Abstract
Gram-negative organisms are a rare cause of infective endocarditis. Escherichia coli, the most common cause of urinary tract infection and gram-negative septicemia involves endocardium rarely. In this case report, we describe infection of native mitral valve by E. coli following septicemia of urinary tract origin in a diabetic male; subsequently, he required prosthetic tissue valve replacement indicated by persistent sepsis and congestive cardiac failure.
Keywords: Escherichia coli, native valve endocarditis, urosepsis
Introduction
The overall incidence of infective endocarditis (IE) caused by non-HACEK gram-negative organisms (organisms other than Hemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, or Kingella species) varies between 3% (community acquired native valve endocarditis) and 13% (prosthetic valve endocarditis in the first two postoperative months). Among 2761 patients with definite IE enrolled in International Collaboration on Infective Endocarditis Prospective Cohort Study (ICE-PCS) database, non-HACEK gram-negative organisms were the cause in 49 (1.8%); Escherichia coli was the cause in 29% of them. Prosthetic valves (59%) and implanted endovascular devices (29%) were more commonly associated with non-HACEK gram-negative bacillus endocarditis.[1]
Case Report
A 54-year-old Indian male with diabetes mellitus and hypertension presented with right loin pain, fever, and tiredness since 1 week. He had undergone meatotomy and circumcision 3 years ago. A year later, there was recurrence of poor urinary stream.
On examination, he was febrile, blood pressure was 140/70 mmHg, chest was clear and there were no murmurs over heart on auscultation. Abdomen was soft on palpation and there was significant tenderness over the right loin. Urethral meatus was pin hole and there were changes of lichen sclerosis in the skin over glans.
Investigations showed 1+ proteinuria, 18-20 white blood cells, and 10-15 red blood cells on urine analysis; hemoglobin, 13 g/dL; total leukocyte count, 17.1 × 103/mm3; differential count polymorphs, 89%; lymphocytes, 9%; eosinophils, 1% and monocytes, 1%; platelet count, 108 × 103/mm3; serum creatinine, 3.5 mg/dL; metabolic acidosis; and serum albumin, 2.2 g/dL. Electrocardiography revealed sinus tachycardia, leftward QRS axis, and poor R wave progression over chest leads. There was right turbid hydro-ureteronephrosis on ultrasonogram abdomen. Transthoracic echocardiogram (TTE) revealed regional wall motion abnormalities, normal valves, and mild left ventricle (LV) systolic dysfunction (ejection fraction 45%).
He was started on cefoperazone-sulbactam and amikacin (with dose adjusted for renal function pending cultures). Insulin infusion was required for control of severe hyperglycemia. Planning ureteric stenting, he underwent cystoscopy on day 0; there was penile urethral stricture and visual internal urethrotomy was performed followed by right ureteric stenting. There was purulent hydronephrotic drip following the stent insertion.
Blood and urine grew E. coli. Levofloxacin was substituted for amikacin based on anti-biogram 5 days after admission to minimize the risk of nephrotoxicity. He was continued on two antibiotics as he was still sick and febrile with persisting leukocytosis (22.0 × 103/mm3; 22.0 × 109/L). Evaluation for focal renal and prostatic abscesses with abdominal and transrectal ultrasound was negative. There was some improvement in renal function following these measures and serum creatinine was 2.4 mg/dL on day 6.
He developed a new grade 3 systolic murmur over the apex of the heart along with clinical and radiological signs of congestive cardiac failure on day 6; repeat TTE revealed flail mitral valve, severe mitral regurgitation, a small echogenic mass on the tip of anterior mitral leaflet suggestive of vegetations on the mitral valve, and dilated left atrium and left ventricle [Figure 1]. Amikacin was restarted with dose modified for renal function and levofloxacin was stopped. Cardiac failure was managed with intravenous frusemide. He had an episode of transient weakness of right upper limb that lasted for less than 12 h on day 7, suggesting a possible embolic transient ischemic attack (TIA) in middle cerebral artery territory. With worsening cardiac failure and respiratory distress, he was intubated and started on dobutamine infusion and ventilator support the same day. There was progressive decline in renal function from day 6. Serum creatinine was 3.2 mg/dL (282.8 μmol/L) on day 9. Repeat cultures were sterile.
Figure 1.
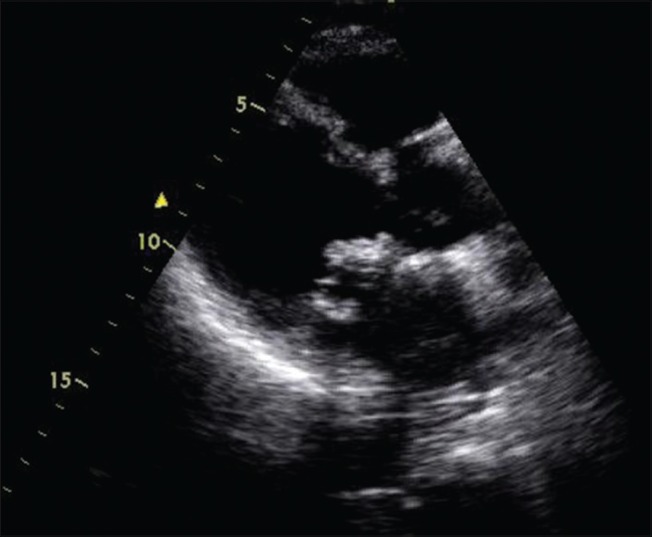
Echogenic mass with ruptured chordae of mitral valve on transthoracic echocardiogram
In view of persistent fever, IE and congestive cardiac failure, valve replacement was carried out on day 9. Mitral valve had vegetations over the anterior leaflet; chordae were necrotic and there were abscesses over the papillary muscles [Figure 2]. Mitral valve excision and replacement with 27 mm St. Jude Medical Biocor™ was carried out along with two vessel coronary artery bypass grafting as there was 100% occlusion of left anterior descending artery and long segment stenosis of proximal left coronary artery on angiogram. Transesophageal echocardiography (TEE) at the end of the procedure showed no residual mitral incompetence.
Figure 2.
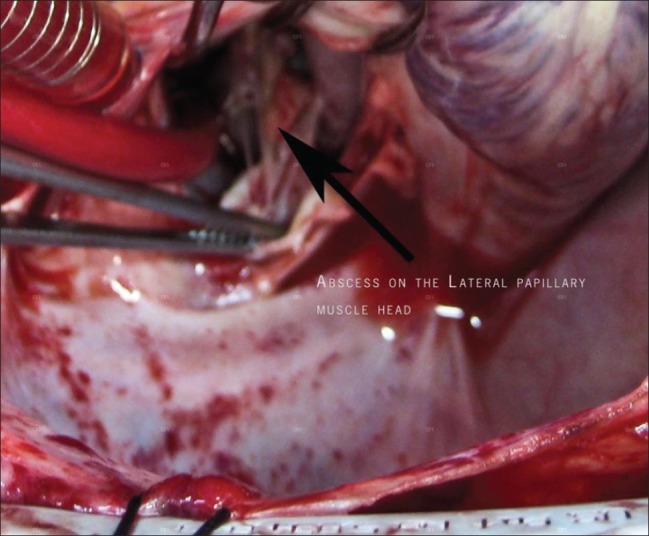
Abscess on the papillary muscle of the mitral valve apparatus observed intraoperatively
His overall clinical status gradually and steadily improved; he could be successfully weaned off ventilator. Smear from the resected valve showed numerous pus cells on gram-stain and scanty growth of E. coli on culture. Repeat cultures on day 16 were sterile. He was continued on cefoperazone sulbactam for 6 weeks and alternate day amikacin for 4 weeks (with periodic monitoring of serum creatinine). Acitrom was added for anticoagulation. Serum creatinine declined to 1.9 mg/dL on day 19 and 1.7 mg/dL on day 50. Cultures continued to be sterile more than 2 weeks after discontinuation of all antibiotics. At last visit (22 months since first presentation), he was clinically comfortable and serum creatinine was 1.6 mg/dL; there was no further episode of urinary infection and there was no valve regurgitation.
Discussion
E. coli native valve endocarditis is extremely rare considering the frequency with which this organism causes bacteremia. In an 18-year prospective survey of 3605 bacteremia episodes, E. coli accounted for 861 (23.9%) and the most common focus of infection was urinary tract. Only two patients had IE.[2] In an exhaustive literature review, only 36 cases had Duke criteria definite E. coli native valve endocarditis between 1909 and 2002,[3] occurring most frequently in immunocompromised elderly females, especially those with DM. The initial event was most often urinary tract infection (UTI). Mitral valve was involved in the majority.[3] Renal involvement in IE can be the consequence of immunologic and embolic phenomena apart from the non-specific effects of sepsis and nephrotoxicity of drugs.
Extra-intestinal pathogenic E. coli strains are phylogenetically and epidemiologically different from commensals and intestinal pathogenic strains. Like commensals, they appear to be incapable of causing intestinal disease and can stably colonize the host intestinal tract.[4] Though they are poorly adherent to endocardium accounting for the low incidence of E. coli IE,[5] they possess various combinations of extra-intestinal virulence factors such as adhesins, iron acquisition systems, host defense avoidance systems, and toxins which make them serious pathogens once they gain entry into a normally sterile extra intestinal site.[4]
Though the overall weight of evidence and opinion regarding management of IE caused by Gram-negative bacilli is in favor of early cardiac surgery in combination with prolonged courses (at least 6 weeks) of combined antibiotic therapy, particularly in the setting of left-sided involvement,[6,7] analysis of ICE-PCS database suggested no major survival benefit with additional cardiac surgery or with combination antibiotic therapy.[1] Aminoglycosides should not be administered as single daily dose in patients with normal renal function and serum concentrations should be periodically monitored. When fever persists for more than 7 days inspite of appropriate antibiotic therapy, the possibilities of paravalvular abscess, extra-cardiac abscess, and embolic events have to be considered. The size of the vegetations may not indicate response. Mortality rate varies from 30% to 83% in different series; it was 21% in the ICE-PCS database for E. coli IE.[1]
The patient discussed in this case report satisfied one major (endocardial involvement as evidenced by oscillating intra-cardiac mass and new onset mitral regurgitation on echocardiogram) and three minor (fever, positive blood culture not meeting major criterion, and embolic TIA) modified Duke criteria for the clinical diagnosis of definite IE; the diagnosis was proved beyond doubt with positive culture from surgical debridement of the valve.
In our case, pyelonephritis was most likely the primary event as there was an underlying predisposing factor for UTI (prior urologic problem with recurrence of thin stream) and there was no predisposition for IE in the form of predisposing heart condition (normal valves observed on the first echocardiography) or intravenous drug use. Renal failure was related to the effects of infection and obstruction. Worsening of renal failure following initial recovery was related to congestive cardiac failure.
The differential diagnosis of persisting fever in any patient with septicemia under treatment with culture specific antibiotic includes IE apart from the difference between in vivo and in vitro antibiotic sensitivity, super infection with fungus or another bacterium that is resistant to the current antibiotic or presence of focal or metastatic abscess or the occurrence of infection in relation to other interventions like central or peripheral line infection. Persistent E. coli bacteremia in an elderly in the absence of cardiac risk factors should prompt echocardiography as this could be a manifestation of native valve endocarditis[3] and this case report illustrates the need to consider the differential diagnosis of IE in any patient with urosepsis having persistent fever while on appropriate antibiotic therapy. Aggressive antibiotic and surgical management of such patients is important to improve patient survival.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Morpeth S, Murdoch D, Cabell CH, Karchmer AW, Pappas P, Levine D, et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829–35. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gransden WR, Eykyn SJ, Phillips I, Rowe B. Bacteremia due to Escherichia coli: A study of 861 episodes. Rev Infect Dis. 1990;12:1008–18. doi: 10.1093/clinids/12.6.1008. [DOI] [PubMed] [Google Scholar]
- 3.Micol R, Lortholary O, Jaureguy F, Bonacorsi S, Bingen E, Lefort A, et al. Escherichia coli native valve endocarditis. Clin Microbiol Infect. 2006;12:401–3. doi: 10.1111/j.1469-0691.2006.01375.x. [DOI] [PubMed] [Google Scholar]
- 4.Russo TA, Johnson JR. Proposal for a new inclusive definition for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181:1753–4. doi: 10.1086/315418. [DOI] [PubMed] [Google Scholar]
- 5.Gould K, Ramirez-Ronda CH, Holmes RK, Sanford JP. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975;56:1364–70. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, et al. AHA Scientific statement Infective Endocarditis Diagnosis, Antimicrobial Therapy and Management of Complications. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 7.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis and treatment of infective endocarditis. Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]


