Abstract
Tobacco smoking has been linked to an increased risk of multiple sclerosis. However, to date, results from the few studies on the impact of smoking on the progression of disability are conflicting. The aim of this study was to investigate the effects of smoking on disability progression and disease severity in a cohort of patients with clinically definite multiple sclerosis. We analysed data from 895 patients (270 male, 625 female), mean age 49 years with mean disease duration 17 years. Forty-nine per cent of the patients were regular smokers at the time of disease onset or at diagnosis (ever-smokers). Average disease severity as measured by multiple sclerosis severity score was greater in ever-smokers, by 0.68 (95% confidence interval: 0.36–1.01). The risk of reaching Expanded Disability Status Scale score milestones of 4 and 6 in ever-smokers compared to never-smokers was 1.34 (95% confidence interval: 1.12–1.60) and 1.25 (95% confidence interval: 1.02–1.51) respectively. Current smokers showed 1.64 (95% confidence interval: 1.33–2.02) and 1.49 (95% confidence interval: 1.18–1.86) times higher risk of reaching Expanded Disability Status Scale scores 4 and 6 compared with non-smokers. Ex-smokers who stopped smoking either before or after the onset of the disease had a significantly lower risk of reaching Expanded Disability Status Scale scores 4 (hazard ratio: 0.65, confidence interval: 0.50–0.83) and 6 (hazard ratio: 0.69, confidence interval: 0.53–0.90) than current smokers, and there was no significant difference between ex-smokers and non-smokers in terms of time to Expanded Disability Status Scale scores 4 or 6. Our data suggest that regular smoking is associated with more severe disease and faster disability progression. In addition, smoking cessation, whether before or after onset of the disease, is associated with a slower progression of disability.
Keywords: smoking; multiple sclerosis; progression; disability, cessation
Introduction
Multiple sclerosis is a chronic inflammatory demyelinating disease of unknown aetiology characterized by a misdirected immune response affecting the CNS. Pathologically, multiple sclerosis is characterized by demyelination, inflammation and axonal damage within the brain and spinal cord. It is widely accepted that multiple sclerosis development and progression depend on a combination of genetic and environmental factors, which are currently incompletely known. The natural history varies widely amongst individuals and around the world. In general, at the disease onset an estimated 85–90% of patients have a relapsing-remitting clinical course that is characterized by clearly defined relapses with full or partial recovery, whereas the remaining 10–15% have primary-progressive multiple sclerosis, which represents a relentless progression from the beginning with no remission (Compston and Coles, 2008). Of the patients with relapsing-remitting multiple sclerosis, 80–90% eventually evolve to secondary-progressive multiple sclerosis within 10 to 20 years from onset (Weinshenker et al., 1989). The causes of transition and the interplay of risk factors are not entirely known. The average annual cost to the National Health Service of £30 263 per individual makes multiple sclerosis one of the most costly conditions in the UK (Kobelt et al., 2006; Orme et al., 2007; Manouchehrinia and Constantinescu, 2012). Given the fact that multiple sclerosis is a life-long chronic disease and with estimated prevalence rates between 84 and 203 per 100 000 population in the UK alone (Ford et al., 1998; Rothwell and Charlton, 1998), the impact of multiple sclerosis, in terms of the strain on health services as well as cost, is considerable. It is therefore important to identify means to prevent the onset, and slow the progression of multiple sclerosis.
Smoking is an avoidable exposure that has previously been linked with an estimated 50% increased risk of developing multiple sclerosis in case-control studies (Antonovsky et al., 1965; Ghadirian et al., 2001; Hernan et al., 2001; Riise et al., 2003, 2011; Hawkes, 2007; Hedstrom et al., 2009, 2011; Simon et al., 2010). However, it is not entirely clear whether smoking also influences the clinical course of the disease, the few studies addressing this issue having yielded conflicting results. The results of a recent meta-analysis of these studies examining the role of smoking in disease progression fell short of statistical significance and showed high heterogeneity (Handel et al., 2011). The possible correlation between smoking and disease progression in multiple sclerosis is of particular interest in view of reports on a negative correlation between smoking and some neurodegenerative conditions (e.g. Parkinson’s disease; Checkoway et al., 2002) and some autoimmune disorders (e.g. ulcerative colitis; Boyko et al., 1987). The evaluation of the magnitude of the effect of cigarette smoking on the clinical course of multiple sclerosis may help to determine underlying disease mechanisms and is important, as studies have reported a high percentage of smokers amongst patients with multiple sclerosis (Marrie et al., 2009). Here we examine the effects of smoking on the disability progression and explore the potential benefit of smoking cessation using data from a well-documented, substantial, clinical cohort of patients with multiple sclerosis.
Materials and methods
Setting and participants
The study population comprised patients registered in the largest East Midlands multiple sclerosis specialist clinic database at Nottingham University Hospital, which contains >98% of the identified patients with multiple sclerosis in Nottinghamshire and certain parts of Lincolnshire and Derbyshire. Patients in this registry must have the diagnosis of multiple sclerosis, clinically isolated syndrome or suspected multiple sclerosis. As the major NHS referral centre in East Midlands, the multiple sclerosis specialist clinic covers the region’s multiple sclerosis population and provides access to therapy. All the clinically definite multiple sclerosis diagnoses in our cohort are supported by a positive result of a MRI scan and made by a multiple sclerosis specialist neurologist. Patients are seen routinely and undergo medical and neurological evaluation including estimation of Expanded Disability Status Scale (EDSS) score (Kurtzke, 1983), with reporting of history, treatments and investigation results. Of all the patients registered in the clinic, 1126 patients were registered on the clinical database created by one multiple sclerosis specialist neurologist (C.S.C.) with the aim of investigating prospectively the comorbid conditions associated with multiple sclerosis (Edwards and Constantinescu, 2004). Of these, we were unable to review and collect data from 94 cases during the study period. This provided 1032 cases for review with a median of 15 years of clinical data. The study was approved by the National Research Ethics Service East Midlands Ethics Committee Derby-1.
Data used in the study
Age, gender, and duration of disease modifying treatment were obtained. Disease modifying treatments were modelled as a binary variable in which patients were grouped into those who received treatment for <1 year and those who received treatment for 1 year or more (≥1). Age at onset was defined as the age at the time of the first manifestation of the disease or any neurological sign indicative of multiple sclerosis identified by a multiple sclerosis specialist neurologist. EDSS scores obtained from the neurologist’s report were used in the analysis. The type of clinical course at the onset of the disease was also collected and patients were classified as relapsing-remitting multiple sclerosis or primary-progressive multiple sclerosis based on the criteria of Lublin and Reingold (1996). Disease duration was calculated from the date of first manifestation of the disease to the date of the last EDSS score on record. We also calculated global Multiple Sclerosis Severity Score (MSSS) from EDSS score and disease duration according to the guidelines by Roxburgh et al. (2005).
Smoking history
Patients were asked about smoking status and, if they were smokers, number of cigarette smoked per day at their first clinic visit at the time of the first manifestation of the disease or time of diagnosis. Patients were grouped as being non-smokers, ex-smokers or current smokers. ‘Ever-smoking’ was defined based on criteria by The European Community Respiratory Health Survey III (Burney et al., 1994) as at least 20 packs of cigarettes or 12 oz (360 g) of tobacco in a lifetime, or at least one cigarette per day or one cigar a week for 1 year.
Statistical methods
Descriptive statistics were used to summarize the baseline demographic characteristics and clinical outcomes. We examined the association between smoking and the type of multiple sclerosis at its onset (relapsing-remitting multiple sclerosis or primary-progressive multiple sclerosis) using logistic regression models. Linear regression models were used to determine the effects of smoking intensity on the severity of the disease measured by MSSS score. Detailed models were adjusted for onset age, sex, type of multiple sclerosis at the onset of the disease (relapsing-remitting versus primary-progressive) and use of treatment. Although regression models of large sample size are robust to some degree of non-normality (Lumley et al., 2002), all the linear models were controlled for homogeneity and distribution of residuals to avoid violation of underlying normality assumption.
Time to two EDSS milestone scores of 4 and 6 was estimated using the Kaplan-Meier method taking into account participation of those that have not yet reached EDSS scores 4 or 6. The smoking-specific rate ratios were calculated using Cox proportional hazard regression models (Cox and Oakes, 1984) controlled for sex, onset age (continuous in years), use of disease modifying treatments (in a binary group of ≥1 year or <1 year) and initial clinical course of the disease (relapsing-remitting versus primary-progressive). Cox regression models were also used to estimate the rate ratios of reaching MSSS score categories 5 or above (patient progress faster than half of the multiple sclerosis population) and 7.5 or above (patients progress faster than 75% of the multiple sclerosis population) between smoking groups. Age is one of the most important factors in accumulation of disabilities in multiple sclerosis and the hazard will significantly change as a function of age. To account for this, patients in the cohort were followed from the date of birth, entered the study at the age at the onset of the disease (left truncation or late entry) and exit at their event/censoring age. This way the impact of age was controlled for more effectively (Korn et al., 1997). The final Cox models were checked for proportionality assumption based on the Schoenfeld residuals and were stratified by sex and/or initial disease type to hold the proportionality assumption. A comparison of the effects of smoking on the likelihood of patients being in the upper quartiles (MSSS > 7.5) versus lower quartiles (MSSS < 2.5) of the MSSS spectrum was made using a logistic regression model to ensure the robustness of the results when using MSSS as an outcome of interest. All statistical analyses were performed with Stata 11 (StataCorp. 2009. Stata Statistical Software: Release 11 StataCorp LP).
Results
Of the 1032 patients, 30 patients (12 clinically isolated syndrome and 18 multiple sclerosis diagnosis suspected only) did not fulfil McDonald and/or Poser criteria (Poser et al., 1983; McDonald et al., 2001) and were therefore excluded from the study. We were unable to collect sufficient demographic data from 33 patients. In addition, smoking data were unavailable for 74 subjects. Thus there were 895 subjects with full data for analysis. Comparison of our final population with the 74 patients excluded due to missing smoking data showed that the two populations were highly similar across all demographic and clinical outcome variables (data not shown). Of 895 patients (270 male, 625 female) with mean standard deviation (SD) age of 49 (11) years and mean disease duration of 17 (10) years, 49% were regular smokers at the time of disease onset or at diagnosis. Approximately half of the participants (49%) had relapsing-remitting multiple sclerosis, 39% secondary-progressive multiple sclerosis, and 11% primary-progressive multiple sclerosis. Sixty-eight patients stopped smoking before the onset of the disease and 117 patients stopped smoking an average of 10 (±8) years after the disease onset. Patients who stopped smoking before disease onset were significantly older [39 (±11) years] at the onset of the disease than those who stopped after the disease onset [31 (±9) years] (P < 0.001). In general, our cohort characteristics were similar to those reported for the typical multiple sclerosis population (Table 1).
Table 1.
Comparison of baseline characteristics between ever-smokers and never-smokers
| Never smokers (n = 451) | Ever smokers (n = 444) | |
|---|---|---|
| Age* (mean, SD) | 49.98 (±10.92) | 48.53 (±11.03) |
| Sex (female) | 348 (77.16%) | 277 (62.39%) |
| EDSS† (median, IQR) | 5.5 (3.5) | 6 (3) |
| MSSS (mean, SD) | 5.13 (±2.70) | 5.93 (±2.52) |
| Disease duration (mean, SD) | 17.70 (±10.15) | 15.89 (±9.51) |
| Age at the onset (mean, SD) | 32.29 (±9.97) | 32.75 (±9.70) |
| Type of the disease | ||
| Relapsing-remitting | 226 (50.11%) | 217 (48.87%) |
| Primary-progressive | 51 (11.31%) | 51 (11.49%) |
| Secondary-progressive | 174 (38.58%) | 176 (39.64%) |
| Disease modifying treatment ≥1 year | 173 (38.36%) | 183 (41.22%) |
*Age at the last clinic visit.
†EDSS recorded at the last clinic visit.
Smoking, disease type and severity of multiple sclerosis
We could not find any association between smoking status and having progressive type of multiple sclerosis at disease onset (relapsing-remitting multiple sclerosis versus primary-progressive multiple sclerosis). In our cohort, after controlling for sex and onset age, the risk of having progressive clinical course (primary-progressive multiple sclerosis) at disease onset was independent of smoking status (odds ratio = 0.82; P = 0.396; 95%CI: 0.52–1.29). There were also no significant differences between the proportion of ever-smokers in relapsing-remitting multiple sclerosis and secondary-progressive multiple sclerosis groups (chi-square with one degree of freedom = 0.1102, P = 0.740). Male patients and those with late onset of the disease were significantly more likely to have progressive disease at onset. Median (interquartile range) diagnosis lag time between onset of symptoms and diagnosis of multiple sclerosis was 3 (6) years in never-smokers and 2 (6) years in ever-smokers (P = 0.2).
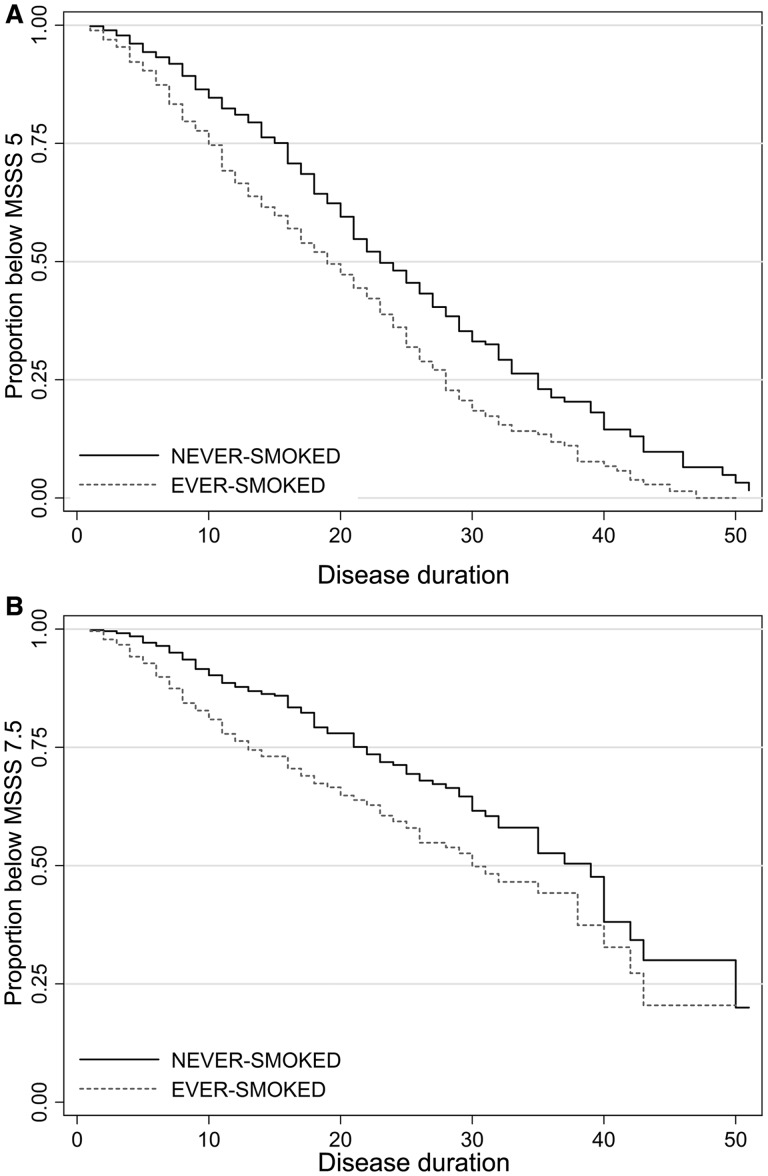
Comparison of patients in the upper and lower quartiles of disease severity, measured by MSSS (MSSS < 2.5, n = 163; and MSSS > 7.5, n = 287), showed a higher proportion of ever-smokers in the upper quartiles MSSS (57% versus 35%, chi-square with one degree of freedom = 18.76, P < 0.001). Using the logistic regression model, we noted that ever-smokers were 2.37 times (P < 0.001, 95%CI: 1.52 to 3.7) more likely to fall in the upper quartile MSSS than lower quartile compared with never-smokers. Median times to MSSS score category 5 or above were 23, 24 and 18 years for non-smokers, ex-smokers and current smokers from the disease onset, respectively. Median times to MSSS score category 7.5 or above were 39, 39 and 26 years for non-smokers, ex-smokers and current smokers from the disease onset, respectively (Fig. 1). Current smokers reached MSSS score categories >5 and >7.5 in a significantly shorter time compared with never-smokers [hazard ratio (HR) = 1.63, P < 0.001, 95%CI: 1.32–2.01 and HR = 1.98, P < 0.001, 95%CI: 1.43–2.75, respectively). There were no differences in the risk of reaching MSSS score categories >5 and >7.5 between ex-smokers and non-smokers. The average MSSS was higher by 0.68 (P = 0.001, 95%CI: 0.36–1.01) for ever-smokers compared with never-smokers while controlling for sex, onset age, initial multiple sclerosis type and use of disease modifying treatment using linear regression model.
Figure 1.
Kaplan–Meier survival graph showing times to MSSS score >5 (A) and MSSS score 7.5> (B) by ever-smokers versus never-smokers from the disease onset.
Time to Expanded Disability Status Scale scores 4 and 6
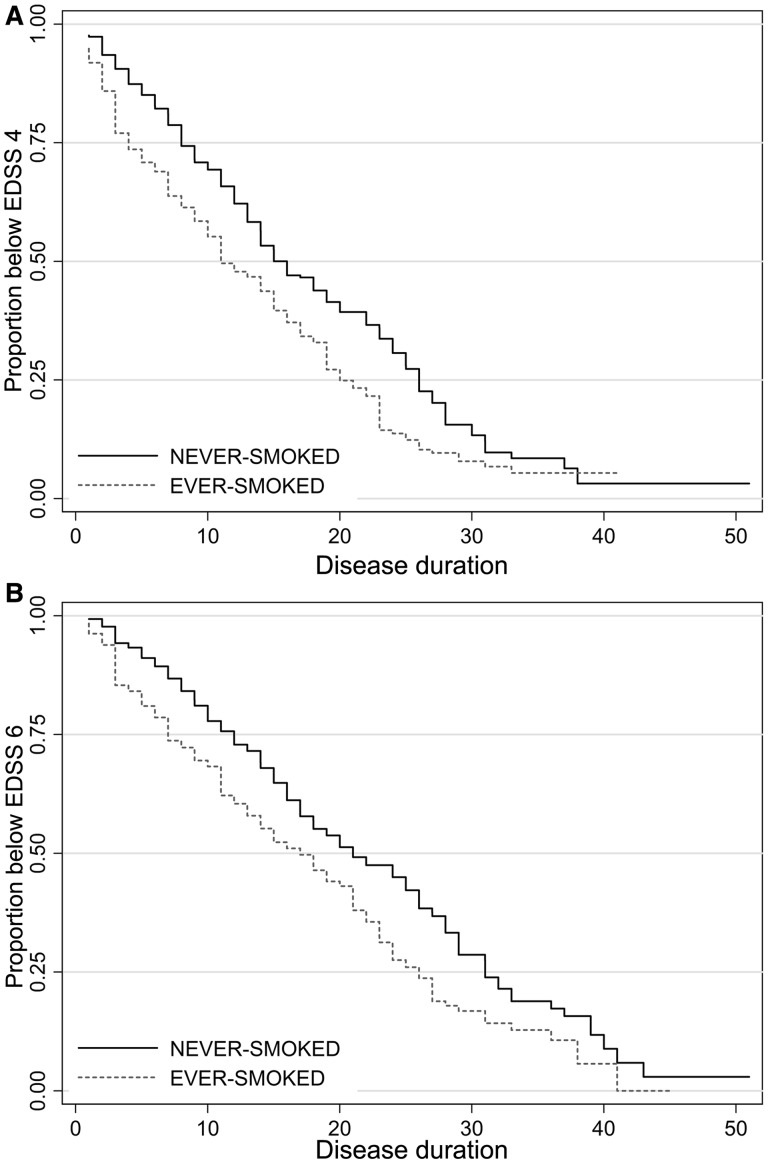
The time to EDSS score 4 was unavailable in 71 patients (8%), and time to EDSS score 6 was unavailable in 29 patients (3.2%). The results of the survival summary and the Cox proportional hazard regression model are listed in Table 2. The final models were adjusted for onset age, use of disease modifying treatment and stratified by sex and/or disease initial type to hold the proportionality assumption. There was a significant difference in time to EDSS 4 and 6 between ever-smokers and never-smokers. Ever-smokers were 1.34 (P = 0.001, 95%CI: 1.12 to 1.60) and 1.25 (P = 0.02, 95%CI: 1.02 to 1.51) times more likely than never-smokers to reach EDSS scores 4 and 6, respectively (Fig. 2). A higher risk of reaching EDSS scores 4 and 6 was found for current smokers compared with non-smokers. Ex-smokers had a significantly lower risk of reaching EDSS scores 4 (HR: 0.65, 95%CI: 0.50–0.83) and 6 (HR: 0.69, 95%CI: 0.53–0.90) than current smokers. There were also no significant differences in the time to EDSS scores 4 and 6 between patients who stopped smoking before multiple sclerosis onset and those who stopped after developing multiple sclerosis.
Table 2.
Kaplan–Meier estimates of the time to EDSS scores 4 and 6 and the results of Cox hazard regression model by smoking status
| Smoking status | n | Median time to EDSS 4 from onset (95%CI) | Median time to EDSS 4 from birth (95%CI) | Hazard ratio (95%CI) |
|---|---|---|---|---|
| Non-smokers | 417 | 16 (14–19) | 50 (48–51) | Reference |
| Stopped before onset | 58 | 11 (9–18) | 52 (47–58) | 1.01 (0.7–1.46) |
| Stopped after onset | 107 | 18 (14–20) | 50 (47–52) | 1.03 (0.8–1.34) |
| Current smokers | 231 | 11 (9–12) | 45 (42–47) | 1.64 (1.33–2.02) |
| n | Median time to EDSS 6 from onset (95%CI) | Median time to EDSS 6 from birth (95%CI) | Hazard ratio (95%CI) | |
| Non-smokers | 439 | 22 (19–25) | 54 (53–55) | Reference |
| Stopped before onset | 64 | 15 (10–19) | 56 (50–60) | 0.97 (0.66–1.4) |
| Stopped after onset | 111 | 23 (20–25) | 54 (51–55) | 1.04 (0.78–1.38) |
| Current smokers | 241 | 16 (13–20) | 50 (47–53) | 1.49 (1.18–1.86) |
Figure 2.
Kaplan–Meier survival graph showing times to EDSS score 4 (A) and EDSS score 6 (B) by ever-smokers versus never-smokers from the disease onset.
Daily cigarette consumption and disability progression
Average daily cigarette use was available for 807 individuals [mean (SD) 6.6 (±9.5) cigarettes per day, range 0 to 60]. We examined the effects of daily cigarette use on the MSSS score. We observed that the average MSSS is increased by 0.04 (P < 0.001, 95%CI: 0.02 to 0.06) for each extra cigarette consumed per day. This means that an increase in the number of cigarettes smoked daily at the time of disease onset or diagnosis by 10 was associated with an increase in the MSSS of 0.4 at an average of 16 years later.
Discussion
In this study, we found that disease progression is more rapid amongst ever-smokers. Ever-smoking in our cohort was associated with a significant increase in both of the clinical outcomes (MSSS and EDSS score). Here we present, to our knowledge for the first time, evidence of the potential beneficial effects of smoking cessation on disability progression in patients with multiple sclerosis. We found that ex-smokers have a significantly reduced risk of reaching EDSS score milestones 4 and 6 compared with current smokers, and that this risk reduction was similar between those who stopped smoking before or after the onset of multiple sclerosis. Thus, there are positive effects of smoking cessation on disease progression even after multiple sclerosis onset. Our study provides new, important clinical findings on the influence of tobacco use in a cohort of patients with multiple sclerosis. We used a population of patients with multiple sclerosis, all with clinically definite multiple sclerosis confirmed by multiple sclerosis specialist neurologists. Most importantly, our clinic and consequently our cohort, has a population-based nature as it is estimated to cover >98% of the patients with multiple sclerosis in Nottinghamshire and defined parts of Lincolnshire and Derbyshire regions. Our findings are based on diagnosis, identification and classification of patients and like any other study may be subject to bias in ascertainment, recruitment and misclassification of status. However, the fact that the confirmation of the diagnosis and the disability scores in our study were obtained from a clinical database of multiple sclerosis specialist neurologists increases the homogeneity and integrity of our results. Thus, our findings are robust, and characteristics such as gender distribution, age of onset and type of multiple sclerosis distribution of our cohort are similar to those reported in most other multiple sclerosis cohorts. Thus our sample population appears to be representative of the multiple sclerosis population at large and our findings can be generalized and are reflective of routine clinical practice. Our study has some limitations that may have influenced our estimates. It is likely that our cohort missed some patients with very severe disease who died before attending our multiple sclerosis clinic. We assessed the potential impact of this survivorship bias on our results by estimating changes in odds ratios or coefficients while adding patients with longer disease duration to the models (data not shown). The effects of smoking on the disease progression of these patients were similar to that of the whole cohort.
We believe our study is one of the most comprehensive studies to examine the correlation of tobacco smoking and multiple sclerosis clinical outcomes, with particular emphasis on disability progression. In a study of a UK population, Hernan et al. (2005) found a risk ratio of 3.6 years (95%CI: 1.3–9.9) for transition to secondary-progressive multiple sclerosis in 179 cases of multiple sclerosis with median 5.3 years follow-up using the General Practice Research Database. In our study we estimated the risk of reaching EDSS score 6, which is a robust outcome measure and a surrogate of time to secondary-progressive multiple sclerosis (Scalfari et al., 2010) and also allows inclusion of primary-progressive multiple sclerosis. Using these outcomes we found a higher risk ratio of reaching EDSS score 6 in smokers, although the risk ratio was lower than the risk ratio reported by Hernan et al. (2005) for development of secondary-progressive multiple sclerosis. Although the, risk ratios were in the same direction, differences in patient sample size [895 in our study versus 179 in the Hernan et al. (2005) study], longer duration of follow-up (15 versus 5.3 years), and number of patients reaching the outcome (450 versus 20) may account for the difference in risk ratios. Others have also used time to EDSS 4 and 6, however with no significant evidence of association between cigarette smoking and progression (Koch et al., 2007). We believe that greater sample size and longer duration of follow-up makes our estimates more robust. Our results are in accordance with the results from an observation by D'hooghe et al. (2012), which showed higher risk of reaching EDSS score 6 amongst occasional and daily cigarette consumers. D'hooghe et al. (2012) relied on questionnaires for obtaining data on disease onset and self-reported disability scores that may introduce some bias. The advantage of our study is its higher homogeneity in terms of clinical data used such as disease type and EDSS scores that were based on face-to-face patient examination and recorded by multiple sclerosis specialist neurologists. By comparing the two lower and upper MSSS quartiles we showed that smokers are more likely to have a severe disease course as shown previously (Gholipour et al., 2011). A higher probability of progressive onset amongst smokers has been observed previously (Sundstrom and Nystrom, 2008; Healy et al., 2009); however, our estimate in a much larger sample showed no evidence that smoking favours a progressive onset of the disease.
Smoking is known to be a significant risk factor for the development and progression of several autoimmune diseases (Prummel and Wiersinga, 1993; Saag et al., 1997; Hardy et al., 1998; Hudson et al., 2011) and is a frequently studied health behaviour because of its well-known associations with chronic diseases. Because disease progression is more rapid in ever-smokers, preventing smoking may be important in reducing the progression of multiple sclerosis. Estimating the impact of smoking in terms of costs shows its relevance. Costs of multiple sclerosis (direct and indirect) can increase by nearly 2-fold in patients with EDSS score 3.5 to 6 compared with those with EDSS score ≤3, from £7273 to £12 875 per patient per year (Kobelt et al., 2006). In the UK, it has been estimated that each quality adjusted life year gained in multiple sclerosis by means of disease modifying treatments costs from £18 700 to £25 500 (Gani et al., 2008). Based on our results and compared to these figures, preventing or stopping smoking could be an economical strategy and an effective way to improve outcomes in multiple sclerosis that can be implemented along other multiple sclerosis therapeutic approaches.
It is not clear whether this additional increase in impairment and disability in ever-smokers is purely due to the biological influence of tobacco smoking on multiple sclerosis specifically, or is due to other underlying factors such as increase in comorbidities associated with smoking. It has been previously reported that smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases (Marrie et al., 2011). Although comorbidity may account for parts of the progression seen in ever-smokers, there are lines of evidence that suggest a potential pathophysiological role of tobacco smoke on the progression of the disease in multiple sclerosis. Of note, findings from MRI studies, evidence on the negative impact of smoked tobacco but not moist snuff on risk of multiple sclerosis as demonstrated by Hedstrom et al. (2009) as well as a significant association between smoking intensity and disease severity (dose-response rate) as demonstrated in our study may suggest a direct impact of tobacco smoke on multiple sclerosis progression. Nevertheless the possibility of an indirect impact of smoking on multiple sclerosis progression, or a combination of direct and indirect effects cannot be excluded.
There are some likely biological explanations for a mechanistic pathway between smoking and disability accumulation in multiple sclerosis (Pryor et al., 1998; Bijl et al., 2001; Malkawi et al., 2009). Exposure to tobacco smoke has been shown to alter the innate and adaptive immune cells (Holt and Keast, 1977). Increased risk of cancer, cardiovascular and other chronic diseases amongst smokers may possibly be related to smoking-induced changes in the immune system. Further work is needed to elucidate the mechanism by which smoking increases the risk of progression in multiple sclerosis.
Conclusions and implications
In summary, we found that ever-smokers with multiple sclerosis accumulate more disability over a shorter period of time and suffer from more severe disease than never-smokers. Our findings point toward the beneficial effect of smoking cessation even after the disease onset in patients with multiple sclerosis. Measures to prevent and reduce smoking may lead to improved outcomes in multiple sclerosis.
Acknowledgements
We thank Maritza Hawkins, Dawn Owen, Max Harrison, and Hannah Sharp, Division of Clinical Neurology, Faculty of Medicine & Health Sciences, University of Nottingham, for help with providing the medical records.
Glossary
Abbreviations
- EDSS
Expanded Disability Status Scale
- MSSS
Multiple Sclerosis Severity Score
References
- Antonovsky A, Leibowitz U, Smith HA, Medalie JM, Balogh M, Kats R, et al. Epidemiologic study of multiple sclerosis in Israel. I. an overall review of methods and findings. Arch Neurol. 1965;13:183–93. doi: 10.1001/archneur.1965.00470020073010. [DOI] [PubMed] [Google Scholar]
- Bijl M, Horst G, Limburg PC, Kallenberg CG. Effects of smoking on activation markers, Fas expression and apoptosis of peripheral blood lymphocytes. Eur J Clin Invest. 2001;31:550–3. doi: 10.1046/j.1365-2362.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Koepsell TD, Perera DR, Inui TS. Risk of ulcerative colitis among former and current cigarette smokers. N Eng J Med. 1987;316:707–10. doi: 10.1056/NEJM198703193161202. [DOI] [PubMed] [Google Scholar]
- Burney PG, Luczynska C, Chinn S, Jarvis D. The European Community Respiratory Health Survey. Eur Respir J. 1994;7:954–60. doi: 10.1183/09031936.94.07050954. [DOI] [PubMed] [Google Scholar]
- Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155:732–8. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of survival data. London, New York: Chapman and Hall; 1984. [Google Scholar]
- D'hooghe MB, Haentjens P, Nagels G, De Keyser J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur J Neurol. 2012;19:616–24. doi: 10.1111/j.1468-1331.2011.03596.x. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Constantinescu CS. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler. 2004;10:575–81. doi: 10.1191/1352458504ms1087oa. [DOI] [PubMed] [Google Scholar]
- Ford HL, Gerry E, Airey CM, Vail A, Johnson MH, Williams DR. The prevalence of multiple sclerosis in the Leeds Health Authority. J Neurol Neurosurg Psychiatry. 1998;64:605–10. doi: 10.1136/jnnp.64.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani R, Giovannoni G, Bates D, Kemball B, Hughes S, Kerrigan J. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26:617–27. doi: 10.2165/00019053-200826070-00008. [DOI] [PubMed] [Google Scholar]
- Ghadirian P, Dadgostar B, Azani R, Maisonneuve P. A case-control study of the association between socio-demographic, lifestyle and medical history factors and multiple sclerosis. Can J Public Health. 2001;92:281–5. doi: 10.1007/BF03404961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour T, Healy B, Baruch NF, Weiner HL, Chitnis T. Demographic and clinical characteristics of malignant multiple sclerosis. Neurology. 2011;76:1996–2001. doi: 10.1212/WNL.0b013e31821e559d. [DOI] [PubMed] [Google Scholar]
- Handel AE, Williamson AJ, Disanto G, Dobson R, Giovannoni G, Ramagopalan SV. Smoking and multiple sclerosis: an updated meta-analysis. PLoS One. 2011;6:e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 1998;57:451–5. doi: 10.1136/ard.57.8.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH. Smoking is a risk factor for multiple sclerosis: a metanalysis. Mult Scler. 2007;13:610–5. doi: 10.1177/1352458506073501. [DOI] [PubMed] [Google Scholar]
- Healy BC, Ali EN, Guttmann CR, Chitnis T, Glanz BI, Buckle G, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66:858–64. doi: 10.1001/archneurol.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology. 2009;73:696–701. doi: 10.1212/WNL.0b013e3181b59c40. [DOI] [PubMed] [Google Scholar]
- Hedstrom AK, Sundqvist E, Baarnhielm M, Nordin N, Hillert J, Kockum I, et al. Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain. 2011;134(Pt 3):653–64. doi: 10.1093/brain/awq371. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Jick SS, Logroscino G, Olek MJ, Ascherio A, Jick H. Cigarette smoking and the progression of multiple sclerosis. Brain. 2005;128(Pt 6):1461–5. doi: 10.1093/brain/awh471. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Olek MJ, Ascherio A. Cigarette smoking and incidence of multiple sclerosis. Am J Epidemiol. 2001;154:69–74. doi: 10.1093/aje/154.1.69. [DOI] [PubMed] [Google Scholar]
- Holt PG, Keast D. Environmentally induced changes in immunological function: acute and chronic effects of inhalation of tobacco smoke and other atmospheric contaminants in man and experimental animals. Bacteriol Rev. 1977;41:205–16. doi: 10.1128/br.41.1.205-216.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M, Lo E, Lu Y, Hercz D, Baron M, Steele R, et al. Cigarette smoking in patients with systemic sclerosis. Arthritis and Rheum. 2011;63:230–8. doi: 10.1002/art.30071. [DOI] [PubMed] [Google Scholar]
- Kobelt G, Berg J, Lindgren P, Kerrigan J, Russell N, Nixon R. Costs and quality of life of multiple sclerosis in the United Kingdom. Eur J Health Econ. 2006;7(Suppl 2):S96–104. doi: 10.1007/s10198-006-0380-z. [DOI] [PubMed] [Google Scholar]
- Koch M, van Harten A, Uyttenboogaart M, De Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology. 2007;69:1515–20. doi: 10.1212/01.wnl.0000277658.78381.db. [DOI] [PubMed] [Google Scholar]
- Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–69. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Malkawi AH, Al-Ghananeem AM, de Leon J, Crooks PA. Nicotine exposure can be detected in cerebrospinal fluid of active and passive smokers. J Pharm Biomed Anal. 2009;49:129–32. doi: 10.1016/j.jpba.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Manouchehrinia A, Constantinescu C. Cost-effectiveness of disease-modifying therapies in multiple sclerosis. Curr Neurol Neurosci Rep. 2012;12:1–9. doi: 10.1007/s11910-012-0291-6. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Smoking status over two years in patients with multiple sclerosis. Neuroepidemiology. 2009;32:72–9. doi: 10.1159/000170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie RA, Horwitz RI, Cutter G, Tyry T, Vollmer T. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology. 2011;36:85–90. doi: 10.1159/000323948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health. 2007;10:54–60. doi: 10.1111/j.1524-4733.2006.00144.x. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993;269:479–82. [PubMed] [Google Scholar]
- Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998;11:441–8. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- Riise T, Kirkeleit J, Aarseth JH, Farbu E, Midgard R, Mygland A, et al. Risk of MS is not associated with exposure to crude oil, but increases with low level of education. Mult Scler. 2011;17:780–7. doi: 10.1177/1352458510397686. [DOI] [PubMed] [Google Scholar]
- Riise T, Nortvedt MW, Ascherio A. Smoking is a risk factor for multiple sclerosis. Neurology. 2003;28(61):1122–4. doi: 10.1212/01.wnl.0000081305.66687.d2. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Charlton D. High incidence and prevalence of multiple sclerosis in south east Scotland: evidence of a genetic predisposition. J Neurol Neurosurg Psychiatry. 1998;64:730–5. doi: 10.1136/jnnp.64.6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- Saag KG, Cerhan JR, Kolluri S, Ohashi K, Hunninghake GW, Schwartz DA. Cigarette smoking and rheumatoid arthritis severity. Ann Rheum Dis. 1997;56:463–9. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914–29. doi: 10.1093/brain/awq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon KC, van der Mei IA, Munger KL, Ponsonby A, Dickinson J, Dwyer T, et al. Combined effects of smoking, anti-EBNA antibodies, and HLA-DRB1*1501 on multiple sclerosis risk. Neurology. 2010;74:1365–71. doi: 10.1212/WNL.0b013e3181dad57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P, Nystrom L. Smoking worsens the prognosis in multiple sclerosis. Mult Scler. 2008;14:1031–5. doi: 10.1177/1352458508093615. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133–46. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]