Abstract
While recently we have learned much about the viral and cellular proteins involved in the initial attachment of rotaviruses to MA104 cells, the mechanism by which these viruses reach the interior of the cell is poorly understood. For this study, we observed the effects of drugs and of dominant-negative mutants, known to impair clathrin-mediated endocytosis and endocytosis mediated by caveolae, on rotavirus cell infection. Rotaviruses were able to enter cells in the presence of compounds that inhibit clathrin-mediated endocytosis as well as cells overexpressing a dominant-negative form of Eps15, a protein crucial for the assembly of clathrin coats. We also found that rotaviruses infected cells in which caveolar uptake was blocked; treatment with the cholesterol binding agents nystatin and filipin, as well as transfection of cells with dominant-negative caveolin-1 and caveolin-3 mutants, had no effect on rotavirus infection. Interestingly, cells treated with methyl-β-cyclodextrin, a drug that sequesters cholesterol from membranes, and cells expressing a dominant-negative mutant of the large GTPase dynamin, which is known to function in several membrane scission events, were not infected by rotaviruses, indicating that cholesterol and dynamin play a role in the entry of rotaviruses.
The initial steps in a viral infection involve the specific attachment of the viral particle to a receptor(s) on the cell surface, followed by internalization of the virus into the cell and the subsequent uncoating of the virion to release the active transcription complex. These events are essential for the successful initiation of a virus replication cycle and play an important role in the tissue tropism and pathogenesis of viruses.
In general, viruses can enter cells by fusion of the viral and cellular membranes at the plasma membrane level or in endocytic vesicles or, more rarely, in the case of nonenveloped viruses, by a direct mechanism at the cell surface by which the viral particles are directly translocated into the cytoplasm. The endocytic pathways used by different viruses include clathrin-mediated endocytosis, uptake via caveolae, macropinocytosis, phagocytosis, and a novel nonclathrin, noncaveolar pathway which is currently not well characterized (55).
Rotaviruses, members of the family Reoviridae, represent the single most important etiologic agents of viral gastroenteritis in the young of many animal species, including humans, and are responsible for about 800,000 deaths a year in children under 2 years of age (24). These nonenveloped viruses are formed by three concentric layers of protein which surround the viral genome, formed by 11 segments of double-stranded RNA. The outermost layer of the virion is formed by two proteins, VP4 and VP7, which are responsible for early interactions of the virus with its host cell, i.e., the attachment of the viral particle to specific cellular receptors and the penetration of the virion into the cell's cytoplasm. The rate of the entry step is increased by, and most probably dependent on, trypsin treatment of the virus, which cleaves VP4 into two polypeptides, VP8 and VP5. The cleavage of VP4 does not affect cell binding and has been associated with entry of the virus by direct cell membrane penetration (23). Despite recent advances in the characterization of the viral and cellular proteins involved in the initial interactions of rotaviruses with host cells (2, 28), the mechanism by which these viruses reach the cell interior is poorly understood.
Early electron microscopy studies of rotavirus-infected cells described the presence of rotavirus particles in coated pits and in a variety of vesicles (46, 47), and by use of this technique, it was also proposed that trypsin-treated, infectious rotavirus particles enter the cells by direct plasma membrane penetration, while untreated noninfectious viral particles are removed from the cell surface by endocytosis, a process that does not lead to a productive infection (57). However, since electron microscopy studies with viruses are performed with a large number of particles and since virus preparations usually contain a vast excess of noninfectious particles, it is not possible to determine by this method alone whether individual events are part of a pathway leading to productive infection. In the case of rotaviruses, it is known that the ratio of physical to infectious viral particles may vary between 100 and 10,000 (33).
Biochemical approaches have also been pursued to determine the entry pathway of rotaviruses. The importance of the acidification of endosomes for the initiation of a productive entry has been analyzed by use of several lysosomotropic agents, such as NH4Cl, chloroquine, methylamine, and amantadine (13, 23, 29). The effects of drugs that block the intracellular traffic of endosomes, such as cytochalasin D, dansylcadaverin, or bafilomycin A1 (which inhibits the endosomal proton-ATP pump), have also been tested (4, 8, 13, 23). Thus far, none of these treatments has resulted in the inhibition of viral entry, arguing against a classical endocytic pathway. Direct cell membrane penetration has thus been alternatively proposed as the mechanism of entry for rotaviruses; however, the evidence that supports this mechanism is rather indirect and mainly suggests that a nonendocytic route is used.
Most of the studies that have addressed the mechanism of rotavirus cell entry have used inhibitory drugs, and they have been focused on the analysis of virus uptake by clathrin-coated pits. Recently, however, new techniques and reagents have allowed the characterization of alternative endocytic pathways, which include uptake by caveolae, which can be experimentally disrupted by depletion of plasma membrane cholesterol (1) and/or by overexpression of dominant-negative caveolin mutants (48, 60), and a novel nonclathrin-, noncaveola-dependent endocytosis pathway, which is ill defined and currently is only described in negative terms (7, 55). For this work, we used a combination of pharmacological, biochemical, and genetic approaches to study the mechanism by which rotaviruses gain access into MA104 cells. We found that rotavirus entry is not mediated by either clathrin-dependent endocytosis or caveola uptake, but it is dependent on the function of the large GTPase protein dynamin.
MATERIALS AND METHODS
Cells and viruses.
The rhesus monkey epithelial cell line MA104 was grown in Eagle's minimal essential medium (MEM) supplemented with 10% fetal bovine serum and was used for all experiments carried out in this work. Rhesus rotavirus (RRV) was obtained from H. B. Greenberg, Stanford University, Stanford, Calif. Simian virus 40 (SV40) was kindly provided by L. Gutiérrez, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, Mexico. Viruses were propagated as previously described (1, 8).
Antibodies and reagents.
Monoclonal antibodies (MAbs) HS2 and 159, directed against VP4 and VP7, respectively, were provided by H. B. Greenberg. A rabbit polyclonal serum against NSP5 has been described previously (16). A MAb against the large antigen of SV40 (anti-SV40 TAg) and mouse and rabbit anti-hemagglutinin (HA) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Alexa 488- and 568-conjugated secondary antibodies, the cholera toxin B subunit (Ctx) and transferrin (Tfr) labeled with Alexa 488, and Alexa 594 were purchased from Molecular Probes (Eugene, Oreg.). Chlorpromazine, dansylcadaverine, methyl-β-cyclodextrin (MβCD), filipin, nystatin, NH4Cl, and sucrose were purchased from Sigma (St. Louis, Mo.).
Infectivity assay.
Confluent MA104 cells in 96-well plates (for rotavirus infection) or 24-well plates (for SV40 infection) were washed twice with phosphate-buffered saline (PBS), and then about 2,000 focus-forming units (FFU) of rotavirus RRV or 6,000 FFU of SV40 virus were adsorbed to the cells for 60 min at 37°C. After the adsorption period, the virus inoculum was removed, the cells were washed once with PBS, MEM was added, and the infection was left to proceed for 14 h for RRV or 18 h for SV40 at 37°C. RRV-infected cells were detected by an immunoperoxidase focus detection assay using a rabbit hyperimmune serum to porcine rotavirus YM, as described previously (43). The FFU were counted in a Visiolab 1000 station (Biocom, Les Ulises, France) as reported previously (17). SV40-infected cells were detected by a fluorescence focus assay using an anti-SV40 TAg MAb as described previously (56).
Inhibition of endocytosis in MA104 cells treated with endocytotic drugs.
Confluent monolayers of MA104 cells in 96-well plates were pretreated or not treated with 10 μg of chlorpromazine/ml, 0.2 mM dansylcadaverine, 10 mM MβCD, 15 μg of filipin/ml, or 50 μg of nystatin/ml for 1 h at 37°C; with 25 mM NH4Cl for 30 min; or with 0.45 M sucrose for 10 min at 37°C. After treatment with the corresponding compound, the cells were infected with rotavirus or SV40, as described above, for 1 h at 37°C, and the drugs were maintained in the cultures during this incubation period. Cells infected with RRV were further incubated with MAb 159 (diluted 1:2,000) for 15 min to neutralize the virus that remained on the cell surface, and after this period the infection was left to proceed for 14 h at 37°C in MEM. At this time, the monolayers were fixed and the virus-infected cells were detected as described above. Infectivity data are expressed as percentages of the virus infectivity obtained when the cells were mock treated with MEM as a control.
Immunofluorescence (IF).
MA104 cells grown on glass coverslips to approximately 80% confluence were transfected for transient expression assays as described below, and at 48 h posttransfection the cells were infected with a multiplicity of infection of 0.5 of either RRV or SV40. At 8 (for RRV) or 18 (for SV40) h postinfection, the cells were fixed with 2% paraformaldehyde in PBS for 20 min at 37°C. After this time, the cells were washed twice with PBS containing 50 mM NH4Cl, permeabilized by incubation with PBS-0.5% Triton X-100-50 mM NH4Cl for 15 min at room temperature, and washed twice with PBS with gentle swirling. The coverslips were then incubated for 1 h at room temperature with primary antibodies diluted in blocking buffer (50 mM NH4Cl, 1% bovine serum albumin in PBS), followed by four rinses with PBS. The coverslips were incubated with the appropriate Alexa-labeled secondary antibodies in blocking buffer for 1 h at room temperature. The cells were washed four times with PBS and were mounted on glass slides with Fluoprep (BioMérieux) and the antifading agent DABCO (100 mg/ml; Sigma). The slides were analyzed with a Bio-Rad MRC-600 confocal microscope and CoMOS MPL-1000 software or with a Nikon E600 epifluorescence microscope coupled to a DXM1200 digital still camera (Nikon). The images were then digitally captured and prepared in Adobe Photoshop 7.0.
Plasmids and transfections.
The dominant-negative, GFP-tagged plasmid construct pEΔ95/295 (Eps15mut), which encodes an Eps15 deletion mutant lacking the second and third EH domains, and the control plasmid pD3Δ2, expressing a C terminally truncated fragment of Eps15 (Eps15 control) (5), were kindly provided by A. Benmerah, INSERM, Paris, France; pCINeo/IRES-GFP/caveolin-1 and pCINeo/IRES-GFP/caveolin-1 DN, which are bicistronic expression vectors expressing green fluorescent protein (GFP) and wild-type caveolin-1 (cav1-wt) or caveolin-1 from which residues 1 to 81 were deleted (cav1-mut), respectively (60), were kindly donated by J. Eggermont, Katholieke Universiteit, Leuven, Belgium; and plasmid pCB6-caveolin-3HA (Cav-3wt), which contains HA-tagged wild-type caveolin-3, and plasmids pCB6-caveolin-3DGVHA (Cav-3DGV) and pCB6-caveolin-3KSYHA (Cav-3KSY), expressing two dominant-negative amino-terminal truncation mutants of caveolin-3 (48), were generously provided by R. Parton, University of Queensland, Brisbane, Australia. Plasmids pUHD15-1, which expresses the tetracycline-controlled transactivator, pUHD10-3/dynamin2 (pDyn-wt), which expresses N-terminally HA epitope-tagged dynamin, and pUHD10-3/K44A (pDynK44A), expressing an N-terminally HA epitope-tagged K44A mutant (9), were kindly provided by S. L. Schmid, Scripps Research Institute, La Jolla, Calif. The pUHD15-1 and pDyn-wt or pDynK44A plasmids were cotransfected at a ratio of 1:1, and the expression of dynamin and the K44A mutant was monitored by using a MAb against the HA tag. Plasmids were transfected in 80% confluent cell monolayers grown on coverslips by using Lipofectamine (Invitrogen) according to the manufacturer's protocols. Usually, the cells were transfected 48 h before virus infection or before the Ctx or Tfr uptake assay.
Uptake assays.
For analysis of the uptake of Ctx and Tfr, cells grown on coverslips and pretreated or transfected as described above were chilled on ice for 10 min and then incubated with 5 μg of Alexa 488- or Alexa 594-Ctx per ml or with 50 μg of Alexa 594-Tfr per ml on ice for 30 min. The cells were washed twice with cold medium and then incubated at 37°C in a CO2 incubator for 30 min. At the assay end point, the samples were fixed in 2% paraformaldehyde in PBS for 20 min and processed for IF as described above. The agents chlorpromazine, dansylcadaverine, MβCD, filipin, nystatin, NH4Cl, and sucrose were maintained in the medium during incubation with the endocytic ligands.
RESULTS
Role of caveolae in rotavirus entry.
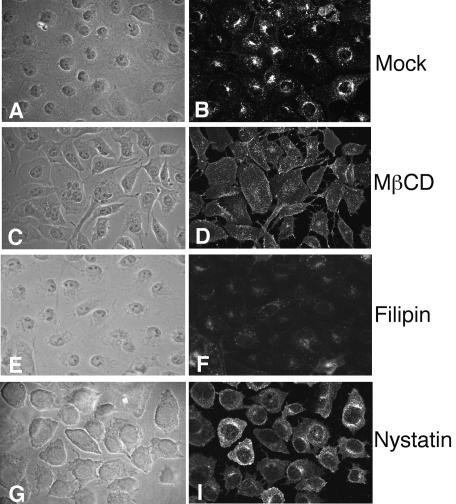
To determine if rotaviruses enter cells through a caveola-mediated pathway, we treated cells with drugs that are known to inhibit this type of endocytosis and then infected them with a rotavirus. Cholesterol is a prominent component of lipid rafts, which are involved in caveola formation, and sequestration of cholesterol with the sterol-binding drugs filipin and nystatin or depletion of this compound from the membranes with MβCD has been shown to impair caveola-mediated endocytosis (55). The effects of these drugs on caveolar endocytosis were examined by measuring the uptake of Alexa-Ctx by cells that were treated or not treated with the drugs, since this toxin is targeted to caveolae through its receptor, the ganglioside GM1, and its uptake is blocked by sterol-binding compounds (41, 59). Figure 1 shows that mock-treated cells internalized Alexa-Ctx, with the fluorescence concentrated around the nuclei. In contrast, the cells that were treated with the sterol-binding drugs (filipin and nystatin) poorly internalized Alexa-Ctx, or the label appeared as diffuse fluorescence in the cell's cytoplasm (nystatin and MβCD), demonstrating the specificity of these drugs for caveola-mediated uptake. Next, we assayed the effects of these drugs on the infectivity of RRV and SV40, which has been shown to enter cells through the caveolar route (1, 56). We found that both filipin and nystatin, at the concentrations used, reduced the infectivity of SV40 by about 50%, as has been previously observed (1, 56), whereas the infectivity of RRV was not significantly affected by either of these drugs (Table 1). In contrast, the treatment of cells with MβCD severely decreased (∼90%) the infectivity of both viruses (Table 1), as was previously reported for rotaviruses (17).
FIG. 1.
Uptake of cholera toxin into MA104 cells treated with sterol-binding drugs. MA104 cells were mock treated (A and B) or pretreated with MβCD (10 mM) (C and D), filipin (15 μg/ml) (E and F), or nystatin (50 μg/ml) (G and H), as detailed in Materials and Methods, and then were incubated with Alexa 488-Ctx. The fluorescent signals are shown in panels B, D, F, and H; panels A, C, E, and G show the corresponding phase-contrast images.
TABLE 1.
Effect of sterol-binding drugs on rotavirus infectivity
| Treatment | Concn (μg/ml) | % Infectivitya
|
|
|---|---|---|---|
| RRV | SV40 | ||
| MβCD | 10 mM | 9 ± 2* | 10 ± 3* |
| Filipin | 5 | 102 ± 5 | ND |
| 15 | 130 ± 11 | 52 ± 4* | |
| Nystatin | 50 | 84 ± 7 | 54 ± 10* |
| 100 | 90 ± 18 | ND | |
Expressed as a percentage of the FFU observed in control, untreated cells. The average numbers of FFU representing 100% infectivity were 269 and 252 for RRV and SV40, respectively. The arithmetic means and standard deviations from three experiments performed in duplicate are shown. ND, not determined. *, P < 0.05 (paired t test).
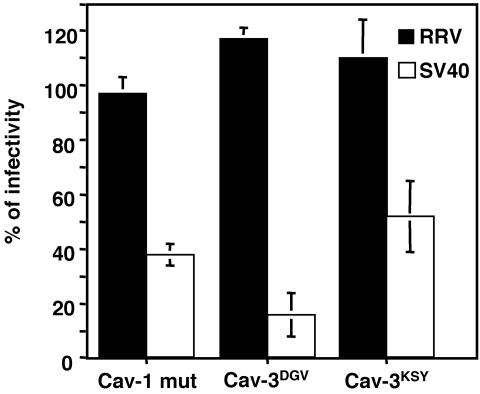
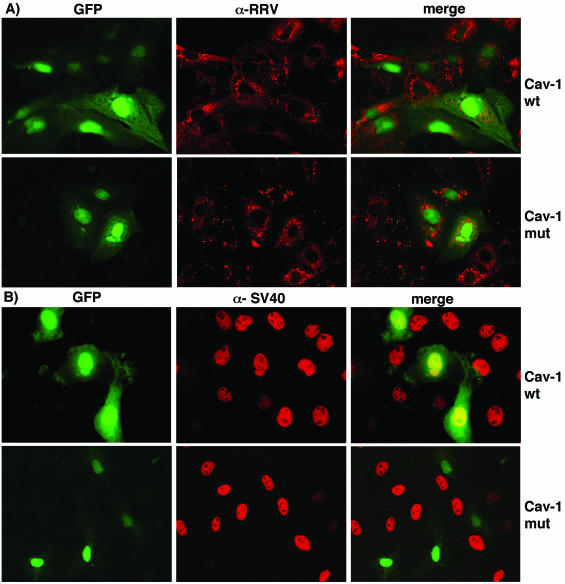
As a more specific way to examine uptake by caveolae, we analyzed the effects of dominant-negative mutants of caveolin-1 and caveolin-3 on the infectivity of RRV and SV40. Cells were transfected with a wild-type (cav-1wt) or dominant-negative form of caveolin-1 (cav-1mut) (60) or with HA-tagged wild-type caveolin-3 (cav-3wt) and dominant-negative mutants Cav-3DGV and Cav-3KSY, which are amino-terminal deletions of caveolin-3 (48). At 48 hours posttransfection, the cells were infected with RRV or SV40, and the fraction of the transfected cells infected by the viruses was scored (Fig. 2). The expression of wild-type caveolin-1 and -3 had no effect on the infectivity of RRV or SV40, whereas in cells transfected with the dominant-negative caveolin mutants, the infectivity of SV40 was clearly decreased (Fig. 2 and 3B). In contrast, none of the caveolin dominant-negative mutants affected the infectivity of RRV, indicating that the entry of this virus is independent of caveolae (Fig. 2 and 3A).
FIG. 2.
Rotavirus infectivity is not mediated by a caveolin-dependent pathway. MA104 cells were transfected with the plasmid cav-1wt, cav-1mut, cav-3wt, cav-3DGV, or cav-3KSY, and at 48 h posttransfection the cells were infected with RRV for 8 h or with SV40 for 18 h. The cells were then fixed and immunostained to detect viral and plasmid-expressed proteins by IF. Cav-1-transfected cells were detected by the coexpression of the GFP protein (see Materials and Methods). Cav-3 expression was detected by an anti-HA tag antibody. The infection by RRV was monitored by using an anti-NSP5 rabbit antibody, and the SV40 infection was detected by using an anti-SV40 TAg MAb. The numbers of infected cells in the positively transfected cells were scored, and the infectivities are expressed as percentages of the infected cell numbers in the control cells transfected with wild-type constructs. The average numbers of FFU representing 100% infectivity for the cells transfected with cav-1wt were 252 and 118 for RRV and SV40, respectively, and for cav-3wt-transfected cells were 89 and 87 for RRV and SV40, respectively. The data shown represent the arithmetic means and standard deviations from three independent experiments.
FIG. 3.
Effect of the expression of caveolin-1 dominant-negative mutants on the infectivity of RRV and SV40. MA104 cells were transfected with the plasmid cav-1wt or cav-1mut, and 48 h after transfection the cells were infected with RRV for 8 h (A) or with SV40 virus for 18 h (B). The cells were then fixed and processed for IF. The expression of cav-1wt or cav-1mut was detected by the coexpression of the GFP protein, since the respective plasmid constructs also directed the synthesis of this protein. The cell infection by RRV was monitored by using an anti-NSP5 rabbit antibody (α-RRV), and the SV40 infection was detected by using an anti-SV40 TAg MAb (α-SV40), followed by incubation with anti-rabbit Alexa 568 and anti-mouse Alexa 568 antibodies, respectively.
Role of clathrin-mediated endocytosis in rotavirus entry.
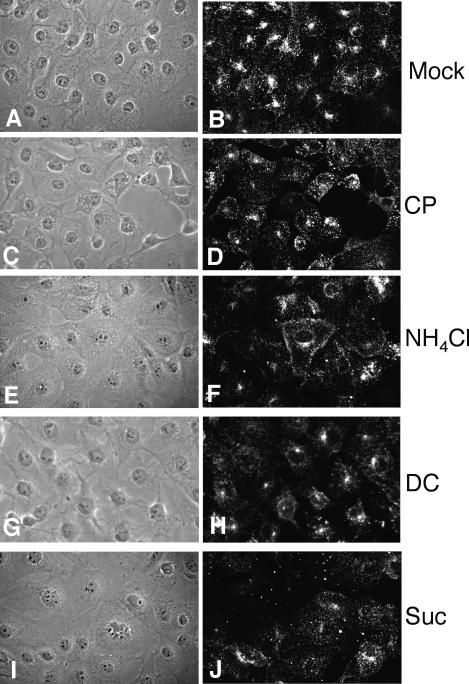
To reevaluate the role of clathrin-dependent endocytosis in the entry of rotaviruses, we undertook several different approaches to avoid a possible misinterpretation of the results as a consequence of pleiotropic effects caused by the treatments, especially when drugs were used. Dansylcadaverine and NH4Cl, which have been previously assayed (4, 8), were included in this study for comparative purposes. We also tested the effect of treating the cells with a hypertonic medium (0.45 M sucrose), which results in the dissociation of clathrin vesicles from the plasma membrane (18, 20), or with chlorpromazine, which causes clathrin lattices to assemble on endosomal membranes and at the same time prevents the assembly of coated pits at the cell surface (62). The effectiveness of these treatments for inhibition of clathrin-mediated endocytosis was first tested by determining the ability of MA104 cells to endocytose Tfr labeled with Alexa 594 (Alexa-Tfr). In control, mock-treated cells, the Alexa-Tfr appeared concentrated around the nuclei, whereas the uptake of Alexa-Tfr in cells treated with the endocytotic drugs appeared as diffuse fluorescence in the cells (Fig. 4), indicating that all treatments were effective. The effects of these treatments on the infectivity of RRV were then determined (Table 2). As previously reported (4, 8), treatment of the cells with dansylcadaverine or NH4Cl did not affect the entry of rotaviruses, while the same treatments reduced the infectivity of reovirus by around 60% compared to the infectivity of this virus in control, untreated cells (results not shown) (8). The two other conditions tested, the sucrose hypertonicity treatment and incubation with chlorpromazine, had no apparent effect on the ability of RRV to infect cells.
FIG. 4.
Uptake of Tfr into MA104 cells treated with drugs that affect clathrin-mediated endocytosis. MA104 cells were mock treated (A and B) or pretreated with chlorpromazine (10 μg/ml) (C and D), NH4Cl (50 mM) (E and F), dansylcadaverine (0.2 mM) (G and H), or sucrose (0.45 M) (I and J), as detailed in Materials and Methods, and then were incubated with Alexa 594-Tfr. The fluorescent signals of Alexa-Tfr are shown in panels B, D, F, H, and J; panels A, C, E, G, and I show the corresponding phase-contrast images.
TABLE 2.
Effect of drugs that affect clathrin-mediated endocytosis on the infectivity of RRV
| Treatment | Concn (mM, unless otherwise indicated) | % RRV infectivitya |
|---|---|---|
| Dansylcadaverine | 0.2 | 109 ± 9 |
| NH4Cl | 25 | 100 ± 6 |
| Sucrose | 0.45 M | 103 ± 7 |
| Chlorpromazine | 10 μg/ml | 116 ± 18 |
Expressed as the percentage of the FFU observed in control, untreated cells. The average number of FFU of RRV representing 100% infectivity was 119. The arithmetic means and standard deviations for three independent experiments performed in duplicate are shown.
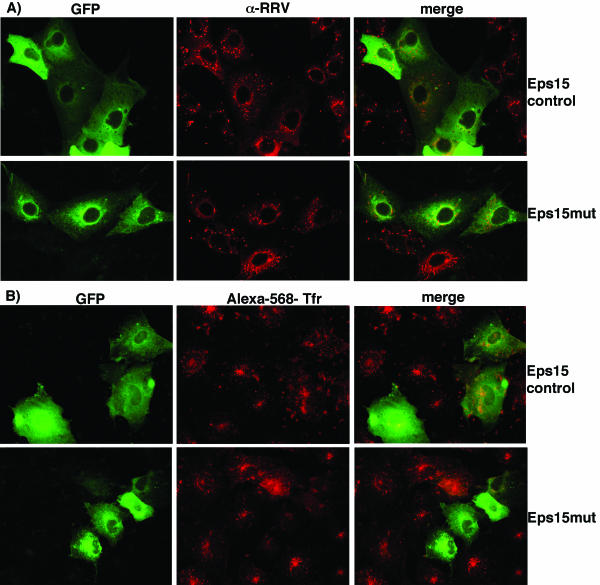
To further explore the role of clathrin-mediated endocytosis in rotavirus entry, we used an expression construct coding for a dominant-negative Eps15 protein. It has been reported that the overexpression of an Eps15 protein lacking the second and third EH domains results in the inhibition of clathrin-mediated endocytosis (5). MA104 cells were transfected with either the GFP-tagged plasmid construct pEΔ95/295 (Eps15mut) or plasmid pD3Δ2 (Eps15control), and the entry of RRV and SV40 and the uptake of Tfr, as a control, were monitored by IF microscopy. Figure 5A shows that RRV was able to infect cells that were transfected either with the control or with the truncated version of Eps15. Cells transfected with the dominant-negative mutant were not able to internalize Alexa-Tfr, while cells transfected with the control construct did, confirming that the dominant-negative mutant of Eps15 prevented the internalization of this protein (Fig. 5B). When the infectivity of RRV and SV40 was scored for these cells, we found that both viruses were able to infect the cells transfected with the mutant protein as effectively as they infected the cells transfected with the control plasmid (Table 3). These results indicate that RRV is capable of entering cells lacking a functional clathrin-mediated endocytic pathway.
FIG. 5.
Rotavirus entry into cells expressing a dominant-negative mutant of Eps15. MA104 cells were transfected with the GFP-tagged construct Eps15D3Δ2 (Eps15 control) or pEΔ95/295 (Eps15-mut) and were infected with RRV (A) or incubated with Alexa-Tfr (B). The expression of Eps15 proteins was monitored by GFP fluorescence. RRV was detected as described in the legend for Fig. 3.
TABLE 3.
Effect of dominant-negative mutants of Eps15 and dynamin on the infectivity of RRV and SV40
| Transfected plasmid | % Infectivitya (no. of transfected cells scored)
|
|
|---|---|---|
| RRV | SV40 | |
| Eps15 controlb | 100 (472) | 100 (286) |
| Eps15mut | 106 ± 12 (279) | 95 ± 14 (137) |
| Eps15 control plus nystatin | 104 ± 20 (220) | 57 ± 11 (296)* |
| Eps15mut plus nystatin | 106 ± 14 (155) | 65 ± 20 (212)* |
| Dynamin wtc | 100 (392) | 100 (193) |
| K44A | 16 ± 2 (454)* | 34 ± 5 (399)* |
Data are expressed as percentages of virus infectivity obtained in cells transfected with the control plasmids. The arithmetic means and standard deviations for three independent experiments are shown. *, P < 0.05 (paired t test).
Control for Eps15mut, Eps control plus nystatin, and Eps15mut plus nystatin. The average numbers of FFU representing 100% infectivity were 148 and 89 for RRV and SV40, respectively.
Control for K44A mutant. The average numbers of FFU were 128 and 60 for RRV and SV40, respectively.
Rotavirus infects cells in which both caveola- and clathrin-mediated endocytosis are inhibited.
Our data indicated that rotavirus infection was not affected by the inhibition of caveola- or clathrin-mediated endocytosis. To determine if rotaviruses were able to use alternatively the caveola- or clathrin-mediated pathway when one of them is blocked, we inhibited both routes at the same time. For this inhibition, MA104 cells were transiently transfected with either the Eps15 control or Eps15mut plasmid for 48 h, and the cells were then treated with nystatin for 1 h at 37°C and infected as described in Materials and Methods, with maintenance of the drug during the infection period. The entry of RRV and SV40 was monitored by IF. Table 3 shows that the infection of rotavirus was not modified under these conditions, as previously observed with each of the individual treatments. In contrast, infection by SV40 was reduced when nystatin was added to the cells, to a level similar to that found when the drug was used alone. Similar to SV40 infection, the uptake of Ctx was inhibited only when nystatin was present, while the uptake of Tfr was inhibited in the Eps15 mutant-expressing cells with or without nystatin in the medium (data not shown). These results indicate that RRV enters cells through a route that is different from the clathrin-mediated and caveolin-mediated endocytic pathways.
Role of dynamin in rotavirus entry.
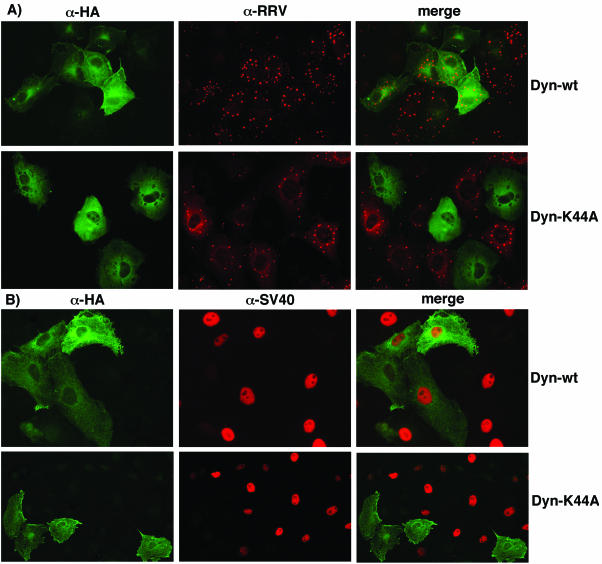
Dynamin is a GTPase that seems to be a master regulator of membrane trafficking events at the cell surface, since it is required for phagocytosis and caveola-mediated and clathrin-mediated endocytosis as well as for some clathrin- and caveola-independent endocytosis pathways (7, 19, 21, 40). A dominant-negative mutant form of dynamin I, the mutant K44A, contains a single amino acid change in the GTPase domain and has been extensively used to inhibit both caveolar and clathrin-mediated endocytosis and to define the role of these pathways in the cell entry of several viruses (14, 22, 27, 32, 45). In order to define the role of dynamin in the entry of rotaviruses, we transiently cotransfected MA104 cells with either plasmid pUHD10-3/dynamin (Dyn-wt) or pUHD10-3/K44A (Dyn-K44A), which expresses the wild-type or mutant K44A form of dynamin, respectively, under the control of the Tet-responsive promoter, and with plasmid pUHD15-1, which expresses the tetracycline-controlled transactivator, which is a regulatory protein that activates the expression of genes under the Tet-responsive promoter (9). Dyn-wt- and Dyn-K44A-transfected cells were then infected with rotavirus or with SV40, and the number of transfected cells (as detected with an anti-HA tag antibody) that were infected was scored (Fig. 6 and Table 3). The cells transfected with the dominant-negative variant of dynamin were found to be less infectable by both RRV and SV40 than cells transfected with the wild-type form of the protein. Table 3 shows that the infection of SV40 was reduced to about 34% with respect to the infectivity obtained in control cells transfected with the wild-type dynamin, as has been previously reported (45). The infectivity of RRV was also reduced, being only 16% that of control cells. The uptake of Alexa-labeled Ctx and Tfr was also reduced in the Dyn-K44A-expressing cells (data not shown). These results suggest that dynamin is involved in the cell entry of rotaviruses.
FIG. 6.
Rotavirus entry into cells expressing a dominant-negative mutant of dynamin. MA104 cells were cotransfected with plasmid pUHD10-3/dynamin (Dyn-wt) or pUHD10-3/K44A (Dyn-K44A) and plasmid pUHD15-1, which expresses the response regulator factor that promotes the expression of Dyn-wt and Dyn-K44A. The transfected cells were infected with RRV (A) or SV40 (B) and processed for IF. Wild-type dynamin and Dyn-K44A proteins were detected with an anti-HA tag MAb. RRV and SV40 infections were detected as described in the legend for Fig. 3.
DISCUSSION
While detailed information about the entry of several enveloped viruses is now available (11, 25, 50, 61), the mechanism by which nonenveloped viruses enter cells is not well understood. Viral fusion proteins at the surfaces of enveloped viruses mediate apposition and fusion of the viral and cellular membranes, allowing the viral nucleocapsid to enter the cell. Whether this fusion event occurs at the cell surface or in an endosomal vesicle is mostly determined by the optimum pH of the viral fusion protein (49). The cell entry mechanism of nonenveloped viruses possesses the complication that a large, hydrophilic virus particle must traverse a lipid membrane without having the resource of fusing two lipid membranes.
In the case of rotaviruses, the reports that have so far evaluated the endocytic route as the pathway for virus cell entry have only explored the involvement of clathrin-mediated endocytosis (8, 23, 29, 47, 57). In this work, by using new molecular and biochemical tools, we reevaluated the role of this type of endocytosis and of other recently described endocytic pathways in the entry of rotaviruses to MA104 cells.
To determine if rotaviruses enter cells through clathrin-mediated endocytosis, we tested the effects of raising the intraendosomal pH and of treating cells with dansylcadaverine, chlorpromazine, or a hypertonic medium on the infectivity of RRV. None of these treatments affected the entry of RRV, even though all of them were able to block the uptake of Tfr into cells, thus demonstrating that the treatments were effective for blocking the clathrin-mediated pathway. Also, we tested the effect of a dominant-negative mutant of Eps15, which arrests clathrin-coated pit assembly (5). The expression of this dominant-negative mutant in MA104 cells did not affect the entry of RRV, contrasting with the negative effect exerted on the uptake of Tfr. Taken together, these results indicate that rotaviruses do not enter cells through classical clathrin-mediated endocytosis.
More recently, caveola-mediated endocytosis and nonclathrin-, noncaveola-mediated endocytosis routes have been described (7, 37, 55). Caveolae are cholesterol- and sphingolipid-rich smooth invaginations of the plasma membrane which are generally associated with caveolin. Caveola-mediated endocytosis can be disrupted either by drugs that sequester the cholesterol from the plasma membrane or by overexpression of dominant-negative mutants of caveolin. By use of these approaches, it was found previously that echovirus 1, filoviruses, respiratory syncytial virus, and SV40 are internalized via caveolae (1, 12, 30, 63). In order to define the role of caveola-mediated endocytosis in the entry of rotavirus RRV into MA104 cells, we used several drugs that affect this pathway, such as nystatin, filipin, and MβCD. In our hands, nystatin and filipin did not affect the infectivity of rotavirus, while MβCD was able to severely block the infectivity of RRV, as was previously shown by Guerrero et al. (17). These results, together with the fact that dominant-negative caveolin-1 or caveolin-3 mutants did not affect virus infectivity, suggest that the depletion of cholesterol by MβCD inhibits rotavirus entry, not by interfering with caveola-mediated endocytosis, but most probably by altering the integrity of membrane lipid microdomains (17). In support of the involvement of rafts in rotavirus entry, we have found that rotavirus infectious particles, as well as the molecules that have been implicated as receptors for rotavirus, such as integrins α2β1, αvβ3, and the heat shock protein hsc70, are associated with lipid rafts (P. Isa, M. Realke, P. Romero, S. López, and C. F. Arias, submitted for publication). Altogether, these findings indicate that caveola-mediated endocytosis, or at least caveolin-1 and -3, is not involved in the productive entry of rotaviruses, in agreement with the observation that rotaviruses are able to efficiently infect the colon carcinoma cell line Caco-2, which is devoid of caveolin-1 and -3 (35, 58).
Furthermore, the fact that a mixed treatment in which both clathrin-mediated endocytosis and entry via caveolae were simultaneously inhibited (by expression of a dominant-negative mutant of Eps15 and treatment of the cells with nystatin) did not block the entry of RRV suggests that the virus might employ a nonclathrin-, noncaveola-mediated endocytic route to enter the cells. Recently, Chemello et al. (6) proposed that since the inhibition of the vacuolar H+-ATPase pump by bafilomycin A1 inhibits rotavirus infectivity, an endocytic mechanism of entry could be involved. It would be interesting to determine if the vacuolar H+-ATPase is present in vesicles that are internalized in a caveola- and clathrin-independent manner.
Interestingly, the overexpression of a dominant-negative form of dynamin blocked rotavirus entry, suggesting that it is involved in this process. Dynamin has been described as a mechanochemical enzyme needed for the release of internalized vesicles from the plasma membrane (21, 51, 52) and as a regulatory molecule that recruits or activates effectors in its GTP-bound form (42, 53, 54). Although dynamin activity was previously thought to be specific for clathrin-mediated endocytosis, it is now clear that it is required for phagocytosis, caveola- and clathrin-mediated endocytosis, and some clathrin- and caveola-independent endocytic pathways (15, 19, 21, 34, 38). Several reports have described that dynamin is needed for the cell entry of different viruses due to its role in either caveola- or clathrin-mediated endocytosis (10, 14, 22, 44, 48).
Altogether, the results described in this work show that rotavirus entry is a clathrin- and caveolin-independent, cholesterol-sensitive pathway which depends on the function of dynamin. In this regard, rotaviruses might use a recently defined cell internalization pathway, referred to as caveola- and raft-dependent endocytosis, that is defined by its clathrin independence, its dependence on dynamin, and its sensitivity to cholesterol depletion (36). We cannot overlook, however, the idea that rotaviruses could enter the cell at the plasma membrane level by using a nondefined direct entry mechanism in which the depletion of cholesterol could either alter the fluidity of the membrane or disrupt the organization of the lipid rafts that might be holding together the rotavirus receptors, thus impairing virus entry. In addition to its property of severing membranes, dynamin has been recently implicated in numerous actin-membrane processes, such as the formation of podosomes, membrane extension and protrusion during lamellipodial advance, and vesicle comet motility (3, 26, 31, 39). Thus, the dynamin dependence of rotavirus entry observed in this work might not only be the result of its participation in the endocytosis pathway per se, but since it is clear that this multidomain GTPase plays an important role in processes that involve membrane dynamics, its role during rotavirus entry might also occur at a later step during the movement of the virus from the plasma membrane to the cytosol. These possibilities could be explored by using different dynamin mutants with functional defects in various domains of the protein which have been shown to have different cell phenotypes.
Acknowledgments
We are grateful to Paul Gaytán and Eugenio López for their help with the synthesis of oligonucleotides, Rafaela Espinosa for her support with cell culturing, and Xóchitl Alvarado for her help with confocal microscopy. We thank A. Benmerah, J. Eggermont, R. Parton, and S. Schmid for their generous donations of dominant-negative mutants.
This work was partially supported by grants 55003662 and 55000613 from the Howard Hughes Medical Institute and grant G37621N from the National Council for Science and Technology of Mexico.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, C. F., C. A. Guerrero, E. Mendez, S. Zárate, P. Isa, R. Espinosa, P. Romero, and S. Lopez. 2001. Early events of rotavirus infection: the search for the receptor(s), p. 47-63. In M. K. Estes and U. Desselberger (ed.), Gastroenteritis viruses. John Wiley and Sons, New York, N.Y. [DOI] [PubMed]
- 3.Baldassarre, M., A. Pompeo, C. Castaldi, S. Cortelino, G. Beznoussenko, M. A. McNiven, A. Luini, and R. Buccione. 2003. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol. Biol. Cell 14:1074-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass, D. M., M. Baylor, C. Chen, and U. Upadhyayula. 1995. Dansylcadaverine and cytochalasin D enhance rotavirus infection of murine L cells. Virology 212:429-437. [DOI] [PubMed] [Google Scholar]
- 5.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 6.Chemello, M. E., O. C. Aristimuno, F. Michelangeli, and M. C. Ruiz. 2002. Requirement for vacuolar H+-ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J. Virol. 76:13083-13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 8.Cuadras, M. A., C. F. Arias, and S. Lopez. 1997. Rotaviruses induce an early membrane permeabilization of MA104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065-9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damke, H., M. Gossen, S. Freundlieb, H. Bujard, and S. L. Schmid. 1995. Tightly regulated and inducible expression of dominant interfering dynamin mutant in stably transformed HeLa cells. Methods Enzymol. 257:209-220. [DOI] [PubMed] [Google Scholar]
- 10.Duan, D., Q. Li, A. W. Kao, Y. Yue, J. E. Pessin, and J. F. Engelhardt. 1999. Dynamin is required for recombinant adeno-associated virus type 2 infection. J. Virol. 73:10371-10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 12.Empig, C. J., and M. A. Goldsmith. 2002. Association of caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuhara, N., O. Yoshie, S. Kitaoka, T. Konno, and N. Ishida. 1987. Evidence for endocytosis-independent infection by human rotavirus. Arch. Virol. 97:93-99. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold, E. S., D. M. Underhill, N. S. Morrissette, J. Guo, M. A. McNiven, and A. Aderem. 1999. Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 190:1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez, R. A., M. A. TorresVega, S. Lopez, and C. F. Arias. 1998. In vivo interactions among rotavirus nonstructural proteins. Arch. Virol. 143:981-996. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero, C. A., S. Zarate, G. Corkidi, S. Lopez, and C. F. Arias. 2000. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 74:9362-9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen, S. H., K. Sandvig, and B. van Deurs. 1993. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuser, J. E., and R. G. Anderson. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinshaw, J. E. 2000. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16:483-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, M., J. Park, S. Lee, B. Park, J. Shin, K. J. Song, T. I. Ahn, S. Y. Hwang, B. Y. Ahn, and K. Ahn. 2002. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294:60-69. [DOI] [PubMed] [Google Scholar]
- 23.Kaljot, K. T., R. D. Shaw, D. H. Rubin, and H. B. Greenberg. 1988. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J. Virol. 62:1136-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotavirus, p. 1787-1833. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 25.Lanzrein, M., A. Schlegel, and C. Kempf. 1994. Entry and uncoating of enveloped viruses. Biochem. J. 302:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, E., and P. De Camilli. 2002. Dynamin at actin tails. Proc. Natl. Acad. Sci. USA 99:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S., Y. Zhao, and W. F. Anderson. 1999. Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway. J. Virol. 73:5994-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez, S., and C. F. Arias. 2003. Attachment and post-attachment receptors for rotavirus, p. 143-163. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis, in press. Elsevier Science, Amsterdam, The Netherlands.
- 29.Ludert, J. E., F. Michelangeli, F. Gil, F. Liprandi, and J. Esparza. 1987. Penetration and uncoating of rotaviruses in cultured cells. Intervirology 27:95-101. [DOI] [PubMed] [Google Scholar]
- 30.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNiven, M. A., L. Kim, E. W. Krueger, J. D. Orth, H. Cao, and T. W. Wong. 2000. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 151:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendez, E., S. Lopez, M. A. Cuadras, P. Romero, and C. F. Arias. 1999. Entry of rotaviruses is a multistep process. Virology 263:450-459. [DOI] [PubMed] [Google Scholar]
- 34.Merrifield, C. J., M. E. Feldman, L. Wan, and W. Almers. 2002. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 4:691-698. [DOI] [PubMed] [Google Scholar]
- 35.Mirre, C., L. Monlauzeur, M. Garcia, M. H. Delgrossi, and A. Le Bivic. 1996. Detergent-resistant membrane microdomains from Caco-2 cells do not contain caveolin. Am. J. Physiol. 271:C887-C894. [DOI] [PubMed] [Google Scholar]
- 36.Nabi, I. R., and P. U. Le. 2003. Caveolae/raft-dependent endocytosis. J. Cell Biol. 161:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 38.Nicoziani, P., F. Vilhardt, A. Llorente, L. Hilout, P. J. Courtoy, K. Sandvig, and B. van Deurs. 2000. Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell 11:481-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ochoa, G. C., V. I. Slepnev, L. Neff, N. Ringstad, K. Takei, L. Daniell, W. Kim, H. Cao, M. McNiven, R. Baron, and P. De Camilli. 2000. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 150:377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh, P., D. P. McIntosh, J. E. Schnitzer, and P. D. Losty. 1998. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlandi, P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orth, J. D., and M. A. McNiven. 2003. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 15:31-39. [DOI] [PubMed] [Google Scholar]
- 43.Pando, V., P. Isa, C. F. Arias, and S. Lopez. 2002. Influence of calcium on the early steps of rotavirus infection. Virology 295:190-200. [DOI] [PubMed] [Google Scholar]
- 44.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 46.Petrie, B. L., D. Y. Graham, and M. K. Estes. 1981. Identification of rotavirus particle types. Intervirology 16:20-28. [DOI] [PubMed] [Google Scholar]
- 47.Quan, C. M., and F. W. Doane. 1983. Ultrastructural evidence for the cellular uptake of rotavirus by endocytosis. Intervirology 20:223-231. [DOI] [PubMed] [Google Scholar]
- 48.Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98-105. [DOI] [PubMed] [Google Scholar]
- 49.Rusell, D. G., and M. Marsh. 2001. Endocytosis in pathogen entry and replication, p. 247-280. In M. Marsh (ed.), Endocytosis. Oxford University Press, New York, N.Y.
- 50.Salminen, A., J. M. Wahlberg, M. Lobigs, P. Liljestrom, and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. II. Cleavage-dependent reorganization of the spike protein complex controls virus entry. J. Cell Biol. 116:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid, S. L., and J. E. Hinshaw. 1995. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature 374:190-192. [DOI] [PubMed] [Google Scholar]
- 52.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10:504-512. [DOI] [PubMed] [Google Scholar]
- 53.Sever, S., H. Damke, and S. L. Schmid. 2000. Dynamin: GTP controls the formation of constricted pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150:1137-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sever, S., H. Damke, and S. L. Schmid. 2000. Garrotes, springs, ratchets, and whips: putting dynamin models to the test. Traffic 1:385-392. [DOI] [PubMed] [Google Scholar]
- 55.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 56.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki, H., S. Kitaoka, T. Konno, T. Sato, and N. Ishida. 1985. Two modes of human rotavirus entry into MA 104 cells. Arch. Virol. 85:25-34. [DOI] [PubMed] [Google Scholar]
- 58.Svensson, L., B. B. Finlay, D. Bass, C. H. von Bonsdorff, and H. B. Greenberg. 1991. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 65:4190-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torgersen, M. L., G. Skretting, B. van Deurs, and K. Sandvig. 2001. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114:3737-3747. [DOI] [PubMed] [Google Scholar]
- 60.Trouet, D., D. Hermans, G. Droogmans, B. Nilius, and J. Eggermont. 2001. Inhibition of volume-regulated anion channels by dominant-negative caveolin-1. Biochem. Biophys. Res. Commun. 284:461-465. [DOI] [PubMed] [Google Scholar]
- 61.Wahlberg, J. M., and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. I. Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J. Cell Biol. 116:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werling, D., J. C. Hope, P. Chaplin, R. A. Collins, G. Taylor, and C. J. Howard. 1999. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J. Leukoc. Biol. 66:50-58. [DOI] [PubMed] [Google Scholar]