Abstract
Adenovirus (Ad) gene transfer vectors can be used to transfer and express antigens and function as strong adjuvants and thus are useful platforms for the development of genetic vaccines. Based on the hypothesis that Ad vectors with enhanced infectibility of dendritic cells (DC) may be able to evoke enhanced immune responses against antigens encoded by the vector in vivo, the present study analyzes the vaccine potential of an Ad vector expressing β-galactosidase as a model antigen and genetically modified with RGD on the fiber knob [AdZ.F(RGD)] to more selectively infect DC and consequently enhance immunity against the β-galactosidase antigen. Infection of murine DC in vitro with AdZ.F(RGD) showed an eightfold-increased transgene expression following infection compared to AdZ (also expressing β-galactosidase, but with a wild-type capsid). Binding, cellular uptake, and trafficking in DC were also increased with AdZ.F(RGD) compared to AdZ. To determine whether AdZ.F(RGD) could evoke enhanced immune responses to β-galactosidase in vivo, C57BL/6 mice were immunized with AdZ.F(RGD) or AdZ subcutaneously via the footpad. Humoral responses with both vectors were comparable, with similar anti-β-galactosidase antibody levels following vector administration. However, cellular responses to β-galactosidase were significantly enhanced, with the frequency of CD4+ as well as the CD8+ β-galactosidase-specific gamma interferon response in cells isolated from the draining lymph nodes increased following immunization with AdZ.F(RGD) compared to Ad.Z (P < 0.01). Importantly, this enhanced cellular immune response of the AdZ.F(RGD) vector was sufficient to evoke enhanced inhibition of the growth of preexisting tumors expressing β-galactosidase: BALB/c mice implanted with the CT26 syngeneic β-galactosidase-expressing colon carcinoma cell line and subsequently immunized with AdZ.F(RGD) showed decreased tumor growth and improved survival compared to mice immunized with AdZ. These data demonstrate that addition of an RGD motif to the Ad fiber knob increases the infectibility of DC and leads to enhanced cellular immune responses to the Ad-transferred transgene, suggesting that the RGD capsid modification may be useful in developing Ad-based vaccines.
Adenovirus (Ad) E1− E3− gene transfer vectors rapidly evoke strong humoral and cellular immune responses to their transgene product and thus are a useful platform for genetic vaccines (11, 26, 29, 35, 40, 47). In part, the effectiveness of Ad-based vaccines results from the ability of Ad vectors to transfer genes to antigen-presenting cells in vivo, particularly dendritic cells (DC) (8, 14, 18, 20, 27, 36, 42, 50). The focus of the present study is to engineer the Ad capsid to enhance the interaction of Ad with DC and thus enhance the effectiveness of Ad vector-based genetic vaccines.
The primary interaction of Ad with cells in vitro is through the affinity of the knob domain of fiber with the coxsackie-adenovirus receptor (CAR) on the target cell (4, 42). A secondary interaction occurs between the RGD motif in the penton base with αvβ3.5-integrin and similar integrins (21, 45). Since DC express low levels of CAR and high levels of surface integrins (3, 10, 42), theoretically they are suitable targets for Ad vectors that have been genetically modified to change their tropism to target integrins. In this context, addition of an RGD binding motif to the Ad fiber has been shown to improve the transduction of a variety of cell types exposing the α-integrins including endothelial cells, smooth muscle, fibroblasts, glioma, and DC (16, 27, 38, 46).
The present study analyzes the immunogenic potential of an Ad vector, expressing the β-galactosidase (β-gal) transgene as a surrogate marker, modified with RGD on the fiber knob [AdZ.F(RGD)], compared to an Ad vector with a wild-type capsid (AdZ). The data show that AdZ.F(RGD) led to increased infection efficiency of murine DC in vitro, as shown by increased binding, cellular uptake, intracellular trafficking, and transgene expression. Subcutaneous (footpad) immunization with AdZ.F(RGD) did not enhance humoral responses but evoked increased cellular responses, with enhanced CD4 and CD8 β-gal-specific gamma interferon (IFN-γ) production with AdZ.F(RGD) compared to AdZ. Most interestingly, the immunization with AdZ.F(RGD) resulted in decreased tumor growth and improved survival in mice with preestablished β-gal-expressing tumors, indicating that the increased cellular immune response can be translated to inhibit tumor growth. The data suggest that fiber-modified Ad vectors to target DC may be useful in the development of Ad-based vaccines.
MATERIALS AND METHODS
Ad vectors.
The recombinant Ad vectors used in this study are E1a, partial E1b, and partial E3 vectors based on the Ad5 genome. The expression cassettes were inserted into the E1 region and contained the human cytomegalovirus intermediate-early enhancer promoter, the transgene, and a simian virus 40 poly(A)/stop signal. The vectors expressed either β-gal (Z), luciferase (L), or no transgene (Null) (15). The Ad vectors contained the following modifications of the capsid proteins: AdZ and AdL, with wild-type capsids (retaining both CAR and integrin binding functions); AdZ.F(RGD), with the high-affinity RGD sequence GCDCRGDCFCA incorporated at the COOH-terminal end of the fiber protein (46); AdL.F*, with capsids defective for CAR binding due to site-directed mutagenesis of the fiber knob (19, 30); AdL.PB*, with capsids defective for integrin binding due to ablation of the RGD sequence in the penton base (44); and AdL.F*PB*, with capsids defective for CAR and integrin binding due to a mutation of the fiber knob and ablation of the RGD sequence (41). The vectors were used on the basis of equal numbers of physical particles and were propagated and purified as described previously (19, 23, 31, 32, 41, 44, 46).
Mice.
Female C57BL/6 and BALB/c mice were obtained from Taconic Farms (Tarrytown, N.Y.). The animals were housed under specific-pathogen-free conditions and used at 6 to 8 weeks of age. They were immunized by injection of 30 μl of Ad diluted in phosphate-buffered saline, pH 7.4 (PBS), subcutaneous into the footpad, intramuscularly into the calf muscle, intravenously via the tail vein, or into the lungs via intratracheal administration.
Cell culture.
Bone marrow-derived DC were generated from bone marrow precursors as described previously (36). In brief, bone marrow cells harvested from C57BL/6 mice were grown in complete RPMI 1640 medium (10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml [GIBCO BRL, Gaithersburg, Md.]) supplemented with 10 ng of recombinant mouse granulocyte-macrophage colony-stimulating factor per ml and 2 ng of recombinant mouse interleukin-4 (IL-4) per ml (both from R&D Systems, Minneapolis, Minn.) and used after culture for 8 days. The A549 lung epithelial cell line (CCL185; American type Culture Collection, Rockville, Md.) was maintained in complete Dulbecco's modified essential medium (10% fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml). The CT26.CL25 cell line, a colon carcinoma cell line syngeneic to BALB/c, modified to express β-gal as a model antigen, was maintained in complete RPMI 1640 medium containing 400 mg of G418 (GIBCO BRL) per ml (6).
Gene transfer to murine DC in vitro.
To determine the role of fiber and penton binding for Ad infection of DC, murine DC were infected with AdL, AdL.F*, AdL.PB*, or AdL.F*PB* at a dose of 104 particle units (PU) for 24 h. Luciferase expression was determined by the luciferase assay (Promega, Madison, Wis.), and expression was adjusted for total protein concentration by using the bicinchoninic acid assay (Bio-Rad Laboratories, Hercules, Calif.).
To quantify the efficiency of gene transfer by AdZ.F(RGD) to DC in vitro, murine DC or A549 cells were infected with AdZ.F(RGD), AdZ, or AdNull at 104 PU (DC) or 102 PU (A549) per cell. β-Gal expression in the cell lysates was determined 24 h later using the Galacto-Light kit (Tropix Inc, Bedford, Mass.) and adjusted for total protein concentration by using the bicinchoninic acid assay as described above. The limit of detection was 103 relative light units (RLU)/mg of protein.
To analyze the binding of AdZ.F(RGD) to DC, murine DC were infected with carboxyfluorescein (CF)-labeled AdZ.F(RGD) or AdZ. The Ad vectors were conjugated with 5-carboxyfluorescein succinimidyl ester (Molecular Probes, Eugene, Oreg.) as previously described (24). The DC were infected at 1011 PU/cell at 4°C for 30 min. At the end of the incubation, the cells were washed three times with PBS and labeled with phycoerythrin (PE)-conjugated antibody against major histocompatibility complex (MHC) class II (Pharmingen, San Diego, Calif.). An isotype PE-labled antibody was used as the control. The cells were then analyzed by flow cytometry (FACScalibur; BD Biosciences, Mountain View, Calif.).
To evaluate the trafficking of RGD-modified Ad in DC, murine DC were infected with AdZ.F(RGD) or AdZ conjugated with Cy3 fluorescent dye (Amersham Life Science, Arlington Heights, Ill.) as previously described (24) to allow assessment by fluorescence microscopy. DC were incubated with Cy3-AdZ or Cy3-AdZ.F(RGD) at 1011 PU/ml for 10 min at 37°C, washed with PBS, and incubated for 60 min at 37°C. At the end of the incubation, the cells were washed three times with PBS and fixed with 4% paraformaldehyde (at 23°C for 15 min). The cells were then treated with 1 μg of the DNA dye 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) per ml in PBS with 0.1% Triton X-100 for 5 min at 23°C to stain the nuclei. The samples were observed by fluorescence microscopy using a Nikon Microphot SA microscope (Nikon, Columbia, Md.), and digital image analysis was performed with Metamorph imaging software (Universal Imaging, West Chester, Pa.).
Transgene-specific humoral responses.
To evaluate the humoral response to the β-gal transgene following immunization with RGD-modified Ad, C57BL/6 mice were immunized subcutaneously in the footpad with AdZ.F(RGD) or AdZ at 108 or 109 PU/mouse. Mice injected with AdNull at equal doses or naive mice (PBS injected) served as controls. Serum was collected from the tail vein 14 and 28 days following immunization. Anti-β-gal-specific total immunoglobulin M (IgM) and IgG antibodies were determined by enzyme-linked immunosorbent assay. Microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 1 μg of β-gal (Roche Molecular Biochemicals, Indianapolis, Ind.) per well in PBS at 4°C. The plates were washed three times with PBS and blocked with 1% bovine serum albumin (Sigma-Aldrich, St. Louis, Mo.) in PBS for 30 min. After three washes with 0.05% Tween 20 in PBS (PBST), the sera were added in sequential twofold dilutions starting at 1:20 and the mixture was incubated for 1 h. After three washes with PBST, goat anti-mouse IgM or goat anti-mouse IgG (Caltag Laboratories, Burlingame, Calif.) was added at 0.5 μg/ml and the mixture was incubated for an additional 1 h. Following five washes with PBST, swine anti-goat IgG (Caltag) was added at 0.2 μg/ml for 1 h, and detection of bound antibody was accomplished using the peroxidase substrate kit (Bio-Rad Laboratories). Absorbance at 415 nm was read after 15 min. Titers were calculated as reciprocal dilutions twofold above background values (substrate only). For titer determination, the absorbance values of all dilutions were extrapolated to the twofold background value by using a linear-fit function (28).
Transgene-specific cellular responses.
To assess transgene-specific cellular immune response, C57BL/6 or BALB/c mice were immunized subcutaneously with 107, 108, or 109 PU (C57BL/6) or 5 × 108 PU (BALB/c) of AdZ.F(RGD), AdZ, or AdNull. The frequency of antigen-specific T lymphocytes was determined with an IFN-γ and IL-4-specific enzyme-linked immunospot (ELISPOT) assay 6 days following Ad administration. MAIPS-45 plates (Millipore, Bedford, Mass.) were coated overnight at 4°C with 5 μg of cytokine-specific capture antibodies AN18 (IFN-γ) or 11B11 (IL-4) (both from Mabtech, Stockholm, Sweden) per ml. Responder cells were isolated from regional lymph nodes by dispersing the tissue with a Potter-Elvehjem tissue grinder (Kontes Glass, Vineland, N.J.) and passing it through nylon gauze (spacing, 100 μm). Subsequently, CD4+ or CD8+ T cells were purified by negative depletion using SpinSep T-cell subset purification kits (StemCell Technologies, Vancouver, B.C., Canada). The purity for T-cell subsets was generally >95%. Splenic DC were purified from naive animals to serve as antigen-presenting cells by passing the spleens through mesh as described above and purifying the DC by positive selection using CD11c MACS beads (Miltenyi Biotec, Auburn, Calif.) and double purification over two consecutive MACS LS+ columns (Milentyi Biotec). Purity for DC was assessed by staining with an anti-CD11c antibody (BD Biosciences) (22) and was always >90%. Prior to the addition of responder T cells and DC, plates were blocked for 3 h with full RPMI medium supplemented with 10 mM HEPES (pH 7.4) (BioSource International, Camarillo, Calif.) and 10−5 M β-mercaptoethanol (Sigma-Aldrich).
For CD4+ ELISPOT assays, 2 × 105 CD4+ T cells were incubated with splenic DC at a ratio of 6:1 with or without β-gal. For CD8+ ELISPOT assays, 105 CD8+ T cells were incubated with β-gal-expressing CT26.CL25 colon carcinoma cells as antigen-presenting cells at a ratio of 3:1. All cell counts were performed using a FACScalibur flow cytometer running at a constant flow rate. The instrument was calibrated using QUANTIBRITE beads (BD Biosciences). The plates were incubated for 20 h (IFN-γ) or 48 h (IL-4). For development of the spots, the cells were flicked off and the plates were washed four times with PBST. Subsequently, 1 μg of biotinylated anti-IFN-γ or anti-IL-4 (both from Mabtech) detection antibodies were added and the plates were incubated for 2 h at 37°C. The plates were then washed four times with PBST, the streptavidin-alkaline phosphatase conjugate (Vectastain-ABC peroxidase kit; Vector Laboratories, Burlingame, Calif.) was added, the mixture was incubated for 1 h at 23°C, and the conjugate was removed by washing twice with PBST and three times with PBS. For final spot detection, the 3-amino-9-ethylcarbazole substrate (Sigma) was added for 4 min and rinsed with H2O. The plates were then dried for 30 min at 37°C in the dark, and spots were counted by computer-assisted ELISPOT image analysis (Zellnet Consulting, New York, N.Y.).
Transgene-specific suppression of tumor growth.
To determine if the enhanced anti-transgene cellular immune response mediated by AdZ.F(RGD) can induce enhanced suppression of established transgene-expressing tumors, the β-gal-expressing, BALB/c-derived tumor cell line CT26.CL25 was used in a model for established tumors. BALB/c mice were injected subcutaneously into the left flank with CT26.CL25 cells (2 × 105 cells/mouse). When the tumors had grown to 20 to 25 mm2 (day 6), the animals were immunized by subcutaneous injection to the right footpad with AdZ.F(RGD), AdZ, or AdNull (all vectors at 1010 PU/mouse). The sizes of the tumors were monitored every other day. The tumor area was calculated and expressed as the average tumor area (square millimeters) ± standard error of the means (SEM). If the animals appeared moribund or the largest diameter reached 15 mm, the animals were sacrificed and this was recorded as the date of death for the survival studies.
Statistical analysis.
The data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using the nonpaired two-tailed Student t test, assuming equal variance. Statistical significance was determined at P < 0.05. Survival estimates and median survivals were determined using the method of Kaplan and Meier.
RESULTS
Increased efficiency of gene transfer of AdZ.F(RGD) to DC.
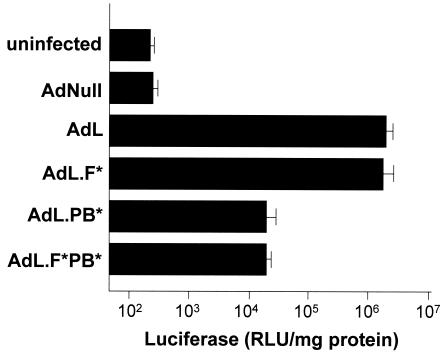
To evaluate the importance of integrin binding for Ad infection of DC, infection efficiency was compared using modified Ad vectors that are ablated in the fiber and/or penton base and are thus unable to bind to the CAR receptor and/or integrins on the cell surface. Murine DC infected with either AdL or AdL.F* showed comparable levels of transgene expression following a 24-h infection (P > 0.6) (Fig. 1), indicating that the interaction of the Ad fiber with the DC is not necessary for infection of the DC. However, transgene expression levels were significantly decreased following infection with equal amounts of AdL.PB* (107-fold compared to AdL; (P < 0.001) and AdL.F*PB* (104-fold compared to AdL; P < 0.001) (Fig. 1). These observations demonstrate that the interaction via the integrin pathway is crucial for infection of DC by Ad vectors and predicts an increased infection efficiency for Ad vectors with additional integrin binding sites, such as AdZ.F(RGD).
FIG. 1.
Role of integrins in Ad vector infection of bone marrow-derived DC. DC were infected with AdL.F* (CAR binding ablated), AdL.PB* (integrin binding ablated), AdL.F*PB* (CAR integrin binding base ablated), or AdL (intact CAR and integrin binding) at 103 PU/cell. All vectors express luciferase (L) as a marker transgene. Infection with equal amounts of AdNull or uninfected cells were used as controls. Luciferase expression was measured in cell lysates 24 h following infection and was normalized to total cellular protein. Data are shown as the mean and SEM of triplicate measurements of one of three independent experiments.
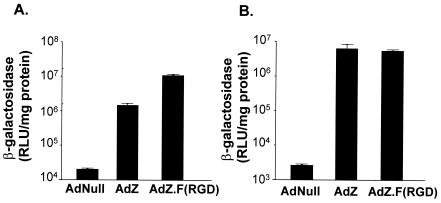
To assess the transduction efficiency of RGD-modified Ad in DC, murine DC were infected with AdZ.F(RGD) or AdZ in vitro. β-Gal expression was increased 7.6-fold following infection of DC with AdZ.F(RGD) compared to AdZ (P < 0.001), (Fig. 2A). In contrast, no increased transgene expression was observed with in A549 cells, which are known to have high levels of the CAR receptor (16) (P > 0.6) (Fig. 2B). This suggests that DC, which are known to be difficult targets for Ad transfer due to their low levels of the CAR Ad receptor (42), can be more efficiently infected by targeting RGD binding integrins.
FIG. 2.
Efficiency of gene transfer to DC with AdZ.F(RGD) compared to AdZ. Bone marrow-derived murine DC (A) or A549 (B) epithelial cells were infected with AdZ, AdZ.F(RGD) or AdNull at 104 PU (DC) or 102 PU (A549) per cell. β-Gal expression was determined 24 h later and was normalized to total cellular protein. Note that the background is higher in the DC than in the A549 cells. Data are shown as mean and SEM of quadruplicate measurements of one of three independent experiments.
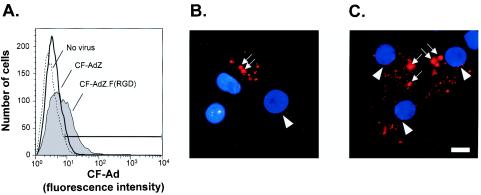
To evaluate the binding and intracellular trafficking of RGD-modified Ad in DC, murine DC were infected with fluorescently labeled Ad vectors. To assess binding, DC were incubated with either CF-AdZ.F(RGD) or CF-AdZ for 30 min at 4°C, conditions that do not permit virus internalization (24), and the association of Ad with the cells was evaluated by flow cytometry. DC incubated with CF-AdZ.F(RGD) showed increased binding of the vectors to class II-positive cells compared to CF-AdZ (33% ± 8% and 6% ± 3%, respectively; P < 0.05) (Fig. 3A).
FIG. 3.
Binding, uptake, and intracellular trafficking of AdZ.F(RGD) compared to AdZ in DC. (A) Bone marrow-derived murine DC were infected with 104 PU of CF-labeled AdZ.F(RGD) or CF-labeled AdZ for 30 min at 4°C and then analyzed. DC were identified by a PE-conjugated antibody against MHC class II. (B and C) To assess cellular uptake and trafficking of Ad vectors in DC by fluorescence microscopy, DC were infected with Cy3-labeled AdZ (B) or Cy3-labeled AdZ.F(RGD) (C) (1011 pu/ml) for 10 min at 37°C, incubated for 60 min, and analyzed by fluorescence microscopy. Fluorescence is shown in red. Nuclei were counterstained with DAPI (blue). Larger, brighter spots reflect sites of intracellular accumulation of Cy3 (arrowheads), consistent with sequestration in the endocytic pathway. Smaller spots at the nuclear periphery are associated with trafficking of Ad to the nuclear envelope (arrowheads). Bar, 10 μm.
To assess the intracellular uptake and trafficking of RGD-modified Ad in DC, murine DC were infected with Cy3-labeled AdZ.F(RGD) or AdZ for 10 min at 37°C and the intracellular localization of the Ad vectors was evaluated after 60 min by fluorescence microscopy. Fluorescence associated with viral particles was observed both in the cytoplasm and at the nuclear envelope in cells infected with Cy3-AdZ.F(RGD) and Cy3-AdZ (Fig. 3B and C). The cytoplasmic distribution was characterized by large, bright fluorescent puncta possibly caused by the accumulation of vectors in intracellular organelles (Fig. 3B and C). The nuclear envelope staining pattern, which was more prevalent in cells infected with Cy3AdZ.F(RGD), was characterized by smaller puncta, consistent with trafficking of individual virions to the nuclear envelope. Together, these data suggest that efficiency for Ad vectors in DC in using pathways that culminate in nuclear trafficking is increased by incorporation of the RGD sequence to the fiber, probably due to increased binding and subsequent cellular uptake of the modified Ad vector via integrins.
Humoral immune responses to AdZ.F(RGD).
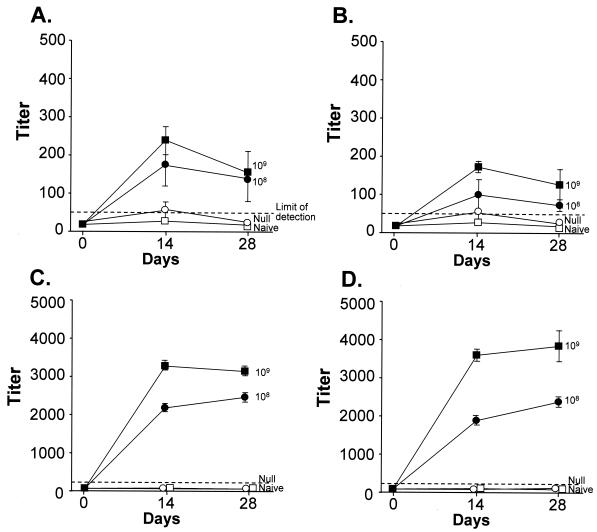
To assess the humoral response to the Ad transgene following immunization with AdZ.F(RGD) compared to AdZ, the serum anti-β-gal IgM and IgG responses in mice immunized by footpad administration of the two vectors were determined. Anti-β-gal-specific IgM and IgG antibodies were detected in the mice immunized with 108 and 109 PU 14 and 28 days following administration (Fig. 4). No significant titers were detectable in naive or AdNull-immunized animals. No significant differences were observed in the titers in of mice immunized with AdZ.F(RGD) and AdZ at any dose or time point evaluated (P > 0.2 for all comparisons) (Fig. 4). Similar results were observed in mice immunized with 109 PU of the Ad vectors via the intravenous, intramuscular, or intratracheal route (data not shown).
FIG. 4.
Humoral response to the β-gal transgene following immunization with RGD-modified Ad vector compared to a wild-type capsid Ad vector. C57BL/6 mice were infected with AdZ.F(RGD), AdZ, or AdNull at 108 or 109 PU/mouse by subcutaneous (footpad) administration. PBS-injected naive mice served as controls. Anti-β-gal total IgM (A and B) and IgG (C and D) antibodies were determined at 2 and 4 weeks by enzyme-linked immunosorbent assay. (A) IgM, AdZ; (B) IgM, AdZ.F(RGD); (C) IgG, AdZ; (D) IgG, AdZ.F(RGD). Data are shown as mean and SEM of four mice per group. The limit of detection is shown as a dashed line.
Cellular immune responses to Ad.F(RGD).
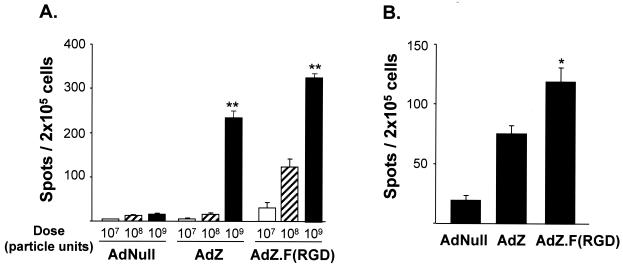
To assess cellular immune responses following immunization with Ad.F(RGD) compared to AdZ, C57BL/6 mice were immunized by subcutaneous administration of 107, 108, or 109 PU of AdZ.F(RGD) or AdZ and the frequency of antigen-specific, CD4+ T cells in the regional lymph nodes was determined by an ELISPOT assay. At 6 days following administration, CD4+ cells were isolated from the draining lymph nodes and the frequency of β-gal-specific IFN-γ and IL-4-producing cells was determined using β-gal-pulsed syngeneic DC as target cells. β-Gal-specific IFN-γ-producing CD4+ cells were detected in the AdZ.F(RGD)- and AdZ-immunized mice for all doses tested (Fig. 5A). The number of CD4+ IFN-γ-producing cells was dose dependent [P < 0.01 for 109 PU compared to 108 PU, for both vectors, and P < 0.01 for 108 PU compared to 107 PU for AdZ.F(RGD)]. Mice immunized with AdNull showed no significant signal above background. For both vectors, no significant number of spots was observed for IL-4-producing CD4 cells or IL-4- and IFN-γ-producing CD8+ cells (data not shown). At doses of 108 and 109 PU, more CD4+ IFN-γ-producing cells were observed with the AdZ.F(RGD) vector than with the AdZ vector (P < 0.01 for both comparisons). Similarly, an increase in the β-Gal-specific IFN-γ response in CD4+ cells following immunization with AdZ.F(RGD) was also observed in BALB/c mice immunized at a dose of 5 × 108 PU (Fig. 5B), although the overall number of spots was smaller compared to that for C57BL/6 mice immunized at a dose of 108 PU. These results indicate that AdZF.F(RGD) can induce a stronger Th1-type CD4+ response in vivo compared to that induced by an Ad vector with a wild-type capsid.
FIG. 5.
Frequency of β-gal-specific CD4+ T cells following immunization with AdZ.F(RGD). C57BL/6 (A) and BALB/c (B) mice were immunized subcutaneously in the footpad with AdZ, AdZ.F(RGD), or AdNull at 107, 108, or 109 PU/mouse (C57BL/6) or 5 × 108 PU/mouse (BALB/c). Naive mice receiving an equal volume of PBS served as controls. At 6 days following immunization, purified CD4+ cells from the draining lymph nodes (popliteal and inguinal) were cocultured with splenic DC with or without β-gal on IFN-γ-coated ELISPOT assay plates for 20 h. Biotinylated anti-IFN-γ antibodies were added and detected using a streptavidin-alkaline phosphatase-based detection system. Shown is the total number of spots without antigen (nonspecific IFN-γ production) and the number of spots from cells derived from naive animals subtracted from the number of positive spots with antigen. Data are presented as mean and SEM of the combined results of three (C57BL/6) or two (BALB/c) independent experiments; each data point represents 10 to 30 mice (cells from 3 mice were pooled for each setup). ** and * denote the significance (P < 0.01 and P < 0.05, respectively) of AdZ.F(RGD) versus Ad.Z.
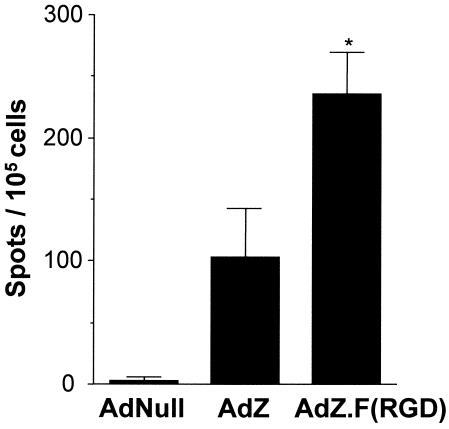
To assess the CD8+ T-cell response following immunization with Ad.F(RGD), BALB/c mice were immunized at a dose of 5 × 108 PU of either AdZ.F(RGD), AdZ, or AdNull. At 6 days following immunization, the CD8+ cells were isolated from the draining lymph nodes and assessed for β-Gal-specific responses following in vitro coculture with the β-gal-expressing cell line CT26.CL25 by using an IFN-γ ELISPOT assay. Transgene-specific IFN-γ production was detected in the cells derived from mice immunized with AdZ and AdZ.F(RGD) compared to the AdNull controls (P < 0.0001) (Fig. 6). The response in the mice immunized with AdZ.F(RGD) was 2.3-fold higher than that in the AdZ-immunized group (P < 0.05).
FIG. 6.
Frequency of antigen-specific CD8+ T cells following immunization with AdZ.F(RGD). BALB/c mice were immunized subcutaneously in the footpad with AdZ.F(RGD), AdZ, or AdNull at 5 × 108 PU/mouse. Naive mice receiving an equal volume of PBS served as controls. At 6 days following immunization, purified CD8+ cells from the draining lymph nodes (popliteal and inguinal) were cocultured with or without β-gal-expressing CT26.CL25 cells on IFN-γ-coated ELISPOT assay plates for 20 h. Biotinylated anti-IFN-γ antibodies were added and detected using a streptavidin-alkaline phosphatase-based detection system. Shown is the total number of spots without antigen (nonspecific IFN-γ production) and spots from cells derived from naive animals subtracted from the number of positive spots with antigen. Data are presented as mean and SEM of the combined results of three independent experiments (each data point represents 12 mice; cells from 3 mice were pooled for each). * denotes significance (P < 0.05) of AdZ.F(RGD) versus Ad.Z.
Transgene-specific antitumor effects following immunization with AdZ.F(RGD).
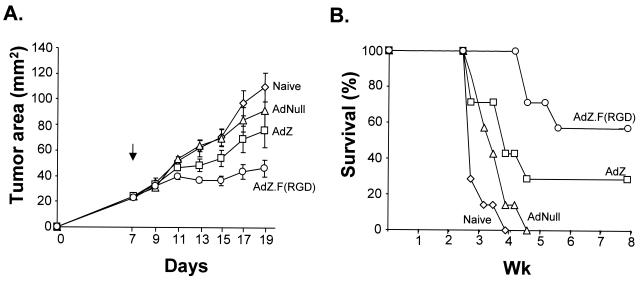
Immunization with AdZ.F(RGD) resulted in specific suppression of the growth of established β-gal-expressing tumors (Fig. 7A). Tumor growth was decreased in mice immunized with AdZ.F(RGD) compared to naive mice or mice immunized with AdNull or AdZ (P < 0.05). Mice immunized with AdZ showed some decrease in tumor growth compared to AdNull or naive mice, although the effect was smaller than that in the mice immunized with AdZ.F(RGD) (P < 0.05 for AdZ compared to AdNull or naive mice on day 13; P > 0.05 for all other time points for both comparisons) (Fig. 7A). Survival of mice immunized with AdZ.F(RGD) and AdZ was prolonged compared to that in the naive or AdNull group, but AdZ.F(RGD)-immunized mice showed prolonged survival compared to mice immunized with AdZ (P < 0.05) or AdNull or naive mice (P < 0.01) (Fig. 7B). These data are consistent with the enhanced antigen-specific cellular responses observed following immunization with AdZ.F(RGD) compared to AdZ and demonstrate that the enhanced immunity evoked by AdZ.F(RGD) compared to AdZ is translated into increased transgene-specific antitumor effects.
FIG. 7.
Suppression of the growth of established tumors (A) and increased survival (B) following subcutaneous immunization with AdZ.F(RGD) compared to AdZ. CT26.CL25 β-gal-expressing tumor cells were implanted subcutaneously (2 × 105) in the midflank of BALB/c mice. On day 7, tumor-bearing mice were treated by subcutaneous (footpad) injection of 1010 PU of AdZ.F(RGD), AdZ, Ad.Null, or PBS (Naive). Mice were euthanized once the tumor size reached 15 mm in one direction.
DISCUSSION
Ad vectors are capable of delivering genes to a variety of cell types. Relevant to the use of Ad as a platform for vaccines, Ad can infect and deliver genes to DC and other antigen-presenting cells in vivo (18, 20, 50), cells critical for the initiation of immune responses. Replication-competent Ad vaccines have been used to immunize U.S. military recruits against Ad, and recombinant Ad vectors expressing genes encoding antigens for a variety of microorganisms and tumors have been used in vaccination studies with rodents, canines, and nonhuman primates (11, 26, 29, 33, 35, 40, 47). The present study is grounded on the hypothesis that increased gene transfer to DC with Ad in vivo will lead to enhanced antigen presentation and thus to increased immune responses. Based on the knowledge that DC are relatively resistant to Ad-mediated gene transduction, partially due to the low level of CAR expression, but express a high level of integrins on the cell surface (3, 10, 42), Ad.F(RGD), an Ad vector modified to express RGD on the fiber and known to transfer genes to a variety of cell types more efficiently, increased the infection efficiency of DC in vitro and led to enhanced CD4+ and CD8+ transgene-specific cellular responses following footpad immunization compared to the effect of a similar Ad vector with a wild-type capsid. The increased cellular responses were functionally significant because they mediated a decrease of the growth of established transgene-expressing syngeneic tumors.
Targeting DC to enhance immune responses.
Various strategies can be used to enhance the effectiveness of Ad as vaccines, including: (i) the genetic manipulation of the antigen to target it to the appropriate antigen presentation pathway in order to enhance immune responses (39, 48), (ii) the use of potent promoters and multicistronic vectors (5), (iii) optimization of the codon usage of the antigen sequences in the expression cassette (2), and (iv) targeting of the Ad vaccine to antigen-presenting cells (7, 27).
The present study focuses on the last strategy to enhance the effectiveness of Ad vectors as platforms for vaccines. Ad vectors enter the cell by using two main receptors: CAR, a specific receptor for the fiber; and αvβ3- or αvβ5-integrin and related surface integrins for the penton base (21, 45). Since DC express a high level of integrins and a small number of CAR, incorporation of an RGD sequence improves the infectivity of DC by Ad in vitro (27). This effect is most probably a result of increased binding and uptake of the modified Ad vectors. Cells expressing a large number of CAR, like A549, can be efficiently infected with small numbers of Ad, and the infection efficiency cannot further be improved by adding the RGD sequence to the fiber. The importance of the integrin-RGD interaction for infection of DC was further illustrated in the present study by using Ad vectors deficient in fiber or penton binding.
Infection of the murine DC line DC2.4 with an RGD-modified Ad expressing ovalbumin resulted in increased activation of these cells, as demonstrated by increases in the surface expression of the activation markers CD40, CD80, CD86, and CD54 as well as MHC classes I and II (27). Intradermal injection of DC2.4 cells modified ex vivo with the RGD-modified Ad expressing ovalbumin resulted in increased antiovalbumin cellular immune responses compared to injection of DC2.4 cells modified with a nonmodified Ad vector. The present study extends this observation by demonstrating its occurrence in vivo where the vector expressing the relevant antigen is directly administered rather than used to infect DC in vitro. The data show increased cellular responses to the Ad transgene following in vivo administration of AdZ.F(RGD), most probably as a result of increased infection and activation of DC in vivo by the RGD-modified Ad.
Role of DC in anti-Ad immune responses.
Host immune responses to Ad vectors are robust and are directed against the transgene expressed by infected cells as well as against the Ad capsid proteins (12, 25, 37, 43, 49). The immune responses to the transgene are humoral and cellular and are both systemic and mucosal (18, 37, 43, 47). The immune response to the transgene is thought to be predominantly via the endogenous pathway, although the exact antigen presentation pathway is not completely understood (12, 18, 43, 47). Ad-infected cells expressing the transgene, leading to degradation of the gene product by the proteosome for presentation on the cell surface by MHC class I proteins, elicit a cellular immune response.
DC in tissue take up and process antigens and then migrate via the afferent lymphatics to the lymphoid tissue, where they present and stimulate the development of antigen-specific T cells (3). Ad administered subcutaneously to mice has been shown to travel to the local lymph nodes (20) and has been found to activate DC in the spleen following systemic or intramuscular administration (18, 50). Direct interaction of DC with Ad seems to be critical for Ad-mediated immunity, and direct infection of DC following intravenous and intramuscular administration with Ad has been demonstrated to lead to antigen presentation via the endogenous pathway (18). It is likely that infection of other cells at the site of administration leads to expression of the transgene, which can then be taken up by the DC as an extracellular antigen and processed via the MHC class II pathway, leading to a predominantly humoral antitransgene response (17). Furthermore, the Ad vector in itself may act in that fashion as an adjuvant by inducing a strong inflammatory response at the injection site, infiltration of immune cells, and apoptosis of infected cells (13, 25, 34). The uptake of apoptotic bodies by DC may transfer the transgenes, enabling them to present the antigen on their MHC class II and I (via cross-presentation) molecules, thereby enhancing the immune response (1, 17).
Immunization with the RGD-modified vectors resulted in a humoral response to the β-gal transgene, but this was not different from the response seen with an unmodified vector. A possible explanation for this is that β-gal as an intracellular protein is mostly processed to induce cellular immune responses. β-Gal is also strongly immunogenic in Ad constructs, and a less immunogenic antigen might have shown differences in the humoral response by adding RGD.
Cellular immunity against modified Ad vectors.
Cellular anti-Ad and antitransgene responses are known to be induced following administration of Ad gene transfer vectors in vivo (12, 25, 37, 47, 49). The cellular immunity against the Ad per se has hampered the use of Ad vectors for gene therapy for applications requiring long-term expression (12, 25, 37, 47, 49). However, the use of Ad for vaccination purposes, especially against intracellular organisms and cancer, requires the most efficient induction of cellular responses. DC activate both cytotoxic T cells (CD8+) and helper cells (CD4+) (3). The CD4+ cells include Th1 and Th2 cells, identified by their cytokine profile (9). The cellular response observed in the present study in cells derived from the draining lymph nodes was a transgene-specific Th1-dominant CD4+ and CD8+ response, which was more pronounced with the RGD-modified vector.
Intravenous administration of a β-gal-expressing unmodified Ad induces transgene-specific cytotoxic T cells and leads to β-gal-specific tumor regression (6). Immunization with Ad-modified DC induces potent antitransgene cellular immunity, which can result in protection against infectious organisms or can inhibit the growth of tumors. Immunization with DC modified with an RGD-modified vector, similar to that used in the present study, expressing ovalbumin, resulted in stronger anti-Ad cellular responses (27). In the present study, the immunization with Ad.F(RGD) not only resulted in stronger CD4+ and CD8+ responses but also led to transgene-specific tumor regression. In this regard, strategies to target DC in vivo to enhance Ad-mediated immune responses have the potential to improve vaccine strategies against intracellular organisms or tumors.
Acknowledgments
We thank R. E. Yamada for technical assistance and N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by U01 HL66952 and M01RR00047; the Will Rogers Memorial Fund, Los Angeles, Calif; the Cystic Fibrosis Foundation, Bethesda, Md.; and GenVec, Inc., Gaithersburg, Md.
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Bonini, C., S. P. Lee, S. R. Riddell, and P. D. Greenberg. 2001. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J. Immunol. 166:5250-5257. [DOI] [PubMed] [Google Scholar]
- 6.Chen, P. W., M. Wang, V. Bronte, Y. Zhai, S. A. Rosenberg, and N. P. Restifo. 1996. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J. Immunol. 156:224-231. [PMC free article] [PubMed] [Google Scholar]
- 7.Denis-Mize, K. S., M. Dupuis, M. L. MacKichan, M. Singh, B. Doe, D. O'Hagan, J. B. Ulmer, J. J. Donnelly, D. M. McDonald, and G. Ott. 2000. Plasmid DNA adsorbed onto cationic microparticles mediates target gene expression and antigen presentation by dendritic cells. Gene Ther. 7:2105-2112. [DOI] [PubMed] [Google Scholar]
- 8.Dietz, A. B., and S. Vuk-Pavlovic. 1998. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood 91:392-398. [PubMed] [Google Scholar]
- 9.Dong, C., and R. A. Flavell. 2001. Th1 and Th2 cells. Curr. Opin. Hematol. 8:47-51. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927-930. [DOI] [PubMed] [Google Scholar]
- 11.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 12.Hackett, N. R., S. M. Kaminsky, D. Sondhi, and R. G. Crystal. 2000. Antivector and antitransgene host responses in gene therapy. Curr. Opin. Mol. Ther. 2:376-382. [PubMed] [Google Scholar]
- 13.Harvey, B. G., S. Worgall, S. Ely, P. L. Leopold, and R. G. Crystal. 1999. Cellular immune responses of healthy individuals to intradermal administration of an E1-E3-adenovirus gene transfer vector. Hum. Gene Ther. 10:2823-2837. [DOI] [PubMed] [Google Scholar]
- 14.Herrera, O. B., S. Brett, and R. I. Lechler. 2002. Infection of mouse bone marrow-derived dendritic cells with recombinant adenovirus vectors leads to presentation of encoded antigen by both MHC class I and class II molecules-potential benefits in vaccine design. Vaccine 21:231-242. [DOI] [PubMed] [Google Scholar]
- 15.Hersh, J., R. G. Crystal, and B. Bewig. 1995. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 2:124-131. [PubMed] [Google Scholar]
- 16.Hidaka, C., E. Milano, P. L. Leopold, J. M. Bergelson, N. R. Hackett, R. W. Finberg, T. J. Wickham, I. Kovesdi, P. Roelvink, and R. G. Crystal. 1999. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J. Clin. Investig. 103:579-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, M. Albert, N. Bhardwaj, I. Mellman, and R. M. Steinman. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188:2163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 2000. Identification of contact residues and definition of the CAR-binding site of adenovirus type 5 fiber protein. J. Virol. 74:2804-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labow, D., S. Lee, R. J. Ginsberg, R. G. Crystal, and R. J. Korst. 2000. Adenovirus vector-mediated gene transfer to regional lymph nodes. Hum. Gene Ther. 11:759-769. [DOI] [PubMed] [Google Scholar]
- 21.Mathias, P., T. Wickham, M. Moore, and G. Nemerow. 1994. Multiple adenovirus serotypes use alpha v integrins for infection. J. Virol. 68:6811-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metlay, J. P., M. D. Witmer-Pack, R. Agger, M. T. Crowley, D. Lawless, and R. M. Steinman. 1990. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 171:1753-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittereder, N., K. L. March, and B. C. Trapnell. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 70:7498-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinier-Frenkel, V., R. Lengagne, F. Gaden, S. S. Hong, J. Choppin, H. Gahery-Segard, P. Boulanger, and J. G. Guillet. 2002. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 76:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 27.Okada, N., T. Saito, Y. Masunaga, Y. Tsukada, S. Nakagawa, H. Mizuguchi, K. Mori, Y. Okada, T. Fujita, T. Hayakawa, T. Mayumi, and A. Yamamoto. 2001. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 61:7913-7919. [PubMed] [Google Scholar]
- 28.Plikaytis, B. D., S. H. Turner, L. L. Gheesling, and G. M. Carlone. 1991. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:1439-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randrianarison-Jewtoukoff, V., and M. Perricaudet. 1995. Recombinant adenoviruses as vaccines. Biologicals 23:145-157. [DOI] [PubMed] [Google Scholar]
- 30.Roelvink, P. W., L. G. Mi, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld, M. A., W. Siegfried, K. Yoshimura, K. Yoneyama, M. Fukayama, L. E. Stier, P. K. Paakko, P. Gilardi, L. D. Stratford-Perricaudet, and M. Perricaudet. 1991. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 252:431-434. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld, M. A., K. Yoshimura, B. C. Trapnell, K. Yoneyama, E. R. Rosenthal, W. Dalemans, M. Fukayama, J. Bargon, L. E. Stier, and L. Stratford-Perricaudet. 1992. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 68:143-155. [DOI] [PubMed] [Google Scholar]
- 33.Rubin, R. A., and L. B. Rourke. 1994. Adenovirus vaccines, p. 475-501. In S. A. Plotkin and E. A. Mortimer (ed.), Vaccines. The W. B. Saunders Co., Philadelphia, Pa.
- 34.Russi, T. J., E. A. Hirschowitz, and R. G. Crystal. 1997. Delayed-type hypersensitivity response to high doses of adenoviral vectors. Hum. Gene Ther. 8:323-330. [DOI] [PubMed] [Google Scholar]
- 35.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 36.Song, W., H. L. Kong, H. Carpenter, H. Torii, R. Granstein, S. Rafii, M. A. Moore, and R. G. Crystal. 1997. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 186:1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song, W., H. L. Kong, P. Traktman, and R. G. Crystal. 1997. Cytotoxic T lymphocyte responses to proteins encoded by heterologous transgenes transferred in vivo by adenoviral vectors. Hum. Gene Ther. 8:1207-1217. [DOI] [PubMed] [Google Scholar]
- 38.Staba, M. J., T. J. Wickham, I. Kovesdi, and D. E. Hallahan. 2000. Modifications of the fiber in adenovirus vectors increase tropism for malignant glioma models. Cancer Gene Ther. 7:13-19. [DOI] [PubMed] [Google Scholar]
- 39.Suhrbier, A. 1997. Multi-epitope DNA vaccines. Immunol. Cell Biol. 75:402-408. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 41.Thomas, C. E., P. Edwards, T. J. Wickham, M. G. Castro, and P. R. Lowenstein. 2002. Adenovirus binding to the coxsackievirus and adenovirus receptor or integrins is not required to elicit brain inflammation but is necessary to transduce specific neural cell types. J. Virol. 76:3452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tillman, B. W., T. D. de Gruijl, S. A. Luykx-de Bakker, R. J. Scheper, H. M. Pinedo, T. J. Curiel, W. R. Gerritsen, and D. T. Curiel. 1999. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J. Immunol. 162:6378-6383. [PubMed] [Google Scholar]
- 43.Tripathy, S. K., H. B. Black, E. Goldwasser, and J. M. Leiden. 1996. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2:545-550. [DOI] [PubMed] [Google Scholar]
- 44.Wickham, T. J., M. E. Carrion, and I. Kovesdi. 1995. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 2:750-756. [PubMed] [Google Scholar]
- 45.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 46.Wickham, T. J., E. Tzeng, L. L. Shears, P. W. Roelvink, Y. Li, G. M. Lee, D. E. Brough, A. Lizonova, and I. Kovesdi. 1997. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 71:8221-8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, J. M. 1996. Adenoviruses as gene-delivery vehicles. N. Engl. J. Med. 334:1185-1187. [DOI] [PubMed] [Google Scholar]
- 48.Wu, T. C., F. G. Guarnieri, K. F. Staveley-O'Carroll, R. P. Viscidi, H. I. Levitsky, L. Hedrick, K. R. Cho, J. T. August, and D. M. Pardoll. 1995. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc. Natl. Acad. Sci. USA 92:11671-11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]