Abstract
Background:
Titanium is the gold standard among materials used for prosthetic devices because of its good mechanical and chemical properties. When exposed to oxygen, titanium becomes an oxide, anatase that is biocompatible and able to induce osseointegration.
Materials and Methods:
In this study we compared the expression profiling of stem cells cultivated on two types of surface: Pure titanium disk and nanotube titanium disk in order to detect if nanotube titanium instead (NTD) surface stimulates stem cells towards osteoblast differentiation.
Results:
Stem cells cultivated on nanotube titanium disks showed the upregulation of bone-related genes RUNX2, FOSL1 and SPP1.
Conclusions:
Results demonstrated that nanotube titanium disk surface is more osteo-induced surface compared to titanium disk, promoting the differentiation of mesenchymal stem cells in osteoblasts.
Keywords: Gene expression, nanotubes, osteoblasts, stem cells, titanium disks
INTRODUCTION
Pure titanium and titanium alloys are materials widely used in orthopedic and dental surgery because of their desirable mechanical properties, chemical stability and biocompatibility.[1] Several authors studied different characteristics of implant morphology in order to value their impact on implant stability.[2,3,4,5,6,7,8] Indeed titanium is used to manufacture joint prosthesis for partial and total joint replacement. Moreover, titanium is also used to produce plates and screws for osteo-synthesis of fractures and for dental implants to substitute lost teeth.[9]
The biocompatibility of titanium is closely related to the properties of the surface oxide layer, in terms of its structure, morphology and composition.[10] Normally on the surface of the prosthesis made of titanium is present a stochastic distribution of two titanium-oxide (rutile and anatase), which are responsible for the properties of the material.[10] In biomedical implants, the anatase phase shows an enhanced ability to induce bone-like apatite in comparison with the rutile phase.[10]
Various physical and chemical treatments of the titanium surface have been proposed with a view to obtaining the most biocompatible one.[10]
We focused our interest on anatase titanium disks. These surfaces have been used as substrate for the nanotubes growth, to implement their characteristics of biocompatibility.
Tissue replacement by culturing autologous cells onto three-dimensional matrixes that facilitate cell progenitor migration, proliferation and differentiation[11] is one of the most promising bone regeneration techniques.
Stem cells are undifferentiated cells with the capability to regenerate into one or more committed cell lineages. Stem cells isolated from multiple sources have been finding widespread use to advance the field of tissue repair.[12]
Dental pulp is a niche housing neural-crest-derived stem cells that display plasticity and multipotential capability. This niche is easily accessible and there is limited morbidity of the anatomical site after collection of the pulp.[13]
Dental pulp stem cells (DPSCs) display noticeable plasticity that is explained by their neural-crest origin.[14] They can extensively proliferate and differentiate into odontoblastic, adipogenic and neural citotype, have a long lifespan and build in vivo an adult bone with Havers channels and an appropriate vascularization.[15]
Adipose tissue is another ideal source of autologous stem cells because it is easily obtainable by lipoaspiration, and its mesenchymal stem cells (MSCs) content is adequate for clinical-grade cell manipulation in regenerative medicine.
These cells, that display a fibroblast-like morphology and lack intracellular lipid droplets seen in adipocytes,[16] can be enzymatically digested out of adipose tissue and separated from the buoyant adipocytes by centrifugation.
A more homogeneous population emerges in culture under conditions supportive of marrow stromal cells growth. This population, termed adipose tissue-derived stem cells (ADSCs), after expansion in culture display a distinct phenotype based on cell surface protein expression and cytokine expression.[17]
In this study we compared the expression profiling of stem cells (DPSCs and ADSCs) cultivated on two type of surface: Pure titanium disk (TD) and nanotube titanium disk (NTD) in order to detect if NTD surface stimulates MSCs towards osteoblast differentiation.
The quantitative expression of the mRNA of specific genes, like transcriptional factors (RUNX2 and SP7), bone-related genes (SPP1, COL1A1, COL3A1, ALPL, and FOSL1) and MSCs marker (ENG) were examined by means of real-time Reverse Transcription-Polymerase Chain Reaction (real-time RT-PCR).
MATERIALS AND METHODS
Titanium nanotubes disks preparation
Disks of commercially pure grade-1 titanium (Titania, Italy) have been used as substrate for the nanotube growth. The disks have diameter of 30 mm with a thickness of 0.5 mm, and were arranged to show an active area of 3.8 cm2. After 3 min. pickling in a HF (Carlo Erba)/HNO3 (Carlo Erba) solution, made by a volumetric ratio of 1:3 and diluted in deionized water until 100 ml, all the titanium sheets have been set in three-electrode cell, containing a KOH 1 M solution (Carlo Erba) and subjected to a prefixed and optimized density current (1 mA/cm2), which is generated by a Potentiostat/Galvanostat Solartron 1286 for 3 min. The counter-electrode is a Platinum sheet, while the reference is a standard calomel electrode (SCE). The growth of the nanotube arrays has been made using a Glycol Ethylene solution with 1 %wt. H2O and 0.2%wt. NH4F for 3 h at 60 V. After the anodization treatment, all the samples are washed in glycol ethylene, left overnight in the dry room, in order to dry them. So to crystallize the TiO2 nanotubes, obtained in amorphous form by anodic growth, after a pre-heat treatment at 80°C in vacuum for 3 h, all the samples have been placed in a tubular furnace (Lenton) for 1 h at 580°C, with a slope of 1°C/min. in air, so as to be transformed into the anatase phase.
DPSCs isolation
Dental germ pulp was extracted from third molars of healthy subjects, following informed consent. Pulp was digested for 1 h at 37°C in a solution containing 3 mg/ml type I collagenase, 4 mg/ml Dispase, in 4 ml phosphate-buffered saline (PBS) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 500 μg/ml clarithromycin. The solution was then filtered with 70 μm Falcon strainers (Sigma Aldrich, Inc., St Louis, Mo, USA). Filtered cells were cultivated in α-MEM culture medium (Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with 20% FCS, 100 μM 2P-ascorbic acid, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and placed in 75 ml flasks. Flasks were incubated at 37°C and 5% CO2 and the medium changed twice a week.
ADSCs isolation
Human ADSCs were isolated from adipose tissue obtained by liposuction of adult volunteer patients. Fat was finely minced with sterile scissors, put in a tube and digested in DMEM supplemented with 1 mg/ml of collagenase type II, in 37°C water bath for 60 min, swirling occasionally.
Once centrifugated at 3000 rpm for 5 min, the sample was removed from centrifuge, shaken vigorously (to completely separate stromal cells from primary adipocytes) and centrifugated again for 5 min. The oily supernatant (which includes primary adipocytes) was aspirated and discarded, while the stromal fraction at the bottom was washed three times with 10 ml of PBSA 1X and centrifugated again for 5 min. Finally the pellet was resuspended in 10 ml of Alpha MEM medium (Sigma Aldrich, Inc., St Louis, Mo, USA) supplemented with 10% fetal calf serum, antibiotics (Penicillin 100 U/ml and Streptomycin 100 micrograms/ml-Sigma, Chemical Co., St Louis, Mo, USA) and amino acids (L-Glutamine - Sigma, Chemical Co., St Louis, Mo, USA). The medium was replaced after 2-3 days. Characterization of staminality was carried out by flow cytometric analyses.
Flow cytometric analyses
The purity of cell cultures was determined by analysis of different antigens after staining with fluorochrome (FITC- or PE-) conjugated mAbs anti-human CD14-FITC, CD14-PE, CD34-FITC, CD45-FITC, CD90-PE, CD105-PE (Immunotech, Marseille, France) and analyzed by FACScan. The nonspecific mouse IgG was used as isotype control (Immunotech). To avoid nonspecific fluorescence from dead cells, live cells were gated tightly using forward and side scatter.
Cell culture
For the assay, ADSCs and DPSCs were trypsinized upon sub-confluence and seeded on NTD and TD.
The medium was changed every 3 days. The cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. Cells were collected for RNA extraction at 15 and 30 days.
RNA processing
For cellular lysis, and subsequent reverse transcription of the RNA released in the solution, the TaqMan Gene Expression Cells-to-Ct Kit (Ambion Inc., Austin, TX, USA) was used. Once cultured cells were lysed with lysis buffer, free RNA was reverse transcribed to cDNA using the RT Enzyme Mix and appropriate RT buffer (Ambion Inc., Austin, TX, USA).
Finally the cDNA was amplified by real-time PCR using the included TaqMan Gene Expression Master Mix and the specific assay designed for the investigated genes.
Real-time PCR
Expression was quantified using real-time PCR. After normalization to the expression of the housekeeping gene RPL13A, the gene expression levels were expressed as fold changes relative to the expression of the untreated cells. Quantification was done with the delta/delta calculation method.[18]
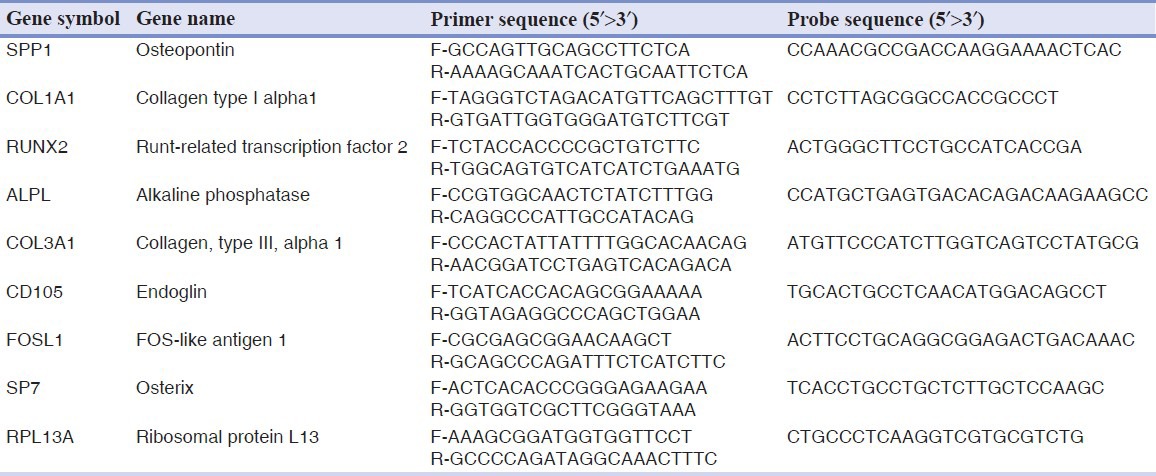
Specific forward and reverse primers and probes for the selected genes were designed using primer express software (Applied Biosystems, Foster City, CA, USA) and are listed in Table 1.
Table 1.
Primer and probes used in real-time Polymerase chain reaction
All PCR reactions were performed in a final volume of 20 μl containing 10 μl 2X TaqMan Universal PCR master mix (Applied Biosystems, Foster City, CA, USA), 400 nM concentration of each primer and 200 nM of the probe, and cDNA. The amplifications were carried out using the ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA). After an initial denaturing step at 95°C for 10 min, amplification profile proceeded with two-step of 15 s at 95°C and 60 s at 60°C for 40 cycles. All experiments were performed including non-template controls to check for the presence of contamination.
RESULTS
ADSCs and DPSCs were cultivated on two type of surface NTD and TD. Gene expression in stem cells cultivated on NTD were compared with that cultivated on TD (control) in order to check whether NTD is more osteoinductive with respect to TD.
Gene expression profiling in ADSCs
After 15 days, ADSCs cultivated on NTD showed the up-regulation of bone-related genes FOSL1, RUNX2, COL1A1, ENG and the down-regulation of SP7, ALPL and SPP1. Expression of COL3A1 was the same in both cells (cultivated on NTD and TD) [Figure 1a].
Figure 1.
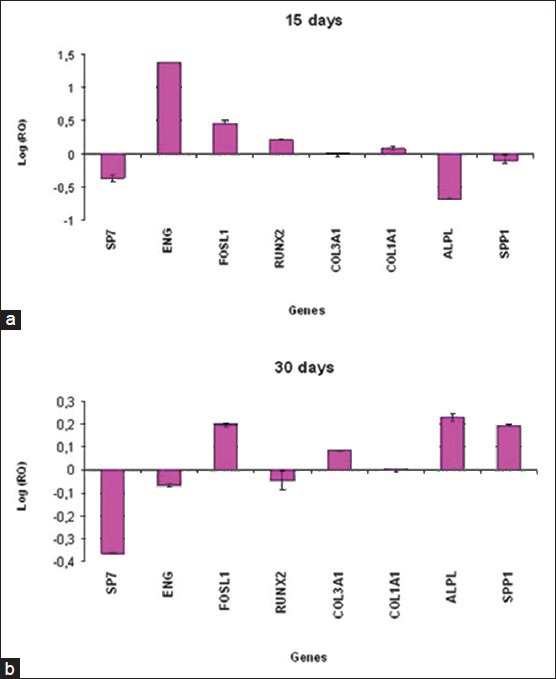
Relative gene expression of ADSCs cultivated on titanium disks for 15 days (a) and 30 days (b)
The results of real-time PCR showed that in ADSCs cultivated on NTD after 30 days of treatment, the bone-related genes FOSL1, COL3A1, COL1A1, ALPL and SPP1 were up-regulated, while SP7, ENG and RUNX2 were down-regulated [Figure 1b].
Gene expression profiling in DPSCs
Different results were obtained for DPSCs. After 15 days, stem cells cultivated on NTD showed the up-regulation of FOSL1 and SPP1 and the down-regulation of SP7, ENG, RUNX2, COL3A1, COL1A1 and ALPL [Figure 2a].
Figure 2.
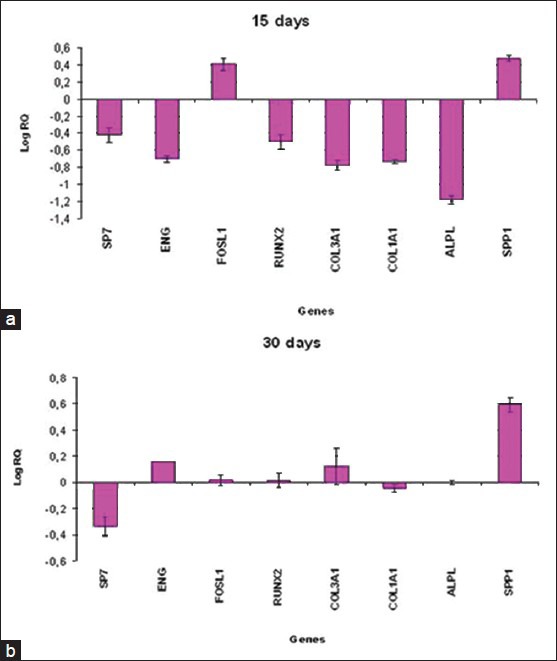
Relative gene expression of DPSCs cultivated on titanium disks for 15 days (a) and 30 days (b)
After 30 days, the bone-related genes ENG, FOSL1, RUNX2, COL3A1 and SPP1 were up-regulated in DPSCs cultivated on NTD, while ENG, FOSL1 and ALPL were decreased [Figure 2b].
DISCUSSION
Titanium proved to be a good material for surgical application. Since thirty years it has been used to produce prosthesis for joint replacement and for dental substitution. Recently, titania film have been used to cover prosthetic implants in medical applications such as orthopedic or dental surgery.[19] In prosthesis made of titanium, the titania film surface is mainly composed by two polymorphs of titanium, rutile and anatase. Anatase titania is more advantageous for medical applications than rutile.
In this study, we tested the osteoinductive property of TDs covered with nanotube in order to detect if this surface is better than pure TDs in terms of osseointegration. Osseointegration is primary element to value when considering implant stability.[20,21,22]
To study the osteoinductive properties of NTD, the expression levels of bone-related genes were measured using real-time RT-PCR in mesenchymal stem cells cultivated on TD.
The study was first conducted in ADSCs. The gene expression levels were measured at two time point: 15 and 30 days.
The up-regulation of the bone-related genes RUNX2, COL1A1 and FOSL1 in the first 15 days of cells culture indicated that NTD enhance osteo-differentiation and proliferation compared to TD.
RUNX2 is an important modulator of osteoblast differentiation that is activated in the first stage of differentiation and plays a fundamental role in osteoblast maturation and homeostasis. RUNX2-null mice have no osteoblasts and consequently bone tissue.[23] Thus we demonstrated that NTD increase the activity of RUNX2 gene in ADSCs, which is a key point in osteo-differentiation.
FOSL1 encodes for Fra-1, a component of the dimeric transcription factor activator protein-1 (AP-1). AP-1 sites are present in the promoters of many osteoblast genes, including alkaline phosphatase, collagen I and osteocalcin.
Type I collagen synthesis is associated with osteoblast differentiation in the early stage, followed by the synthesis of ALP.[24]
In fact ALPL was up-regulated after 30 days in stem cells cultivated on NTD. Increasing in ALPL expression is associated with osteoblast differentiation.[25]
After 30 days SPP1 and collagens were up-regulated too.
SPP1 encodes osteopontin, which is a phosphoglycoprotein of bone matrix and it is the most representative non-collagenic component of extracellular bone matrix.[26]
The present study demonstrated that NTD strongly influences the behavior of ADSCs in vitro by enhancing proliferation, differentiation and deposition of matrix as demonstrated by the activation of osteoblast-related genes RUNX2, COL1A1 and SPP1.
Osteo-differentiation promoted by NTD was observed in DPSCs too, after 15 and 30 days of treatment.
After 15 days, the up-regulation of the bone-related genes FOSL1 and SPP1 was observed.
Their up-regulation continued in the next 30 days of treatment, associated to an increasing of two osteoblasts-related genes, RUNX2 and COL3A1.
CONCLUSION
These results demonstrated that NTD surface is more osteo-induced surface compared to TD, promoting the differentiation of mesenchymal stem cells in osteoblasts.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Carinci F, Guidi R, Franco M, Viscioni A, Rigo L, De Santis B, et al. Implants inserted in fresh-frozen bone: A retrospective analysis of 88 implants loaded 4 months after insertion. Quintessence Int. 2009;40:413–9. [PubMed] [Google Scholar]
- 2.Fanali S, Carinci F, Zollino I, Brugnati C, Lauritano D. One-piece implants installed in restored mandible: a retrospective study. Eur J Inflamm. 2012;10:37–41. [Google Scholar]
- 3.Fanali S, Carinci F, Zollino I, Brugnati C, Lauritano D. A retrospective study on 83 one-piece implants installed in resorbed maxilla. Eur J Inflamm. 2012;10:55–8. [Google Scholar]
- 4.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Effect of distance between one piece implants on crestal bone resorption. Eur J Inflamm. 2011;9:1–6. [Google Scholar]
- 5.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Effect of one-piece implant diameter on clinical outcome. Eur J Inflamm. 2011;9:7–12. [Google Scholar]
- 6.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Impact of one-piece implant lenght on clinical outcome. Eur J Inflamm. 2011;9:13–8. [Google Scholar]
- 7.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Welding improbe the success rate of one-piece implants. Eur J Inflamm. 2011;9(S3):19–24. [Google Scholar]
- 8.Fanali S, Carinci F, Zollino I, Brunelli G, Minguzzi R. Bio-grip and machined titanium stimulate dental pulp stem cells towords osteoblast differentiation. Eur J Inflamm. 2011;9:25–30. [Google Scholar]
- 9.Gapski R, Wang HL, Mascarenhas P, Lang NP. Critical review of immediate implant loading. Clin Oral Implants Res. 2003;14:515–27. doi: 10.1034/j.1600-0501.2003.00950.x. [DOI] [PubMed] [Google Scholar]
- 10.Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, et al. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25:2867–75. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990;87:4473–5. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan TF, Poon A, Basu A, Addleman NR, Chen J, Phong A, et al. Natural variation in four human collagen genes across an ethnically diverse population. Genomics. 2008;91:307–14. doi: 10.1016/j.ygeno.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–85. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: A useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 15.Laino G, Graziano A, d’Aquino R, Pirozzi G, Lanza V, Valiante S, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Moritz N, Areva S, Wolke J, Peltola T. TF-XRD examination of surface-reactive TiO2 coatings produced by heat treatment and CO 2 laser treatment. Biomaterials. 2005;26:4460–7. doi: 10.1016/j.biomaterials.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. Peri-Implantitis: A case report and literature review. Eur J Inflamm. 2012;10:1–6. [Google Scholar]
- 21.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. SEM evaluation of 10 infected implants retrived from man. Eur J Inflamm. 2012;10:7–12. [Google Scholar]
- 22.Scarano A, Murmura G, Carinci F, Lauritano D. Immediately loaded small diameter dental implants: Evaluation of retention, stability and comfort for the edentulous patient. Eur J Inflamm. 2012;10:19–24. [Google Scholar]
- 23.Ziros PG, Basdra EK, Papavassiliou AG. Run×2: Of bone and stretch. Int J Biochem Cell Biol. 2008;40:1659–63. doi: 10.1016/j.biocel.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Harris SE, Bonewald LF, Harris MA, Sabatini M, Dallas S, Feng JQ, et al. Effects of transforming growth factor beta on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res. 1994;9:855–63. doi: 10.1002/jbmr.5650090611. [DOI] [PubMed] [Google Scholar]
- 25.Turksen K, Bhargava U, Moe HK, Aubin JE. Isolation of monoclonal antibodies recognizing rat bone-associated molecules in vitro and in vivo. J Histochem Cytochem. 1992;40:1339–52. doi: 10.1177/40.9.1506671. [DOI] [PubMed] [Google Scholar]
- 26.McKee MD, Farach-Carson MC, Butler WT, Hauschka PV, Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993;9:485–96. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]


