Abstract
Allospecific recruitment of T cells is primary to the pathogenesis of renal transplant rejection. Chemokines and their receptors inducing a Th1 cytokine response play a central role in this recruitment. Renal allograft biopsies of 28 patients with acute cellular rejection and 10 protocol biopsies (controls) were examined in accordance with Banff grading 2007 schema. Immunohistochemistry for CD3 and CC chemokine receptor 5 (CCR5) in sequential sections was performed and quantitatively assessed in the glomeruli, tubules, and interstitium. Histopathologic and clinical correlations were carried out. CD3- and CCR5-positive cells were observed in significantly higher numbers in rejection cases than in controls (P = 0.010). A larger proportion of CCR5-positive cells were noted in the foci of tubulitis compared to the interstitial infiltrates and glomeruli in all cases, and it correlated with the grade of cellular rejection (P = 0.010). A greater number of CCR5-positive cells were seen in early rejection (<6 months posttransplant) compared to late rejection. No clinical correlation with serum creatinine levels was found. CCR5-positive cells represent the alloaggressive subset of T cells in ACR, and their numbers correlate with rejection severity. CCR5 may be used as a marker of early acute rejection and may be an important target for future antirejection therapies.
Keywords: Acute cellular rejection, Banff grading, CC chemokine receptor 5
Introduction
Cellular allograft rejection is one of the commonest causes of graft dysfunction in the early as well as late posttransplant period.[1] Primary to the pathogenesis of this rejection is allospecific recruitment of peripheral T cells.[2] Chemokines induce migration of lymphocytes[3] bearing specific chemokine receptors, which is central to allograft rejection, as has been emphasized in many experimental and human studies.[4–6] The chemokine receptor CC chemokine receptor 5 (CCR5) is expressed on the alloaggressive Th1 cells, which are responsible for activating macrophages and mediating cytotoxicity via Fas-Fas ligand in the graft.[7] This study aimed to determine the distribution of CCR5-positive cells in the graft and correlate it with the degree of cellular rejection.
Subjects and Methods
In this prospective study, 28 patients who had presented with graft dysfunction and demonstrated features of acute cellular rejection (ACR) on biopsy [Figure 1a and b] were included. C4d immunostaining was performed in all cases to exclude a concomitant antibody-mediated rejection. Cases of graft dysfunction attributable to calcineurin inhibitor toxicity or viral infection on biopsy were also excluded. All biopsies were classified according to Banff 2007.[8] Ten protocol biopsies at 1 month posttransplant were included in the control group. Details of immunosuppression and serum creatinine levels of each patient were recorded.
Figure 1.
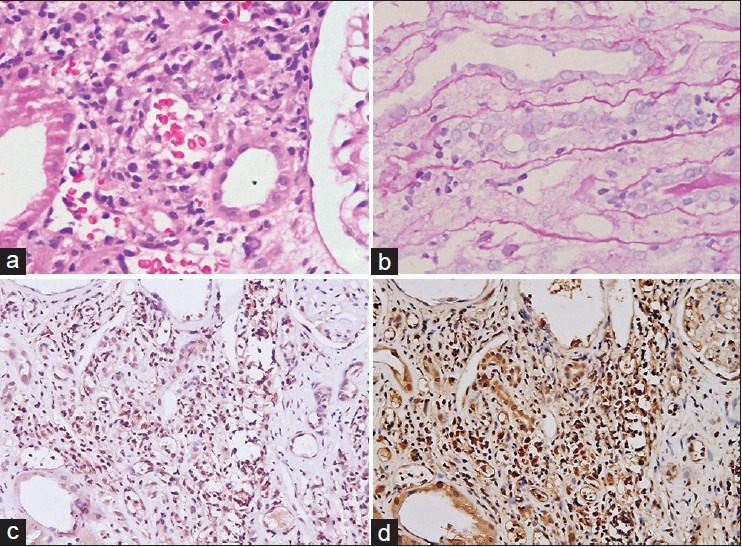
(a) Foci of tubulitis (H and E, 200); (b) foci of tubulitis (PAS, 200); (c) lymphocytes in interstitium and foci of tubulitis (anti-CCR5, 200); (d) lymphocytes in interstitium and foci of tubulitis (anti-CD3, 200)
Immunohistochemistry
Sequential slides were subjected to immunohistochemistry for CD3 (rabbit polyclonal Abcam ab5690 1:100) and CCR5 (rabbit polyclonal AbD Serotec AHP568 1:150) using conventional techniques. CCR5 antibody was standardized using formalin-fixed spleen tissue from splenectomy specimens. The slides were examined by two pathologists (AKD and RG). In the same area of the section, both CD3- and CCR5-positive cells were counted separately in the tubules, interstitium, and glomeruli, and expressed as number of cells per high power field (hpf).
Banff grading of ACR depends on the quantity of leukocytes infiltrating the interstititum, tubules, and arterioles. Thus, we correlated CCR5-positive T lymphocytes with the total grade of rejection as well as the individual components such as tubulitis, intimal arteritis, and interstitial inflammation using statistical tools of correlation such as Spearman correlation and Pearsons correlation. Statistical analysis was performed using SPSS 17.
Results
The clinical characteristics of the cases and controls are elucidated in Table 1. On histopathologic examination, the cases showed ACR of variable Banff grades, as detailed in Table 2a and b. None of the protocol biopsies showed features to suggest borderline/ACR.
Table 1.
Clinical characteristics of the study population
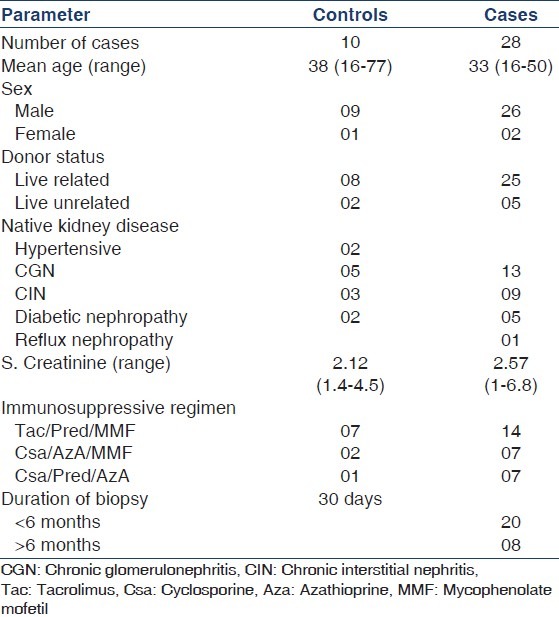
Table 2a.
Histological and immunohistochemical analysis of Banff grade I cases
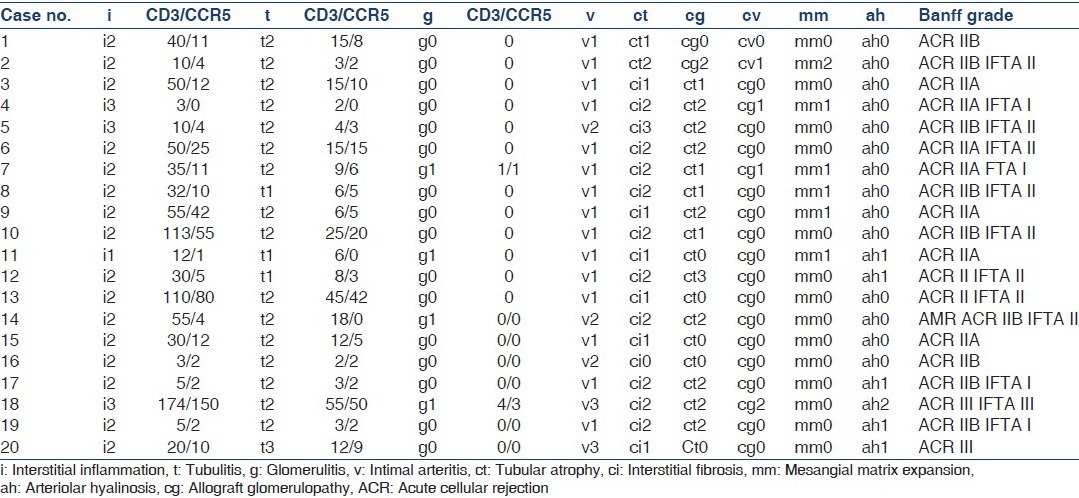
Table 2b.
Histological and immunohistochemical analysis of Banff grade II-III cases
Immunohistochemical analysis revealed that the cases had a significantly higher number of CD3-positive (35.16 vs. 2.82) and CCR5-positive cells (15.48 vs. 0.64) compared to the control group (P = 0.010), and the pattern of distribution between CCR5- and CD3-positive cells in sequential sections was closely comparable (P < 0.01)[Figure 1c and d].
Distribution of CC chemokine receptor 5-positive cells and histopathologic correlations
In cases with rejection, the CCR5-positive lymphocytes were observed to be significantly more in the tubulointerstitium than in the foci of glomerulitis (15.48 vs. 0.32; P = 0.007) and also more in the foci of tubulitis compared to the interstitium, although this did not reach levels of significance (P = 0.351). Quantitation of CD3- and CCR5-positive cells infiltrating the arterial intima could not be performed due to very small numbers of infiltrating cells; however, few infiltrating CCR5-positive cells were noted [Figure 2].
Figure 2.
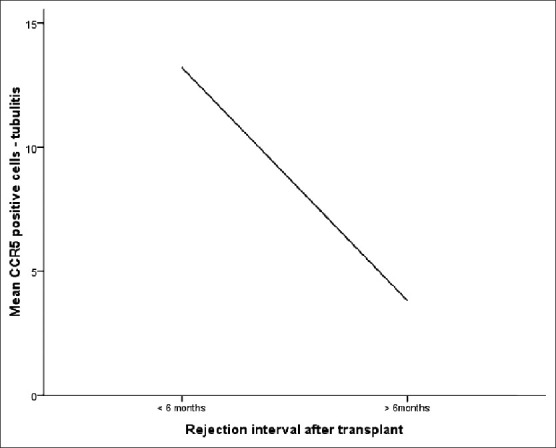
Correlation of chemokine receptor 5-positive cells in foci of tubulitis with interval after transplant
A positive correlation was found between CCR5-positive cells and grading of rejection by Banff 2007 (P < 0.05), that is, higher grade of rejection had higher number of CCR5-positive cells. Percent of T cells expressing CCR5 in the foci of tubulitis positively correlated with the severity of tubulitis, and also with the severity of interstitial inflammation (P < 0.05). No significant correlation with chronic features such as interstitial fibrosis and tubular atrophy was noted.
Clinical correlations
No correlation was noted with the serum creatinine levels at the time of the biopsy, and the number of CCR5-positive cells did not predict response to antirejection therapy determined by follow-up serum creatinine levels (P = 0.134). Thus, although the number of CCR5-positive cells correlated with the grade of rejection, it did not correlate with the degree of graft dysfunction or response to therapy within rejection cases. The patient population was divided into two groups: One containing tacrolimus and the other containing cyclosporine. Patients on tacrolimus containing regimens were observed to have less total number of CCR5-positive cells, particularly in the foci of tubulitis (P < 0.05). In relation to the time interval after transplantation, the mean count of T cells expressing CCR5 in the foci of tubulitis was significantly more in rejection occurring within 6 months of transplantation than in that occurring after a period of 6 months (P < 0.05).
Discussion
As the renal allograft is recognized as foreign, an allospecific immune response is triggered which is largely mediated by chemokines. An increase in concentration of the CC chemokine regulated and normal T cell expressed and secreted (RANTES) in the allograft has been demonstrated in cases of cellular rejection.[9–11] This chemokine is secreted by activated fibroblasts, and mesangial and tubular epithelial cells in the graft,[5,6,11] and induces the migration of CD3-positive T cells expressing the CCR5 receptor.[10,11]
Segerer et al. studied the distribution of CCR5-positive cells by immunohistochemistry in cases of glomerulonephritis, interstitial nephritis, and transplant rejection.[11] In the 18 transplant cases (9 acute and 9 chronic), CCR5-positive cells were seen in all interstitial areas, with mononuclear cell infiltrates constituting approximately 90% of the CD3-positive cells in cases of acute rejection and approximately 65% in cases of chronic rejection. CCR5-positive cells were also seen in foci of tubulitis, glomerulitis, and endothelitis. Seven of the nine cases of acute rejection were of Banff grade I. However, no correlations with histopathology were made. In clinical correlations, they found that the mean CCR5-positive cells per cortical hpf was significantly higher in cases with creatinine more than 1.2 mg/dl (P < 0.01).
In another study, Segerer et al. showed an increase in mRNA expression of CCR5, CCR2B, and CXCR4 in allograft infiltrating leukocytes, compared to that in controls.[12] Expression of CCR1 was documented in both allograft rejections and controls. CCR3 and CCR8 were absent. CXCR4 was most diffusely expressed. These findings were indicative of a Th2 response in rejection. Panzer et al.[10] compared CCR5 and CXCR3 expression in 13 cases of acute rejection and an equal number of cases without rejection in biopsies. They found significantly higher numbers of CCR5-positive cells in acute rejection (160.9 ± 33.1/30 hpfs) compared to no rejection cases (36.2 ± 10.7, P < 0.007), similar to that in this present study. No predictive value could be assigned to CCR5. Their cases varied from Banff grade I a to II b; however, no histopathologic correlations were made.
We also found significantly increased number of CCR5-positive cells in patients on cyclosporine compared to those on tacrolimus containing regimen. Daniel Seron et al. reported increased CD3- and CD68-positive allograft infiltrating cells in patients on cyclosporine compared to those on tacrolimus,[13] similar to our study. However, ours is the first study correlating CC chemokine receptor 5 in these two groups and shows significantly more CCR5-positive cells in patients on cyclosporine containing regime compared to those on tacrolimus.
One important observation in cases 1 and 8 [Table 2a] and cases 4, 11, and 14 [Table 2b] was the presence of very few or no CCR5-positive cells. A possible explanation to this could be that CCR5, which is an early marker of rejection responsible for recruitment of the leukocytes, may not be observed in large numbers once rejection is for a longer time and well established. Almost all of these cases had intimal arteritis (except case 1), pointing toward increased severity of the cases.
In this study, it was again confirmed that CCR5-positive cells are significantly increased in rejecting compared to stable grafts and are found predominantly in the foci of interstitial inflammation and tubulitis. In addition, histopathologic correlations showed significant increase in the infiltrating CCR5-positive cells with worsening Banff grades.
Early ACR had significantly more CCR5-positive T cells in foci of tubulitis compared to late ACR. With animal model experiments demonstrating that blockade of CCR5 receptors leads to retardation of rejection episodes,[14,15] the findings of this study also indicate a future role of anti-CCR5 therapy in the treatment of rejection, particularly early episodes. In addition, as CCR5 represents the actively alloaggressive subset of T cells and increases with worsening of rejection, the authors propose that it be added to the ever-expanding list of activation markers for T cells, and future studies on cases of borderline rejection are warranted.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Gulanikar AC, MacDonald AS, Sungurtekin U, Belitsky P. The incidence and impact of early rejection episodes on graft outcome in recipients of first cadaver kidney transplants. Transplantation. 1992;53:323–8. doi: 10.1097/00007890-199202010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Hancock WW. Analysis of intragraft effector mechanisms associated with human renal allograft rejection: Immunohistological studies with monoclonal antibodies. Immunol Rev. 1984;77:61–84. doi: 10.1111/j.1600-065x.1984.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 3.Segerer S, Nelson PJ, Schlöndorff D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–76. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PJ, Krensky AM. Chemokines, chemokine receptors, and allograft rejection. Immunity. 2001;14:377–86. doi: 10.1016/s1074-7613(01)00118-2. [DOI] [PubMed] [Google Scholar]
- 5.Colvin BL, Thomson AW. Chemokines, their receptors, and transplant outcome. Transplantation. 2002;74:149–55. doi: 10.1097/00007890-200207270-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hancock WW. Chemokines and transplant immunobiology. J Am Soc Nephrol. 2002;13:821–4. doi: 10.1681/ASN.V133821. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 8.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant. 2008;8:753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 9.Pattison J, Nelson PJ, Huie P, von Leuttichau I, Farshid G, Sibley RK, et al. RANTES chemokine expression in cell-mediated transplant rejection of the kidney. Lancet. 1994;343:209–11. doi: 10.1016/s0140-6736(94)90992-x. [DOI] [PubMed] [Google Scholar]
- 10.Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–50. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 11.Segerer S, MacK M, Regele H, Kerjaschki D, Schlöndorff D. Expression of the CC chemokine receptor 5 in human kidney diseases. Kidney Int. 1999;56:52–64. doi: 10.1046/j.1523-1755.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 12.Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, et al. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am J Kidney Dis. 2001;37:518–31. [PubMed] [Google Scholar]
- 13.Serón D, O’Valle F, Moreso F, Gomà M, Hueso M, Grinyó JM, et al. Immunophenotype of infiltrating cells in protocol renal allograft biopsies from tacrolimus-versus cyclosporine-treated patients. Transplantation. 2007;83:649–52. doi: 10.1097/01.tp.0000253760.35580.d3. [DOI] [PubMed] [Google Scholar]
- 14.Gröne HJ, Weber C, Weber KS, Gröne EF, Rabelink T, Klier CM, et al. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: Blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371–83. [PubMed] [Google Scholar]
- 15.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]


