Abstract
A syndrome of rapid-onset end-stage renal disease (SORO-ESRD) following acute kidney injury (AKI) in native kidneys was described recently. To what extent this syndrome of unanticipated and rapidly irreversible ESRD impacts renal allograft survival is unknown. Over 6 months, we managed two deceased donor renal transplant recipients (RTRs) with rapid acceleration of previously stable allograft chronic kidney disease to abruptly terminate in irreversible ESRD following AKI. These are the first reports of SORO-ESRD in RTRs. More research is needed to ascertain the contribution of SORO-ESRD to renal allograft loss.
Keywords: Acute kidney injury, chronic kidney disease, end-stage renal disease, renal transplant recipient
Introduction
In 2010, we described for the first time, the previously unrecognized syndrome of rapid- onset end-stage renal disease (SORO-ESRD) in chronic kidney disease (CKD) patients. This syndrome was described distinct from the classic CKD-ESRD progression profile of a linear, predictable, smooth, and simply time-dependent CKD progression, with progressively increasing serum creatinine values over time, leading inexorably to ESRD and the requirement for renal replacement therapy.[1] Furthermore, we have defined the syndrome of rapid onset end stage renal disease SORO-ESRD as the unpredictable, nonlinear, abrupt, and rapid acceleration to permanent irreversible ESRD, often over a period of less than 2–4 weeks, following acute kidney injury (AKI) in CKD patients with previous stable estimated GFR (eGFR).[1–3] Moreover, our working diagnosis of SORO-ESRD is any CKD patient with an otherwise a priori stable eGFR of ≥30 ml/min/1.73 sq. m body surface area BSA, on or before the 90th day preceding first renal replacement therapy (RRT) following AKI, and who has remained permanently on RRT for >90 days.[1–3] To what extent this syndrome of rapidly irreversible ESRD similarly impacts renal allograft survival is unknown. In June 2011, we completed a retrospective analysis of the serum trajectories of the last 100 ESRD patients who had been on maintenance hemodialysis for >90 days and who were seen between February 2010 and January 2011 in a Northwestern Wisconsin Mayo Clinic Hemodialysis Group. We had identified 31 patients who satisfied the diagnosis of SORO-ESRD.[2,3] Two of these 31 (6.5%) SORO-ESRD patients were renal transplant recipients (RTRs).[2,3] For purposes of clarification, we have included a composite figure here showing the serum creatinine trajectories of two of our previous study patients, one (top) showing the so-called classic CKD-ESRD progression pattern, and the second (lower) showing the SORO-ESRD pattern of rapid irreversible ESRD following an AKI event [Figure 1].
Figure 1.
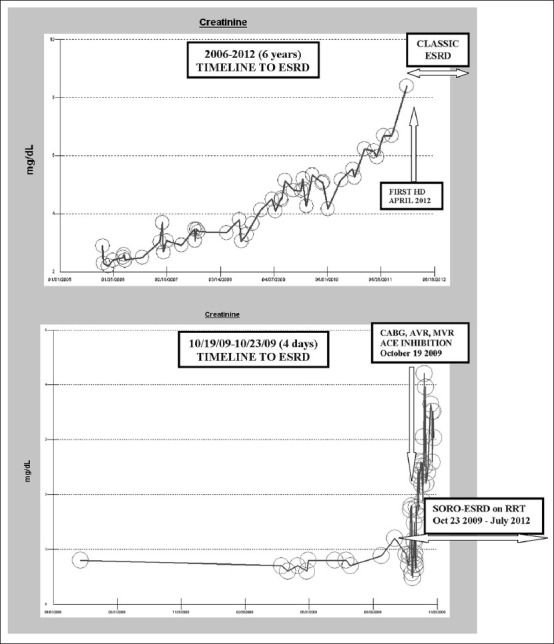
Composite showing serum creatinine trajectories in classic ESRD and in SORO-ESRD
Case Reports
Patient I is a 53-year-old Caucasian type 1 diabetic woman with simultaneous pancreas-kidney transplantation (SPK) in 2000 for ESRD and was maintained on chronic transplant immunosuppression with tacrolimus, cellcept, and prednisone. Through most of 2010, her allograft CKD stage III had remained stable; serum creatinine was 1.6 mg/dL dl on November 23, 2010. In January 2011, she experienced transplant pyelonephritis precipitating AKI, further complicated by dehydration from 1one week of nausea, vomiting, and diarrhea. The patient’s serum creatinine rose quickly within days to 5.16 mg/dLdl. [Figure 2] depicts the eGFR trajectory over time. Concurrently, she developed progressive oliguria, anorexia, and volume overload, requiring emergent hemodialaysis via a tunneled central dialysis catheter on January 8, 2011. Kidney allograft biopsy carried out the following week at Mayo Clinic, Rochester, following a referral, revealed acute tubular necrosis (ATN) and chronic transplant glomerulopathy without rejection, even though she received empiric high-dose, short-course tapered steroid therapy for possible rejection. She remained on maintenance hemodialysis for oliguric irreversible ESRD until January 2012, a year later, when she was successfully re-transplanted with a living-related kidney allograft from her 32-year year-old son, again at Mayo Clinic, Rochester. It is noteworthy that her pancreas allograft has, however, remained functional throughout this period with a current normal hemoglobin A1c (HbA1c) of 5.5% as at May 2012.
Figure 2.
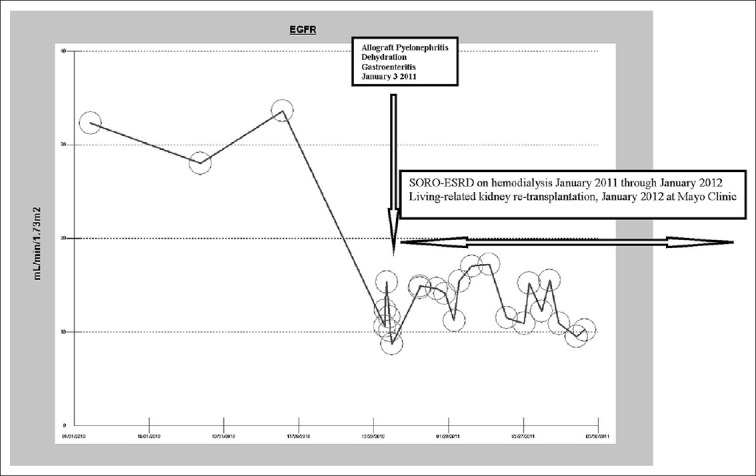
eGFR trajectory in the first RTR with SORO-ESRD, before and after January 2011
Patient II is a 56-year-old Caucasian hypertensive diabetic female patient, with SPK from 1991 for ESRD and was maintained on chronic transplant immunosuppression with cyclosporine and prednisone. During the first quarter of 2010, allograft CKD stage III was stable; serum creatinine was 1.8 mg/dL in April 2010. She suffered from pneumonia and congestive heart failure (CHF) exacerbation that same month, complicated by AKI and a transient but reversible rise in serum creatinine. This illness was then subsequently followed by vomiting from symptomatic diabetic gastroparesis, together with dehydration, and this time serum creatinine rose further to about 3.4 mg/dL the following month, in May 2010. Her renal allograft function again improved and serum creatinine had returned to 1.8 mg/dLdl, her previous baseline, as at of June 14, 2010. She then experienced two back-to-back episodes of AKI associated with pneumonia in July 2010, and this time serum creatinine rose quickly to 4.8 mg/dL, further complicated by oliguria and volume overload, and her renal allograft function has remained irreversibly damaged. Through July 2012, 24 months later, she has since remained on maintenance hemodialysis for irreversible ESRD. Also, remarkably, her pancreas allograft has however remained functional with a current normal HbA1c of 4.7%.
Discussion
These two cases represent the first reports of the syndrome of rapid onset end stage renal disease (SORO-ESRD) among RTRs.[1–3] The second RTR had experienced multiple back-to-back episodes of AKI with renal recovery in between before the final rapid crash to irreversible SORO-ESRD. This clinical picture resonates very closely with the description of Kelly and Dominguez who had analyzed serum creatinine changes in CKD patients with native kidneys and had demonstrated that CKD did not progress linearly, but, rather, that CKD was characterized by a succession of seemingly random acute episodes of renal functional loss.[4] Conversely, the first RTR presented here suffered a single AKI episode resulting from allograft pyelonephritis and dehydration and never recovered allograft function [Figure 2].
These observations of two RTRs with SORO-ESRD in a Northwestern Wisconsin four-nephrologist private practice group, ostensibly serving about 50,000 population, over a relatively short period of six months, suggest that this syndrome of rapid onset end stage renal disease may represent an unrecognized contributor to renal allograft loss. Multicenter studies of this syndrome are mandated to accurately identify accurately the extent to which this syndrome contributes to renal allograft loss within the general renal transplant population.[2,3] Moreover, the identification of susceptible RTRs and plausible potential risk or trigger factors associated with this phenomenon of SORO-ESRD would prove beneficial to renal transplantation medicine. The renal transplant community continues to struggle with identifying and preventing the various factors that contribute to renal allograft loss with the aim of improving renal allograft survival.[5–9] The information resulting from the extended investigation of SORO-ESRD in the general transplant population would be useful to transplant nephrologists as we strive to improve renal allograft survival, in general.[5–9]
Furthermore, we should begin to expend more energy and research resources towards elucidating more aggressive approaches to achieving effective renoprevention measures, in order to reduce AKI-triggered ESRD or SORO-ESRD.[1–3,10,11] These new preventive innovations, if confirmed by further research to be effective, could subsequently mandate major makeovers and process re-engineering of current accepted paradigms and norms in transplant nephrology practice.[1–7,10,11]
Acknowledgments
This work is dedicated to the memory of a very dear friend, Ikechukwu Ojoko (Idejuogwugwu), who passed away back home in Port Harcourt, Nigeria, some years ago, after a reported brief illness. Idejuogwugwu, you are truly missed. This work is also dedicated to the memories of the 153 Nigerians who died in a fiery plane crash in Lagos, Nigeria, on June 3, 2012. May their souls rest in perfect peace.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Onuigbo MA. Syndrome of rapid-onset end-stage renal disease: A new unrecognized pattern of CKD progression to ESRD. Ren Fail. 2010;32:954–8. doi: 10.3109/0886022X.2010.502608. [DOI] [PubMed] [Google Scholar]
- 2.Onuigbo MA, Onuigbo N. The syndrome of rapid onset end-stage renal disease (SORO-ESRD): A new previously unrecognized pattern of CKD to ESRD progression – A call for rethinking and re-engineering of current paradigms in CKD management. In: Onuigbo MA, Onuigbo N, editors. Chronic Kidney Disease and RAAS Blockade: A New View of Renoprotection. London, England: Lambert Academic Publishing GmbH 11 and Co. KG; 2011. pp. 188–236. [Google Scholar]
- 3.Onuigbo MA, Onuigbo NT. The syndrome of rapid onset end-stage renal disease (SORO-ESRD) – A new Mayo Clinic dialysis services experience, January 2010-February 2011. In: Di Iorio B, Heidland A, Onuigbo M, Ronco C, editors. Hemodialysis, When, How, Why. New York, NY: NOVA Science Publishers; 2012. pp. 443–85. [Google Scholar]
- 4.Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol. 2010;32:469–75. doi: 10.1159/000320749. [DOI] [PubMed] [Google Scholar]
- 5.Lo A. Strategies to prevent chronic allograft nephropathy in kidney transplantation: Focus on calcineurin inhibitors. Prog Transplant. 2004;14:157–64. doi: 10.1177/152692480401400210. [DOI] [PubMed] [Google Scholar]
- 6.Spinner ML, Saab G, Casabar E, Bowman LJ, Storch GA, Brennan DC. Impact of prophylactic versus preemptive valganciclovir on long-term renal allograft outcomes. Transplantation. 2010;90:412–8. doi: 10.1097/TP.0b013e3181e81afc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378:1428–37. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 8.Lamattina J, Sollinger H, Becker Y, Mezrich J, Pirsch J, Odorico J. Long-term pancreatic allograft survival after renal retransplantation in prior simultaneous pancreas-kidney recipients. Am J Transplant. 2012;12:937–46. doi: 10.1111/j.1600-6143.2011.03916.x. [DOI] [PubMed] [Google Scholar]
- 9.Arulkumaran N, West S, Chan K, Templeton M, Taube D, Brett SJ. Long-term renal function and survival of renal transplant recipients admitted to the intensive care unit. Clin Transplant. 2012;26:E24–31. doi: 10.1111/j.1399-0012.2011.01520.x. [DOI] [PubMed] [Google Scholar]
- 10.Onuigbo MA. Reno-prevention vs. reno-protection: A critical re-appraisal of the evidence-base from the large RAAS blockade trials after ONTARGET - A call for more circumspection. QJM. 2009;102:155–67. doi: 10.1093/qjmed/hcn142. [DOI] [PubMed] [Google Scholar]
- 11.Onuigbo MA. Can ACE inhibitors and angiotensin receptor blockers be detrimental in CKD patients? Nephron Clin Pract. 2011;118:c407–19. doi: 10.1159/000324164. [DOI] [PubMed] [Google Scholar]


