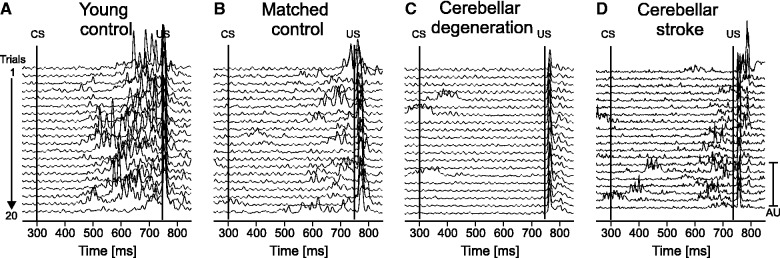
Rectified EMG recordings of orbicularis oculi muscle in (A) a young control (24 years old, male), (B) a matched control (59 years old, male), (C) a patient with cerebellar degeneration (Patient cer-deg-8 in Table 1) and (D) a patient with cerebellar stroke (Patient cer-foc-11 in Table 1). Recordings of the right eye are shown in A–C, of the left eye in D (ipsilateral to the lesion). Each line represents one trial. All 20 trials are shown with the first on top, and the last on bottom of each stack plot. The first line indicates the onset of the conditioned stimulus (CS, ball begins to move) and the second line the onset of the unconditioned stimulus (US, ball touches the forehead).
Conditioned eye-blink response incidence differed significantly among the four groups [H(3) = 36.5; P < 0.001; Kruskal-Wallis H test]. Mann-Whitney U-tests revealed significant group effects when comparing patients with cerebellar degeneration to age-matched control subjects (U = 66; P < 0.001) and when comparing the age-matched and younger-aged controls (U = 229.5; P = 0.015), but not when comparing patients with focal lesions to age-matched control subjects (U = 252; P = 0.174). When pooling all control subjects (n = 55), conditioned eye-blink response incidence correlated negatively with their age (rho = -0.261, P = 0.027 one-tailed; Spearman’s rank correlation coefficient; R = 3.74, P = 0.005; linear regression; Fig. 4).
Figure 4.
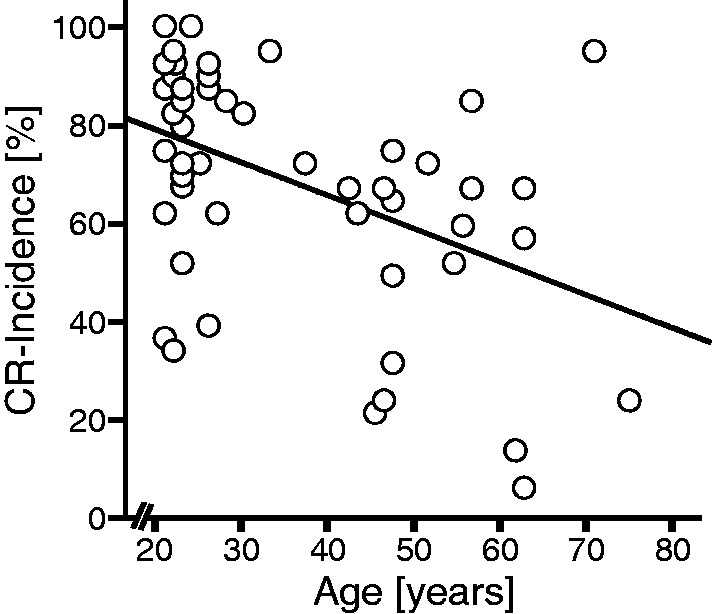
Scatterplot comparing conditioned eye-blink response (CR) incidence and age within the group of all control subjects (n = 55). Linear regression line is also shown (R = 3.74, P = 0.005).
There were no significant correlations between total International Cooperative Ataxia Rating Scale or any of the subscores and conditioned eye-blink response incidence in patients with cerebellar degeneration and patients with focal lesions (all P-values > 0.07 one-tailed; Spearman’s rank correlation coefficient).
Conditioned eye-blink response incidence in patients with unilateral stroke lesions
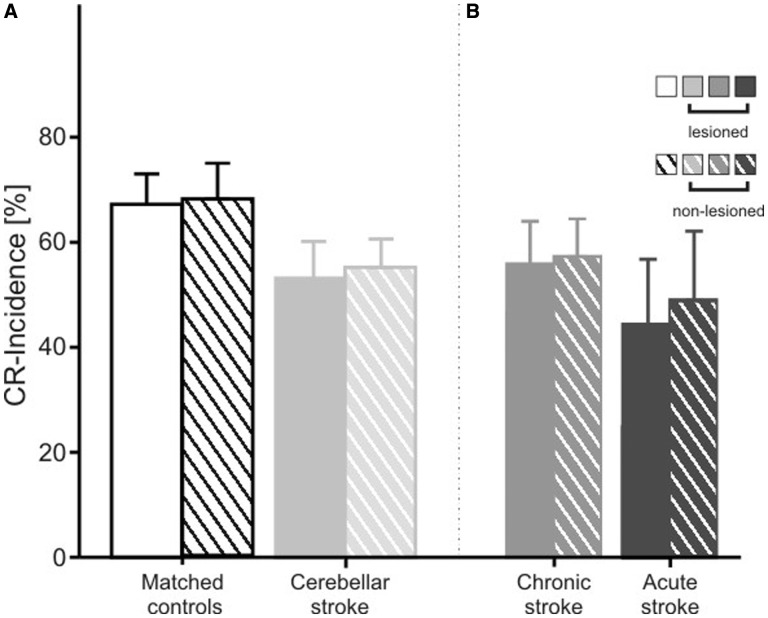
In stroke patients lesions were unilateral in all cases except one (patient cer-foc-4 in Table 1). Because unilateral lesions may primarily affect the ipsilesional side, additional analysis compared the lesioned and non-lesioned sides in the 26 unilateral patients and matched sides in controls (that is, for a lesion on the left, the left eye of the control was matched to the lesioned and the right eye to the non-lesioned eye of the patient). Mean conditioned eye-blink response incidence tended to be lower in focal patients compared to matched control subjects on both sides [Fig. 5A; F(1,48) = 2.53, P = 0.118]. There was no significant difference between the lesioned and non-lesioned side and no side-by-group interaction (P-values > 0.5).
Figure 5.
Mean conditioned eye-blink response (CR) incidence (plus standard error) of the visual threat eye-blink response (A) in matched control subjects (n = 24), all patients with focal lesions due to stroke (n = 26), and (B) the subgroups of patients with chronic (n = 18) and acute/subacute stroke (n = 8). In focal patients, mean conditioned eye-blink response incidence is shown for the eye ipsilateral to the lesion and the eye contralateral to the lesion. Sides were matched in the controls. Filled columns refer to the lesioned eye and hatched columns to the non-lesioned eye. Only data from the 26 patients with a unilateral lesion are shown.
Because time since stroke differed among stroke patients, and disorders may be more prominent in more acute patients with less time for recovery, stroke patients were subdivided into two groups. Studies of reorganization following cerebellar stroke are sparse. Clinical observations, however, indicate that most changes occur within the first weeks following the insult. In patients 6 months after stroke, changes may still occur, but they are thought to be small (Schoch et al., 2007; Konczak et al., 2010). In accordance with the cerebral stroke literature, patients with time since insult >6 months were considered chronic (n = 19; see Table 1). In the remaining eight patients with more acute lesions stroke had occurred between 9 and 56 days since stroke. Figure 5B shows that the reduction in conditioned eye-blink response incidence was most marked in the patients with acute lesions. Reduced conditioned eye-blink response incidence occurred on both the lesioned and non-lesioned side, but appeared to be more prominent on the lesioned side. ANOVA revealed a significant difference comparing acute patients and matched control subjects [F(1,14) = 5.1, P = 0.04], but not between chronic patients and matched control subjects [F(1,34) = 0.91, P = 0.345]. Side effects and group × side interactions did not reach significance (all P-values > 0.5).
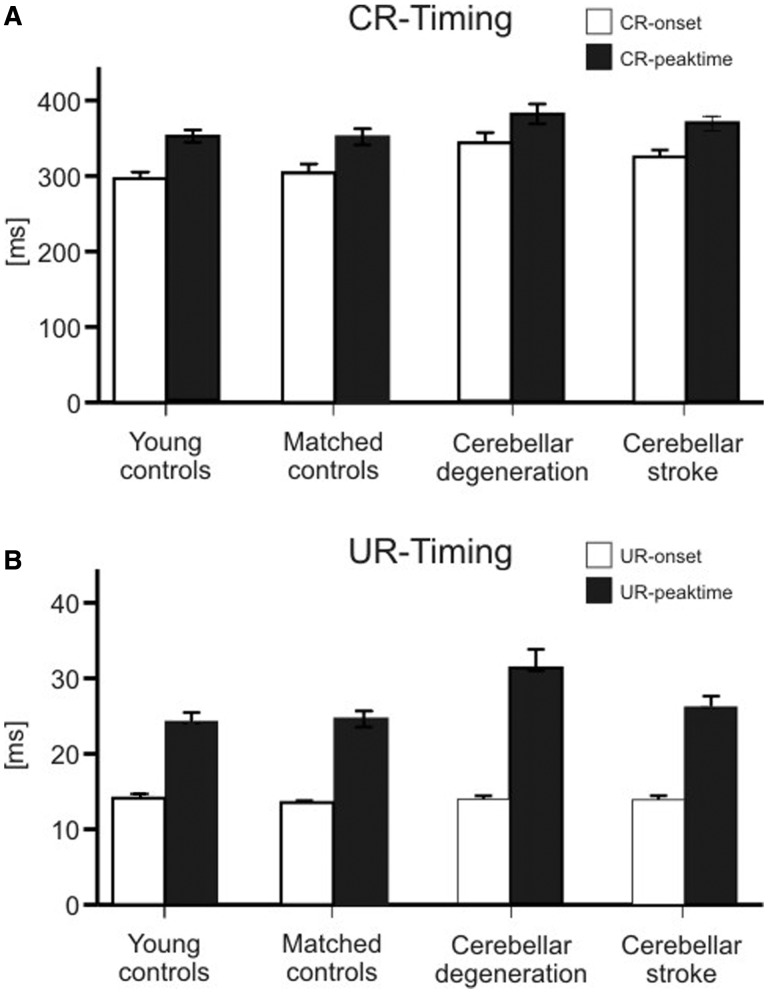
Conditioned eye-blink response and unconditioned response timing
Mean latency for conditioned eye-blink response onset was slightly longer [H(3) = 11.2, P = 0.011; Kruskal-Wallis H test], and mean conditioned eye-blink response peaktime tended to occur later [H(3) = 4.8, P = 0.18], in cerebellar patients compared with controls (Fig. 6A). Post hoc comparisons comparing patients with cerebellar degeneration to age-matched controls, patients with focal cerebellar lesions to age-matched controls, and age-matched controls to younger-aged controls did not reveal significant differences (all P-values > 0.05; Mann-Whitney U-test).
Figure 6.
Conditioned eye-blink response (CR, A) and unconditioned response (UR, B) timing parameters (mean and standard error) in the group of younger-aged control subjects, age-matched control subjects, patients with cerebellar degeneration and cerebellar stroke. Conditioned eye-blink response onset and peak time are expressed as time (ms) after conditioned stimulus onset. Unconditioned response onset and peak time are expressed as time (ms) after unconditioned stimulus onset. White columns = onset latency; filled columns = peak time.
Mean latencies of unconditioned response onset were not different between groups. Unconditioned response peak time was later in patients with cerebellar degeneration than in controls, with no difference between focal patients and controls (Fig. 6B). The group effect was not significant considering unconditioned response onset [H(3) = 4.4, P = 0.21], but was significant considering unconditioned response peaktime [H(3) = 8.58, P = 0.035; Kruskal-Wallis H-test]. Post hoc comparison showed that unconditioned response peaktime was significantly later in patients with cerebellar degeneration compared with control subjects [U = 136, P = 0.024; Mann-Whitney U-test]. All other comparisons were not significant (all P-values > 0.05; Mann-Whitney U-test].
Alpha blinks
The incidence of alpha blinks was reduced in patients (cerebellar degeneration: 7.6% SD 17.4; cerebellar stroke: 14.4% SD 19.1) compared with control subjects (age-matched control subjects: 19.7% SD 17.6; young control subjects: 20.1% SD 19.8). The group difference was significant [H(3) = 15.24, P = 0.002; Kruskal-Wallis H test]. Post hoc comparison revealed a significant difference between patients with cerebellar degeneration and age-matched control subjects (U = 91.0; P = 0.001; Mann-Whitney U-test), but not between patients with cerebellar stroke and matched control subjects, nor between younger-aged and matched control subjects (P-values > 0.14). The mean onset and peak time of alpha responses were not significantly different between groups (P-values > 0.1; Kruskal-Wallis H-test).
Lesion–symptom mapping in patients with cerebellar degeneration
We were able to perform lesion–symptom mapping in patients with cerebellar degeneration because conditioned eye-blink responses were severely reduced, but not absent. The conditioned eye-blink response incidence was above their spontaneous blink rate (21.8 SD 17.5 blinks per minute, assessed 1 min before and after the experiment): conditioned eye-blink response incidence (mean 23.8%) was quantified in a time interval of ∼ 315 ms per trial (see ‘Materials and methods’ section). Assuming spontaneous blinks are random, there would be one blink per 2.75 s. One would expect ∼0.11 blinks per 315 ms conditioned eye-blink response interval, which equals 11% incidence.
Conventional volumetry
As expected, volume of the cerebellum was significantly reduced in patients with cerebellar degeneration (6.6% SD 0.93 of total intracranial volume) compared with age-matched control subjects (8.8% SD 0.62 total intracranial volume; U = 8, P = 0.001, Mann-Whitney U-test). There was no significant difference comparing volume of the cerebrum between groups (cerebellar degeneration: 84.8% SD 2.0 total intracranial volume; control subjects: 83.56% SD 4.3 total intracranial volume; U = 163, P = 0.62). Among all subjects, conditioned eye-blink response incidence was positively correlated with volume of the whole cerebellum (rho = 0.736, P < 0.001 one-tailed; Spearman’s rank correlation coefficient), but not the cerebrum (rho = −0.2, P = 0.114; Fig. 7). Likewise, significant positive correlations with volume of the whole cerebellum, but not the cerebrum, were found when considering patients and control subjects separately [whole cerebellum % total intracranial volume: rho = 0.464, P = 0.023 (cerebellar degeneration), rho = 0.516, P = 0.012 (control subjects); cerebrum % total intracranial volume: rho = −0.225, P = 0.177 (cerebellar degeneration), rho = −0.142, P = 0.28 (control subjects)].
Figure 7.
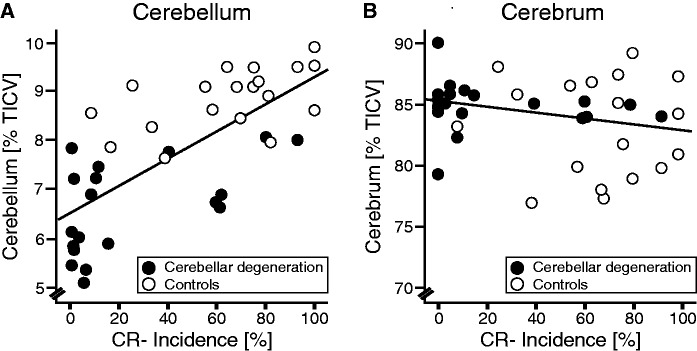
Scatterplots comparing conditioned eye-blink response (CR) incidence and total cerebellar volume (A), and cerebral volume (B) in patients with cerebellar degeneration (filled circles) and matched control subjects (open circles). All volumes are expressed in percentage of total intracranial volume (% total intracranial volume, TICV). Linear regression lines are shown considering both patients and control subjects (cerebellar volume: R = 0.718, P < 0.001; cerebral volume: R = 0.246, P = 0.136).
In the age-matched control subjects, there was no significant correlation between cerebellar volume and age (whole cerebellum % total intracranial volume: rho = 0.043, P = 0.43; cerebellar cortex % total intracranial volume: rho = 0.97, P = 0.361; Spearman rank correlation). Note, however, that no MRI scans had been performed in the young control group. In a previous study of our group including 63 control subjects with a more extended age range (22–71 years) a significant negative correlation between cerebellar volume and age was found (Dimitrova et al., 2006).
Voxel-based morphometry
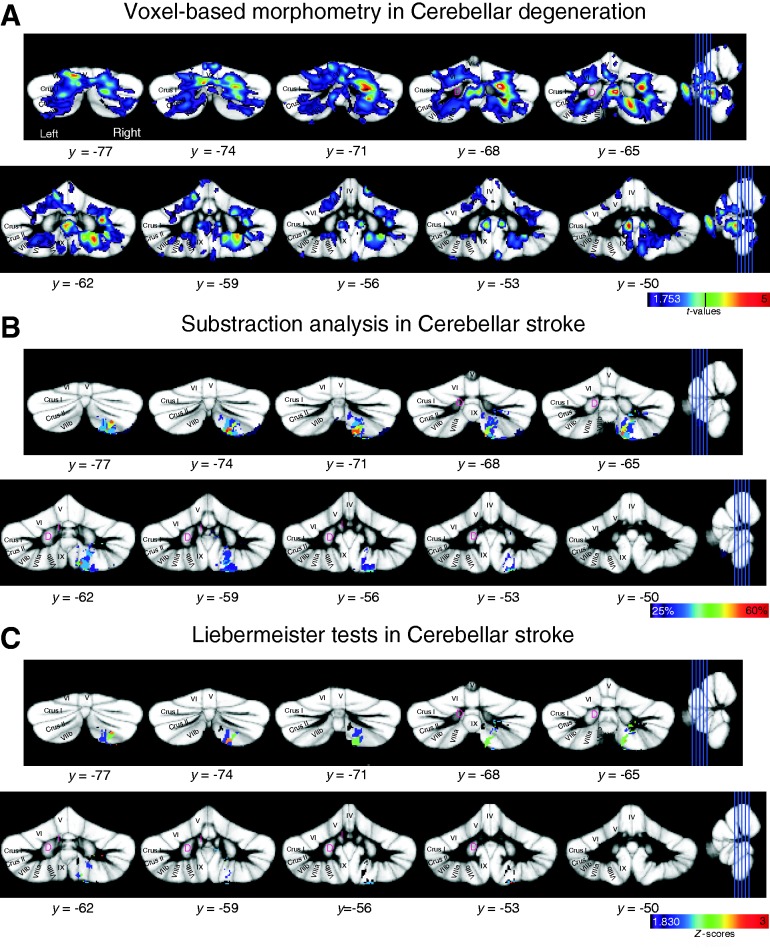
In patients with cerebellar degeneration, we found positive correlations between conditioned eye-blink response incidence and grey matter volume within the cerebellar posterior lobe bilaterally. On the right, a cluster was found in lobule VI extending into Crus I (Fig. 8A; see Table 2 for details). A second cluster was present in Crus II extending into lobule VIIb. Additional correlations were found in vermal and intermediate parts of lobule VIIIa, lobule VIIIb and IX. On the left, a positive correlation was found in intermediate parts of lobule VI proximal to Crus I. In addition, grey matter volume reduction was correlated with decreased conditioned eye-blink response incidence in vermal lobule IX.
Figure 8.
Results of lesion–symptom mapping superimposed on the SUIT probabilistic atlas template (Diedrichsen et al., 2009). Y-values indicate the coordinate in SUIT space. (A) Voxel-based morphometry in patients with cerebellar degeneration. Cerebellar areas are shown with a positive correlation between conditioned eye-blink response incidence and grey matter values. Results are thresholded at P < 0.05, uncorrected. Colour code indicates t-values, and t-value corresponding to P < 0.001 uncorrected is indicated by a vertical line (t = 3.73). (B) Subtraction analysis in patients with focal lesions due to stroke. Cerebellar areas are shown that are more likely to be lesioned in patients with an abnormally low conditioned eye-blink response incidence. Colour code represents % consistency with a threshold of 25%. All lesions were flipped to the same side. (C) Liebermeister tests in patients with focal lesions due to stroke. Cerebellar areas are shown that are more likely to be lesioned in patients with an abnormally low conditioned eye-blink response incidence. Results are thresholded at P < 0.05 FDR corrected, Z-score = 1.83. Colour code indicates Z-scores. All lesions were flipped to the same side.
Table 2.
Summary of voxel-based morphometry analysis in patients with cerebellar degeneration
| Cerebellar lobule | Side | Peak coordinate (mm) |
kE | t-value | Z-score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| VIIb, Crus II | Right | 24 | −68 | −42 | 101 | 6.66 | 4.48 |
| VI, Crus I | Right | 23 | −71 | −34 | 422 | 5.39 | 3.96 |
| Vermal VIIIa | Right | 5 | −65 | −36 | 136 | 5.25 | 3.90 |
| Vermal IX | Left | −4 | −51 | −37 | 72 | 4.89 | 3.73 |
| VI | Left | −14 | −79 | −20 | 59 | 4.82 | 3.69 |
| VIIIa | Right | 19 | −64 | −48 | 241 | 4.63 | 3.60 |
| VIIIb, IX | Right | 15 | −42 | −48 | 84 | 4.48 | 3.51 |
Positive correlations between conditioned eye-blink response incidence and grey matter volume are thresholded at P < 0.001 (partially corrected with an extent threshold of 50 contiguous voxels). Anatomical locations of peak voxels in SUIT space (x, y, z), cluster extent (kE) and peak t-values and Z-scores are listed. L = left; R = right.
Lesion-symptom mapping in patients with cerebellar stroke
Considering all patients with focal lesions, subtraction analysis revealed that patients with a reduced conditioned eye-blink response incidence were more likely to have a lesion within the inferior posterior lobule. The main area was found in the more intermediate parts of the cerebellum and extended from Crus II to lobules VIIb, VIIIa and VIIIb (maximum 56%; x = 15 mm, y = −73 mm, z = −58 mm; Fig. 8B). Smaller areas were found in Crus II at the border of Crus I (maximum 38%; x = 27 mm, y = −68 mm, z = −39 mm), and in lobule VIIB extending into Crus II (maxima 29%; x = 40 mm, y = −70 mm, z = −55 mm; 28%; x = 10 mm, y = −71 mm, z = −43 mm). More correlations were found with lesions in the dentate nucleus (33%; x = 16 mm, y = −59 mm, z = −43 mm) and interposed nucleus (29%; x = 5 mm, y = −60 mm, z = −32 mm).
Relationships between similar cerebellar areas and reduced conditioned eye-blink response incidence were found using Liebermeister tests for binomial data at a threshold of P < 0.05 FDR corrected for multiple comparisons (Z-score = 1.83; Fig. 8C). The main area was found extending from Crus II to lobules VIIb, VIIIa, and VIIIb (maximum Z-score = 2.80; x = 15 mm, y = −57 mm, z = −45 mm), with additional smaller areas in Crus II bordering Crus I (maximum Z-score = 2.23; x = 27 mm, y = −68 mm, z = −39 mm) and dentate nucleus (Z-score = 2.23; x = 16, y = −59, z = −43 mm).
Considering only patients with chronic lesions (4 of 19 showed abnormal values) revealed comparable areas. Again the main area was found extending from Crus II to lobules VIIb, VIIIa and VIIIb (maximum 68%; P < 0.05 FDR corrected, Liebermeister test). The subgroup of patients with acute lesions was small (three of eight showed abnormal behaviour) and did not allow for meaningful subtraction analysis.
Discussion
We found that the visual threat response, a conditioned eye-blink response, which is naturally acquired in early childhood, was significantly reduced in patients with adult-onset cerebellar degeneration. Impaired acquisition of conditioned eye-blink responses has consistently been shown in various human cerebellar lesion studies (Daum et al., 1993; Topka et al., 1993; Bracha et al., 1997; Gerwig et al., 2003, 2010). In agreement with the animal literature, the present findings provide evidence that the human cerebellum is involved in storage-related processes of conditioned responses as well.
Age effects are also consistent with the cerebellar involvement in storage of previously learned responses. In a larger group of healthy subjects that included an additional group of younger-aged control subjects, a significant reduction of visual threat responses was found with increasing age. A similar age-dependent decline has been found for the acquisition of conditioned eye-blink responses in previous studies (Woodruff-Pak and Thompson, 1988), and has been shown to be related to an age-dependent decline of Purkinje cells within the cerebellar cortex (Woodruff-Pak et al., 1990, 2001).
Similar to the previous observation, we found that visual threat responses were largely preserved in patients with focal lesions of the cerebellum (Bracha et al., 1997). Differences in aetiology likely explain differences in findings between the two patient groups. One main difference is that patients with cerebellar degeneration have more diffuse disorders of the cerebellum, whereas disorders are focal in patients with stroke. Another difference is that in degeneration the process is slowly progressive, whereas following a stroke some reorganization takes place. Although more extensive cerebellar injuries severely suppressed conditioned eye-blink response expression in cerebellar degeneration, visual threat responses were likely retained in focal disease because lesions only partly affected the critical areas. As outlined in more detail below, different areas within the posterior lobe bilaterally contribute to expression of the visual threat response.
Chronic focal lesions of the cerebellum, on the other hand, are sufficient to significantly reduce the acquisition of conditioned eye-blink responses in humans (Bracha et al., 1997; Gerwig et al., 2003, 2010). Conditioned eye-blink response acquisition appears to be more sensitive to cerebellar injury, where partial damage to learning networks affects acquisition, but the remaining part of the circuit is sufficient for retention, retrieval and expression. In chronic focal lesions there is time for recovery and reorganization. In fact, in the present subgroup with chronic lesions, the incidence of visual threat responses was not significantly different from controls. Considering the subgroup of patients with acute or subacute focal lesions, however, revealed a significant reduction. There was no significant difference between the lesioned and non-lesioned side suggesting bilateral contribution of the cerebellum to visual threat eye-blink response storage. This conclusion is supported by the findings in patients with cerebellar degeneration. The most parsimonious explanation would be the bilateral (midline) nature of the unconditioned stimulus. Likewise, using a midline unconditioned stimulus, Bracha et al. (1997) found bilateral deficits of conditioned eye-blink response acquisition in focal cerebellar disease. Because the subgroup of patients with acute/subacute lesions was small, however, findings need to be confirmed in a larger group of patients.
Storage disorder was significantly related to the degree of atrophy in patients with cerebellar degeneration. Using conventional volumetry, a significant positive correlation was found with more atrophy being associated with a more severe reduction of learned responses. Voxel-based morphometry confirmed and extended these findings. Here, the degree of atrophy in bilateral areas of the posterior cerebellar cortex was related with expression deficit. There were two main regions, one in lobule VI bordering Crus I, and one more inferiorly, extending from Crus II to VIII and IX (Fig. 9). Lesion-symptom mapping in focal patients revealed a comparable area in the more inferior part of the posterior lobe.
Figure 9.
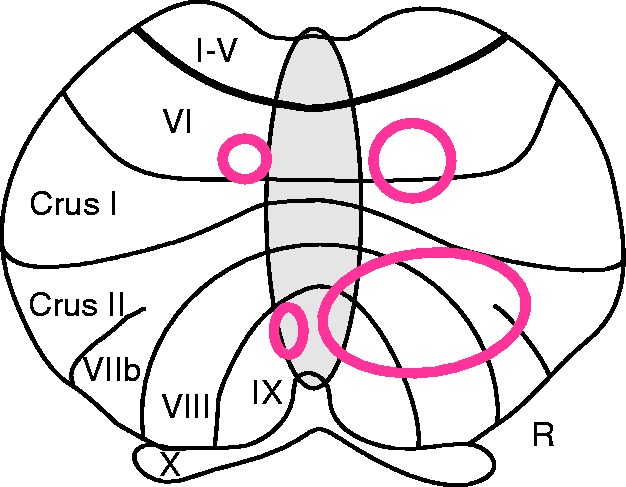
Summary diagram based on Fig. 8 superimposed on a flat map of the cerebellar cortex. Two main areas in the cerebellum are related to visual threat eye-blink response storage: one in lobule VI bordering Crus I, and one extending from lobule Crus II to VIII and IX. R = right.
Storage-related processes of conditioned eye-blink responses appear to take place within the same cerebellar areas known to be involved in the control of unconditioned eye-blinks. In very good accordance with the present findings, based on functional MRI in healthy human subjects, two main regions in the posterior lobe were found to be related to unconditioned eye-blinks, one in lobule VI and Crus I, and the other in lobules Crus II-VIIIa with an additional area in IX (Dimitrova et al., 2002). In their study in cats, Hesslow (1994) reported eye-blink related areas in intermediate and lateral lobule VI, in Crus I and VIIb. Hesslow could not investigate all cerebellar areas, and therefore, more posterior areas may contribute. Likewise, storage-related areas overlap with cerebellar cortical areas known to contribute to acquisition of the conditioned eye-blink response. Human cerebellar lesion studies show that lobule VI and Crus I are important (Gerwig et al., 2003, 2005). Animal studies of the cerebellar cortex focused on the anterior lobe and lobule VI. The anterior lobe has been related to timing of conditioned responses and lobule VI to conditioned stimulus-unconditioned stimulus association (Yeo et al., 1985a; Perrett et al., 1993; Green and Steinmetz, 2005). In the present study, timing parameters of both conditioned and unconditioned responses were delayed, making a specific timing disorder of visual threat responses unlikely. In earlier studies, Hardiman and Yeo (1992) reported that in addition to lobule VI, lobules Crus I and Crus II contribute to conditioned eye-blink response acquisition. Again, it has not been investigated whether circumscribed lesions of more posterior areas lead to disordered acquisition in animals. Thus, although lesions in VI are sufficient to impair acquisition of conditioned eye-blinks in both humans and animals, additional areas likely contribute. In fact, most PET and functional MRI studies in healthy human subjects show more extended areas (Blaxton et al., 1996; Knuttinen et al., 2002). Overall, the present data are in line with the animal literature that the same areas are involved in acquisition and retention of conditioned responses, and overlap with areas related to the unconditioned response (Hesslow, 1994; Kellett et al., 2010; Mostofi et al., 2010).
The interposed nuclei are well known to contribute to eye-blink conditioning, with the relative contributions of the cerebellar cortex and nuclei being a matter of ongoing discussion (McCormick and Thompson, 1984; Yeo et al., 1985b; Christian and Thompson, 2003). Reduced expression of conditioned eye-blink responses may at least in part be caused by associated lesions of the cerebellar nuclei. Cerebellar degeneration is generally considered a human lesion condition of the cerebellar cortex (Timmann et al., 2009). It cannot be excluded, however, that degeneration of the cerebellar cortex induces changes in the cerebellar nuclei. As yet, no method has been developed to perform lesion–symptom mapping at the level of the cerebellar nuclei in cerebellar degeneration. In the patients with focal lesions, parts of the dentate and interposed nuclei appeared to contribute to visual threat eye-blink response retention. The subset of focal patients affecting the interposed nuclei was small, and therefore, conclusions must be validated in future studies.
The present findings strongly support the cerebellum’s role in storage-related processes of learned motor associations. Some limitations of the study have to be acknowledged. Based on the present human lesion data alone, it cannot be decided whether the failure of patients to respond to visual threat is a problem with retention, retrieval or expression, or with a combination of these processes. In addition, it cannot be excluded that motor performance deficits of the eye-blink response contribute to reduction of visual threat eye-blink responses. Furthermore, it cannot be decided whether storage takes place within the cerebellum or these parts are needed to support storage in extracerebellar areas. Likewise, it cannot be excluded that cerebro-cerebellar diaschisis effects contribute to reduced visual threat responses (Baillieux et al., 2010; Komaba et al., 2000). Finally these data cannot distinguish between a single site involved in retention of conditioned responses or multiple learning sites (Christian and Thompson, 2003; Bracha et al., 2009).
The cerebral cortex likely contributes to different levels of control of visual threat responses. The prefrontal cortex, posterior parietal cortex and the visual cortex are known to be involved in the visual threat response in humans (Liu and Ronthal, 1992). Lesions in any of these areas are followed by absent or reduced visual threat responses. The more lateral areas of lobule VI and Crus I, and possibly VIIIa, are connected with the prefrontal cortex, including the premotor cortex and prefrontal eye field (Hashimoto et al., 2010; Buckner et al., 2011; Glickstein et al., 2011). Lobules Crus II and VIIb on the other hand are connected with the posterior parietal cortex (area 7; Clower et al., 2005; Prevosto et al., 2010). There are no known connections between the cerebellum and primary visual cortex. Visual association areas, however, are connected with lobule IX (Glickstein et al., 2011). Transforming visual information of the approaching threat into motor commands for eyelid closure likely depends on the posterior parietal cortex and connected areas in the premotor cortex. The cerebellum may support these processes. The primary motor cortex does not seem to be involved in visual threat responses, and it is unknown how the motor command finds its way to brainstem areas for eyelid closure (Liu and Ronthal, 1992). Primary facial motor areas in intermediate parts of cerebellar lobules VI and VIIIb may be involved. Findings are in good accordance with cerebellar areas found to contribute to other forms of motor behaviours. For example, the primary cerebellar hand areas in the anterior and posterior cerebellum together with Crus I/II in the posterolateral cerebellum are involved in visual control of reaching movements and visuomotor adaptation (Donchin et al., 2012; Taig et al., 2012).
In sum, the present data provide evidence that the human cerebellum is involved in storage of learned associations. Storage-related processes take place at least in part in the cerebellar cortex. The same cerebellar areas are also known to contribute to the acquisition of conditioned responses, and in executing the unconditioned response.
Acknowledgements
The authors like to thank Gary D. Zenitsky for careful editing of the manuscript.
Funding
The study was supported by a grant of the German Research Foundation (DFG TI 239/10-1; FOR 1581).
References
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–20. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, Mariën P. Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex. 2010;46:869–79. doi: 10.1016/j.cortex.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bastian AJ. Moving, sensing and learning with cerebellar damage. Curr Opin Neurobiol. 2011;21:596–601. doi: 10.1016/j.conb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, et al. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J Neurosci. 1996;16:4032–40. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V. Role of the cerebellum in eyeblink conditioning. Prog Brain Res. 2004;143:331–9. doi: 10.1016/S0079-6123(03)43032-X. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zbarska S, Parker K, Carrel A, Zenitsky G, Bloedel JR. The cerebellum and eye-blink conditioning: learning versus network performance hypotheses. Neuroscience. 2009;162:787–96. doi: 10.1016/j.neuroscience.2008.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Irwin KB, Bloedel JR. The human cerebellum and associative learning: dissociation between the acquisition, retention and extinction of conditioned eyeblinks. Brain Res. 2000;860:87–94. doi: 10.1016/s0006-8993(00)01995-8. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Wunderlich DA, Morrissy SJ, Bloedel JR. Patients with cerebellar lesions cannot acquire but are able to retain conditioned eyeblink reflexes. Brain. 1997;120:1401–13. doi: 10.1093/brain/120.8.1401. [DOI] [PubMed] [Google Scholar]
- Brandauer B, Hermsdörfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, et al. Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clin Neurophysiol. 2008;119:2528–37. doi: 10.1016/j.clinph.2008.07.280. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–45. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP'. Cereb Cortex. 2005;15:913–20. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993;107:748–56. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–74. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–38. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dimitrova A, Weber J, Maschke M, Elles HG, Kolb FP, Forsting M, et al. Eyeblink-related areas in human cerebellum as shown by fMRI. Hum Brain Mapp. 2002;17:100–15. doi: 10.1002/hbm.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, et al. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30:12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, et al. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol. 2012;107:134–47. doi: 10.1152/jn.00007.2011. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem. 2011;18:666–77. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain. 2003;126:71–94. doi: 10.1093/brain/awg011. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, et al. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci. 2005;25:3919–31. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Guberina H, Esser AC, Siebler M, Schoch B, Frings M, et al. Evaluation of multiple-session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res. 2010;212:143–51. doi: 10.1016/j.bbr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2011;47:59–80. doi: 10.1016/j.cortex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem. 2005;12:260–9. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman MJ, Yeo CH. The effect of kainic acid lesions of the cerebellar cortex on the conditioned nictitating membrane response in the rabbit. Eur J Neurosci. 1992;4:966–980. doi: 10.1111/j.1460-9568.1992.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Takahara D, Hirata Y, Inoue K, Miyachi S, Nambu A, et al. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur J Neurosci. 2010;31:1402–13. doi: 10.1111/j.1460-9568.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol. 1994;476:229–44. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–60. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Fukunaga I, Chen-Kubota E, Dean P, Yeo CH. Memory consolidation in the cerebellar cortex. PLoS One. 2010;5:e11737. doi: 10.1371/journal.pone.0011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaba Y, Osono E, Kitamura S, Katayama Y. Crossed cerebellocerebral diaschisis in patients with cerebellar stroke. Acta Neurol Scand. 2000;101:8–12. doi: 10.1034/j.1600-0404.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- Konczak J, Pierscianek D, Hirsiger S, Bultmann U, Schoch B, Gizewski ER, et al. Recovery of upper limb function after cerebellar stroke: lesion symptom mapping and arm kinematics. Stroke. 2010;41:2191–200. doi: 10.1161/STROKEAHA.110.583641. [DOI] [PubMed] [Google Scholar]
- Knuttinen MG, Parrish TB, Weiss C, LaBar KS, Gitelman DR, Power JM, et al. Electromyography as a recording system for eyeblink conditioning with functional magnetic resonance imaging. Neuroimage. 2002;17:977–87. [PubMed] [Google Scholar]
- Liu GT, Ronthal M. Reflex blink to visual threat. J Clin Neuroophthalmol. 1992;12:47–56. [PubMed] [Google Scholar]
- Mac Keith RC. The eye and vision in the newborn infant. In: Gardiner P, Mac Keith RC, Smith VH, editors. Aspects of developmental and pediatric ophthalmology. London: Spastics International Medical Publications in association with Heinemann; 1969. pp. 9–14. [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–9. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Mostofi A, Holtzman T, Grout AS, Yeo CH, Edgley SA. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J Neurosci. 2010;30:8920–34. doi: 10.1523/JNEUROSCI.6117-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–18. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–28. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:813–9. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Schoch B, Regel JP, Frings M, Gerwig M, Maschke M, Neuhäuser M, et al. Reliability and validity of ICARS in focal cerebellar lesions. Mov Disord. 2007;22:2162–9. doi: 10.1002/mds.21543. [DOI] [PubMed] [Google Scholar]
- Taig E, Küper M, Theysohn N, Timmann D, Donchin O. Deficient use of visual information in estimating hand position in cerebellar patients. J Neurosci. 2012;32:16274–84. doi: 10.1523/JNEUROSCI.1153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. A role for the cerebellum in learning movement coordination. Neurobiol Learn Mem. 1998;70:177–88. doi: 10.1006/nlme.1998.3846. [DOI] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–7. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Timmann D, Konczak J, Ilg W, Donchin O, Hermsdörfer J, Gizewski ER, et al. Current advances in lesion-symptom mapping of the human cerebellum. Neuroscience. 2009;162:836–51. doi: 10.1016/j.neuroscience.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Topka H, Valls-Solé J, Massaquoi SG, Hallett M. Deficit in classical conditioning in patients with cerebellar degeneration. Brain. 1993;116:961–9. doi: 10.1093/brain/116.4.961. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Weier K, Beck A, Magon S, Amann M, Naegelin Y, Penner IK, et al. Evaluation of a new approach for semi-automatic segmentation of the cerebellum in patients with multiple sclerosis. J Neurol. 2012;259:2673–80. doi: 10.1007/s00415-012-6569-4. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Classical conditioning. Int Rev Neurobiol. 1997;41:341–66. doi: 10.1016/s0074-7742(08)60359-1. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Cronholm JF, Sheffield JB. Purkinje cell number related to rate of classical conditioning. Neuroreport. 1990;1:165–8. doi: 10.1097/00001756-199010000-00020. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18-83 years. Psychol Aging. 1988;3:219–29. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, 3rd, Ewers M, Coffey J, Boyko OB, Lemieux SK. MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol Learn Mem. 2001;76:342–57. doi: 10.1006/nlme.2001.4026. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exp Brain Res. 1985a;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp Brain Res. 1985b;60:87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]