Abstract
Background:
Titanium is used worldwide to make osseointegrable devices, thanks to its favorable characteristics as mechanical proprieties and biocompatibility, demonstrated by in vivo studies with animal models and clinical trials over a forty-year period. However, the exact genetic effect of the titanium layer on cells is still not well characterized.
Materials and Methods:
To investigate how titanium nanotubes stimulate osteoblasts differentiation and proliferation, some osteoblast genes (SP7, RUNX2, COL3A1, COL1A1, ALPL, SPP1 and FOSL1) were analyzed by quantitative Real Time RT- PCR.
Results:
After 15 days, osteoblasts cultivated on titanium naotube showed the up-regulation of bone related genes SP7, ENG, FOSL1 and SPP1 and the down-regulation of RUNX2, COL3A1, COL1A1, and ALPL. After 30 days of treatment, the bone related genes SP7, ENG, FOSL1 and RUNX2 were up-regulated while COL3A1, COL1A1, ALPL and SPP1 were down-regulated.
Conclusions:
Our results, demonstrates that titanium nanotubes can lead to osteoblast differentiation and extracellular matrix deposition and mineralization in dental pulp stem cells by the activation of osteoblast related genes SPP1, FOSL1 and RUNX2.
Keywords: Gene expression, nanotubes, osteoblasts, titanium disks
INTRODUCTION
Titanium and its alloys are employed as implant materials because of their desirable mechanical properties and “biocompatibility”.[1,2,3] For decades, oral, maxillofacial and orthopedic surgeons have utilized dental implants, screws and plates, and prostheses to substitute lost teeth, to fix bone fragments and to replace joints, respectively. Also, many surgical instruments, such as drills and saws, are made with titanium alloys. The “biocompatibility” of titanium has been demonstrated by in vivo studies with both animal models and clinical trials, for more than forty years.[1,4] Several authors studied other different aspect of implant morphology in order to value their impact on implant stability.[5,6,7,8,9,10,11] Macro-, mini-, micro- and nano-design characterizes prostheses. Macro-design is the shape of the prosthesis: some examples are offered by the cylindrical or root form of dental implants. Mini-design is the dimension of the threads or the shape of the neck of the dental fixture. The dimensions range from 1 to 0.1 mm. Micro-design is the shape of the implant surface: An example of which is provided by the “grooves and holes” resulting from surface treatments like machination, acid etching and sand blasting procedures. These treatments determine the roughness of the surface and the “holes” have a cellular dimension. Nano-design is determined by the molecular composition of the surface, such as those composed of: hydroxyapatite, zirconium and titanium. Usually, mechanical proprieties are related to macro- and mini-design, whereas biological properties are related to micro- and nano-design. Mechanical properties are responsible for primary implant stability, whereas biological properties act on the osseointegration process.[12]
When titanium is exposed to oxygen a stochastic distribution of three titanium-oxide isoforms (i.e., rutile, brookite and anatase) are available on the surface of the prosthesis. These isoforms are responsible for its material biological properties.[12]
Although previous studies have demonstrated that titanium surfacing is capable of modulating osteoblast gene expression,[13] they failed to separate the effect of roughness (i.e., micro-dimension) from that of composition (i.e., nano-dimension).
Effect of the titanium nanotubes layer on osteoblast gene expression has been studied, comparing the expression profiling of osteoblasts (HOb) cultivated on two type of surface: pure titanium disk (TD) and nanotubes titanium disk (NTD) in order to detect if NTD surface stimulates osteoblast proliferation.
The quantitative expression of the mRNA of specific genes, like transcriptional factors (RUNX2), bone related genes (SPP1, COL1A1, COL3A1, ALPL, and FOSL1) was examined by means of Real Time Reverse Transcription-Polymerase Chain Reaction (Real Time RT-PCR).
MATERIALS AND METHODS
Titanium nanotubes disks preparation
Disks of commercially pure grade-1 titanium (Titania, Italy) have been used as substrate for the nanotube growth. The disks have diameter of 30 mm with a thickness of 0.5 mm, and were arranged to show an active area of 3.8 cm2. After 3 minutes pickling in a HF (Carlo Erba)/HNO3 (Carlo Erba) solution, made by a volumetric ratio of 1:3 and diluted in deionized water until to 100 ml, all the titanium sheets have been set in three-electrode cell, containing a KOH 1 M solution (Carlo Erba) and subjected to a prefixed and optimized density current (1 mA/cm2), which is generated by a potentiostat/galvanostat Solartron 1286 for 3 minutes. The counter-electrode is a Platinum sheet, while the reference is a standard calomel electrode (SCE). The growth of the nanotube arrays has been made using a Glycol Ethylene solution with 1 %wt. H2O and 0, 2% wt. NH4F for 3 hours at 60 V. After the anodization treatment, all the samples are washed in glycole ethylene, left overnight in the dry room, in order to dry them. So to crystallize the TiO2 nanotubes, obtained in amorphous form by anodic growth, after a pre-heat treatment at 80°C in vacuum for 3 hours, all the samples have been placed in a tubular furnace (Lenton) for 1 hour at 580°C, with a slope of 1°C/minute in air, so to be transformed into the anatase phase.
Primary osteoblasts (HOb) cell culture
Fragments of bone derived from skull of an healthy volunteer were sampled during operation and transferred in 75 cm2 culture flasks containing DMEM medium supplemented with 20% fetal calf serum, antibiotics (Penicillin 100 U/ml and Streptomycin 100 micrograms/ml - Sigma Aldrich, Inc., St Louis, Mo, USA) and amminoacids (L-Glutamine - Sigma Aldrich, Inc., St Louis, Mo, USA). Cells were grown in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed the next day and every 3 days thereafter. After 15 days, the pieces of bone tissue were removed from the culture flask. Cells were harvested after 30 days of incubation.
Cell culture
For the assay, HOb were trypsinized upon subconfluence and seeded on NTD and TD.
The medium was changed every 3 days. The cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. Cells were collected, for RNA extraction at 15 and 30 days.
RNA processing
Reverse transcription to cDNA was performed directly from cultured cell lysate using the TaqMAN Gene Expression Cells-to-Ct Kit (Ambion Inc., Austin, TX, USA), following manufacturer's instructions. Briefly, cultured cells were lysed with lysis buffer and RNA released in this solution. Cell lysate were reverse transcribed to cDNA using the RT Enzyme Mix and appropriate RT buffer (Ambion Inc., Austin, TX, USA).
Finally, the cDNA was amplified by real-time PCR using the included TaqMan Gene Expression Master Mix and the specific assay designed for the investigated genes.
Real time PCR
Expression was quantified using real time RT-PCR. The gene expression levels were normalized to the expression of the housekeeping gene RPL13A and were expressed as fold changes relative to the expression of the untreated cells. Quantification was done with the delta/delta calculation method.[14]
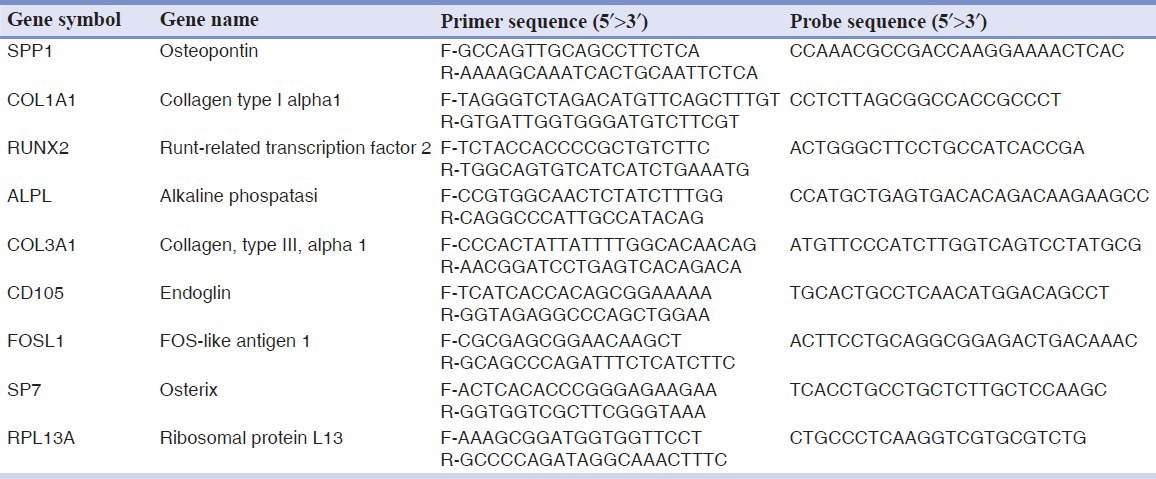
Forward and reverse primers and probes for the selected genes were designed using primer express software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 1.
Table 1.
Primer and probes used in real time PCR
All PCR reactions were performed in a 20 μl volume using the ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). Each reaction contained 10 μl 2X TaqMan universal PCR master mix (Applied Biosystems, Foster City, CA, USA), 400 nM concentration of each primer and 200 nM of the probe, and cDNA. The amplification profile was initiated by 10-minute incubation at 95°C, followed by two-step amplification of 15 seconds at 95°C and 60 seconds at 60°C for 40 cycles. All experiments were performed including non-template controls to exclude reagents contamination.
RESULTS
HObs were cultivated on two type of surface, NTD and TD. Gene expression in HOb cultivated on NTD was compared with that cultivated on TD (control) in order to check whether NTD is more osteoinducent respect TD.
After 15 days, HOb cultivated on NTD showed the up-regulation of bone related genes SP7, ENG, FOSL1 and SPP1 and the down-regulation of RUNX2, COL3A1, COL1A1, and ALPL [Figure 1a].
Figure 1.
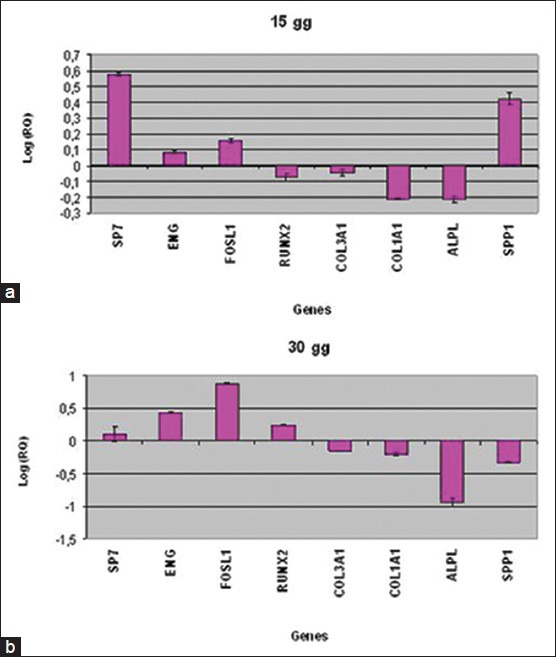
Relative gene expression of HOb cultivated on titanium disks for 15 days (a) and 30 days (b)
After 30 days of treatment, the bone related genes SP7, ENG, FOSL1 and RUNX2 were up-regulated in HOb cultivated on NTD, while COL3A1, COL1A1, ALPL and SPP1 were down-regulated [Figure 1b].
DISCUSSION
Titanium is well tolerated by the bone and is able to induce osseointegration of the implant. Osteointegration is primary element when considering implant stability.[15,16,17] Recently titania film have been used to cover prosthetic implants in medical applications such as orthopedic or dental surgery.[18] Titania film creates an active surface that is able to improve the biocompatibility of titanium implants.[19] In prostheses made of titanium, the titania film surface is mainly composed of two polymorphs of titanium, rutile and anatase. Rutile is considered the stable form of titania while anatase is metastable and converts to rutile at high temperatures.[19] Anatase titania is more advantageous for medical applications than rutile. Compared to rutile, anatase exhibits stronger interactions between metal and support, and the surface of anatase titania can absorb more OH- and PO43- than that of rutile titania in body fluid, which favors the depositing of bone-like apatite.[20]
Effect of the titanium nanotubes on HOb gene expression has been studied, comparing the expression profiling of osteoblasts cultivated on two type of surface: Pure titanium disk (TD) and nanotubes titanium disk (NTD) in order to detect if NTD surface stimulates HOb proliferation.
The quantitative expression of the mRNA of specific genes, like transcriptional factors (RUNX2 and SP7) and bone related genes (SPP1, COL1A1, COL3A1, ALPL, ENG and FOSL1) was examined by means of real time Reverse Transcription-Polymerase Chain Reaction (real time RT-PCR).
After 15 days of treatment the bone related genes SP7, ENG, FOSL1 and SPP1 were up-regulated in HOb cultivated on NTD respect to HOb cultivated on TD. These results indicated that NTD enhance osseointegration.
SP7 is a zinc finger transcription factor that regulates bone formation and osteoblast differentiation in vitro and in vivo and that is expressed in the early stage of osteogenic differentiation.
ENG is a receptor for TGF-β1 and -β3[21] and modulates TGF-β signaling by interacting with related molecules, such as TGF-β1, -β3, BMP-2, -7, and activin A. It is speculated that these members of the TFG-β super family are mediators of cell proliferation and differentiation and play regulatory roles in cartilage and bone formation.[22]
NTD also modulate the expression of FOSL1 that encodes for Fra-1, a component of the dimeric transcription factor activator protein-1 (Ap-1), which is composed mainly of Fos (c-Fos, FosB, Fra-1 and Fra-2) and Jun proteins (c-Jun, JunB and JunD).
AP-1 sites are present in the promoters of many developmentally regulated osteoblast genes, including alkaline phosphatase, collagen I, osteocalcin (OC).
Maccabe et al.[23] demonstrated that differential expression of Fos and Jun family members could play a role in the developmental regulation of bone-specific gene expression and as a result, may be functionally significant for osteoblast differentiation.
SPP1 encodes osteopontin, which is a phosphoglycoprotein of bone matrix and it is the most representative non collagenic component of extracellular bone matrix.[24] Osteopontin is actively involved in bone resorbitive processes directly by ostoclasts.[25] Osteopontin produced by osteoblasts, show high affinity to the molecules of hydroxylapatite in extracellular matrix and it is chemo-attractant to osteoclasts.[26]
After 30 days of treatment these genes, SP7, ENG and FOSL1, continue to be up-regulated in HOb cultivated on NTD, indicating the osteodifferentiative properties of titanium nanotubes.
In addition another bone related genes, RUNX2, was over-expressed in HOb cultivated on NTD respect to control. RUNX2 is a transcriptional factor activated in the first stage of differentiation and plays a fundamental role in osteoblast maturation and homeostasis. RUNX2-null mice have no osteoblasts and consequently bone tissue.[27]
CONCLUSION
Our results, demonstrates that NTD can lead to osteoblast differentiation and extracellular matrix deposition and mineralization in dental pulp stem cells by the activation of osteoblast related genes SPP1, FOSL1 and RUNX2.
These results showed that NTD is more osteoinducent surface compared to TD, promoting the osseointegration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: A review. Eur Cell Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 2.Fathi MH, Moosavi SB, Mortazavi V. Novel material for endodontic implant, in vitro and in vivo tests. Dent Res J. 2003:1. [Google Scholar]
- 3.Fathi MH, Mortazavi V. Tantalum, niobium and titanium coatings for biocompa improvement of dental implants. Dent Res J. 2007;4:74–82. [Google Scholar]
- 4.Degidi M, Piattelli A, Carinci F. Parallel screw cylinder implants: Comparative analysis between immediate loading and two-stage healing of 1,005 dental implants with a 2-year follow up. Clin Implant Dent Relat Res. 2006;9:151–60. doi: 10.1111/j.1708-8208.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 5.Fanali S, Carinci F, Zollino I, Brugnati C, Lauritano D. One-piece implants installed in restored mandible: A retrospective study. Eur J Inflamm. 2012;10:37–41. [Google Scholar]
- 6.Fanali S, Carinci F, Zollino I, Brugnati C, Lauritano D. A retrospective study on 83 one-piece implants installed in resorbed maxilla. Eur J Inflamm. 2012;10:55–8. [Google Scholar]
- 7.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Effect of distance between one piece implants on crestal bone resorption. Eur J Inflamm. 2011;9:1–6. [Google Scholar]
- 8.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Effect of one-piece implant diameter on clinical outcome. Eur J Inflamm. 2011;9:7–12. [Google Scholar]
- 9.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Impact of one-piece implant lenght on clinical outcome. Eur J Inflamm. 2011;9:13–8. [Google Scholar]
- 10.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Welding improbe the success rate of one-piece implants. Eur J Inflamm. 2011;9(S3):19–24. [Google Scholar]
- 11.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Bio-grip and machined titanium stimulate dental pulp stem cells towards osteoblast differentiation. Eur J Inflamm. 2011;9:25–30. [Google Scholar]
- 12.Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, et al. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25:2867–75. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 13.Carinci F, Pezzetti F, Volinia S, Francioso F, Arcelli D, Marchesini J, et al. Analysis of MG63 osteoblastic-cell response to a new nanoporous implant surface by means of a microarray technology. Clin Oral Implants Res. 2004;15:180–6. doi: 10.1111/j.1600-0501.2004.00997.x. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Scarano A, Murmura G, Carinci F, Lauritano D. Immediately loaded small diameter dental implants: Evaluation of retention, stability and comfort for the edentulous patient. Eur J Inflamm. 2012;10:19–24. [Google Scholar]
- 16.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. Peri-Implantitis: A case report and literature review. Eur J Inflamm. 2012;10:1–6. [Google Scholar]
- 17.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. SEM evaluation of 10 infected implants retrived from man. Eur J Inflamm. 2012;10:7–12. [Google Scholar]
- 18.Han Y, Hong SH, Xu KW. Porous nanocrystalline titania films by plasma electrolytic oxidation. Surf Coat Technol. 2002;154:314–8. [Google Scholar]
- 19.Tang GT, Zhang R, Yan Y, Zhang Z. Preparation of porous anatase titania film. Mater Lett. 2004;58:1857–60. [Google Scholar]
- 20.Akin FA, Zreiqat H, Jordan S, Wijesundara MB, Hanley L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone-tissue implants. J Biomed Mater Res. 2001;57:588–96. doi: 10.1002/1097-4636(20011215)57:4<588::aid-jbm1206>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–9. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 22.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–77. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.McCabe LR, Banerjee C, Kundu R, Harrison RJ, Dobner PR, Stein JL, et al. Developmental expression and activities of specific fos and jun proteins are functionally related to osteoblast maturation: role of Fra-2 and Jun D during differentiation. Endocrinology. 1996;137:4398–408. doi: 10.1210/endo.137.10.8828501. [DOI] [PubMed] [Google Scholar]
- 24.McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultra structural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993;9:485–96. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- 25.Dodds RA, Connor JR, James IE, Rykaczewski EL, Appelbaum E, Dul E, et al. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: an in vitro and ex vivo study of remodeling bone. J Bone Miner Res. 1995;10:1666–80. doi: 10.1002/jbmr.5650101109. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuki C, Kamitakahara M, Miyazaki T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J R Soc Interface. 2009;6(Suppl 3):S349–60. doi: 10.1098/rsif.2008.0419.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziros PG, Basdra EK, Papavassiliou AG. Runx2: of bone and stretch. Int J Biochem Cell Biol. 2008;40:1659–63. doi: 10.1016/j.biocel.2007.05.024. [DOI] [PubMed] [Google Scholar]


