Abstract
Background:
Picibanil (OK-432) is a lyophilized mixture of group A Streptococcus pyogenes with antineoplastic activity. Because of its capacity to produce a selective fibrosis of lymphangiomas (LMs), it has been approved by Japanese administration in 1995 for the treatment of LMs.
Materials and Methods:
We treated 15 children (age range: 6-60 months) affected by head and neck macrocystic LMs with intracystic injections (single dose of 0.2 mL) of Picibanil (1-3 injections).
Results:
Complete disappearance of the lesion was noticed in eight (53.33%) cases, a marked (>50%) reduction of LMs was found five (33.33%) cases, while a moderate (<50%) response was recorded in two (13.33%) cases. Picibanil side effects included fever, local inflammation, and transitory increase of blood platelets’ concentration; a single case of anemia was resolved with concentrated red blood cells transfusion.
Conclusions:
Intracystic injection of Picibanil is an effective and safe treatment for macrocystic LMs in pediatric patients and may represent the treatment of choice in such cases, especially where surgical excision is associated with the risk of functional/cosmetic side effects.
Keywords: Children, lymphangiomas, pediatric age, picibanil (OK-432)
INTRODUCTION
Lymphangiomas (LMs) are congenital malformations of the lymphatic system consisting of lymphatic channels and cystic spaces of varying size. They often occur in the head and neck region (75% of all cases) and represent about 5% of all benign neoplasm in infants and children. Their incidence in the literature is quite variable raging from 4/10,000 to 1/16,000 births and no significant gender or racial difference in LMs incidence has been reported. Up to 90% of LMs are diagnosed before the age of 2 years and up to 50% at birth. Morbidity and mortality are related to the size and site of the lesion.[1,2,3,4]
The ordinary clinical presentations of LMs are slowly growing, painless, soft, and compressible masses, which are usually asymptomatic. They often cause cosmetic deformities in the maxillofacial area, while lesions of the upper aero-digestive tract (mouth floor, hypo-pharynx, and larynx) may have functional consequences, such as airway obstruction or dysphagia. Neck lesions may cause torcicollis, whereas peri-orbital LMs may produce globe displacement and decrease visual acuity.[1,2,3,4]
Basing on cysts’ size, LMs are divided into macrocystic (lesions with cysts larger than 2 cm), microcystic (cysts smaller than 2 cm), and mixed lesions.[5,6]
The diagnosis of LMs is both clinical and instrumental. In particular, ultrasound (US) and magnetic resonance imaging (MRI) are essential for LMs identification and differential diagnosis from other vascular malformations (VMs), branchial and thyroglossal cysts or thyroid mass.[7]
Treatment of LMs ranges from observation to surgical excision and/or sclerosis of the lesion. Observation is usually not recommended especially in case of enlarging LMs, since spontaneous regression occurs in only 6-15% of LMs.[2,3,4,5,6,8,9,10,11,12,13,14,15,16,17,18,19,20,21]
Even though the surgical removal is generally considered the first choice treatment of LMs, the need of extensive approaches to attain the complete removal of the lesion may cause anesthetic and functional consequences.[22,23,24,25] In order to avoid such consequences, nonsurgical approaches have been proposed, such as Picibanil (OK-432), a lyophilized biological preparation containing cells of Streptococcus Pyogenes su-strain treated with Benzylpenicillin. Even though some Japanese authors reported the efficacy of OK-432 to produce a sclerosis of cystic spaces in LMs, the cumulative literature on this topic remains limited, particularly in pediatric patients outside Japan.[3,21]
We present our experience in children and infants affected by macrocystic LMs treated with local injections of OK-432 at the Department of Pediatric Maxillofacial Surgery, Children's Hospital of Brescia (Italy). Local response and possible side effects of our treatment have been recorded. The consequences of our findings are discussed.
MATERIALS AND METHODS
Between September 2005 and October 2010, 15 consecutive Caucasian children (7 M, 8 F; mean age: 28.9 months; age range: 6-60 months) with head and neck macrocystic LMs. were treated with OK-432 at the Department of Pediatric Maxillofacial Surgery, Children's Hospital of Brescia (Italy).
No child had a story of allergy to Penicillin. All the patients underwent physical examination, laboratory analysis (complete blood cell count, electrolyte analysis, serum creatinine, and urinalysis), medical photography, and imaging (US and MRI).[7]
LMs location was as follows: Lateral-cervical region (4/15 cases), sovraclavear region (2/15), submandibular region (3/15), and maxillofacial region (6/15). Among them, 3/15 patients had been previously submitted to LM surgical excision with local recurrence within 6 months [Table 1].
Table 1.
Consecutive LM patients treated with Picibanil (OK-432)
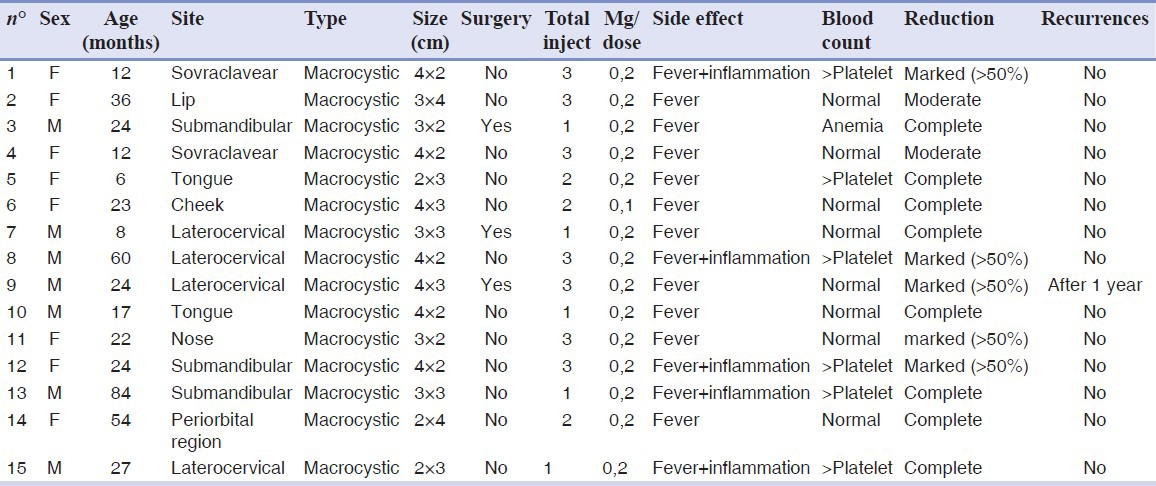
After an accurate informed consent (including treatment modalities and possible side effects), in which patients’ parents were offered to submit their kids to OK-432 LM injection as a “not currently approved (for the Italian Law)” alternative to traditional surgical excision, the parents accepted to submit their children to Picibanil treatment. After an accurate examination of the medical literature, the Ethics Committee of our Hospital approved the use of OK-432 intralesional injection in children affected by LMs, even though such use is not among the currently approved indications for Picibanil.
All 15 children were submitted to intralesional injection of OK-432 (Chugai Pharmaceutical Co, Tokyo, Japan)[3,26] in the operating room in general anesthesia. Under US guidance, a 25-G needle was introduced into LM cystic spaces, the cystic fluid was aspirated, and the OK-432 solution was instilled. In each session, 0.2 mg of Picibanil was injected. Based on the literature, in case of LM incomplete shrinkage, second and third OK-432 injections were performed 6 months after the previous session.[3,21]
Antibiotic prophylaxis (Amoxicillin + Clavulanic acid 50 mg/kg twice a day per os for 6 days) was administered. All side-effects and complications, such as fever, swelling, pain, redness, indurations, or respiratory difficulties, were recorded [Table 1].
All patients were discharged from hospital after 4 or 11 days from the injection.
Clinical, photographic, and imaging assessment was performed 3, 12, and 24 weeks after each session to determine LM shrinkage. As suggested by Rautio et al., LMs shrinkage was defined as “complete” (total disappearance), “marked” (>50% of the initial volume), “moderate” (<50% of the initial volume), or “no response”.[18]
RESULTS
Three weeks after the first injection of 0.2 mg Picibanil, we observed a complete clinical disappearance of LM in 5 (33.33%) cases [Figures 1-3], a marked reduction in 2 (13.33%) patients, a moderate response in 2 (13.33%), and no response in 6 (40%) children. Among the 10 patients submitted to a second OK-432 injection 6 months after the first session, 3 children presented LM complete disappearance, 1 a marked reduction, 3 a moderate shrinkage, and 3 no response. Among the 7 patients submitted to a third Picibanil injection 6 months after the second session, 5 patients presented a marked reduction and 2 patients showed a moderate response [Table 1]. Among the 8 kids who had a complete clinical response, 1 (12.5%) patient presented a recurrence 1 year after treatment. Regardless of the medical evaluation, all children's parents reported a satisfactory aesthetic results and an improvement of their kid's quality of life after the treatment.
Figure 1.
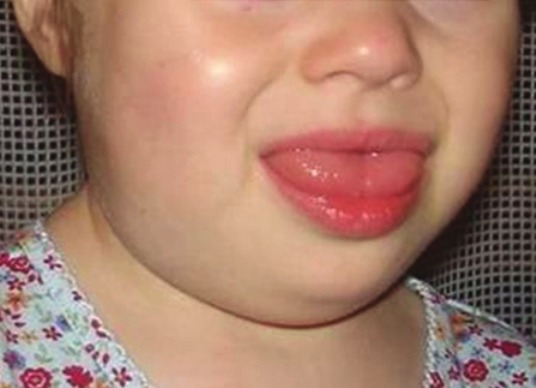
A 24-month-old female with macrocystic lymphangioma of the tongue
Figure 3.
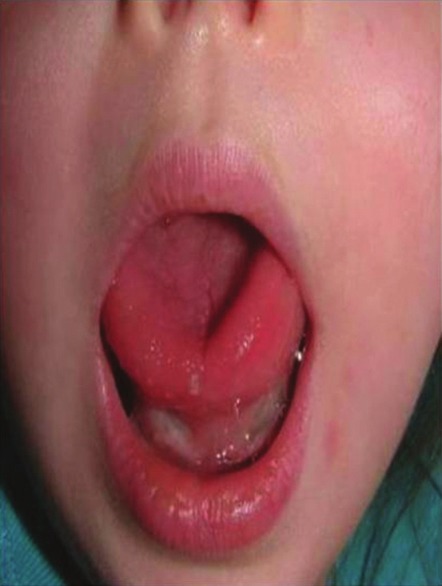
Clinical resolution of swelling due to tongue lymphangioma
In two cases (children with tongue LM), the patients were transferred from the operating room to the pediatric intensive care unit for the first 12 hours for a better postoperative management of the upper airway [Figure 2].
Figure 2.
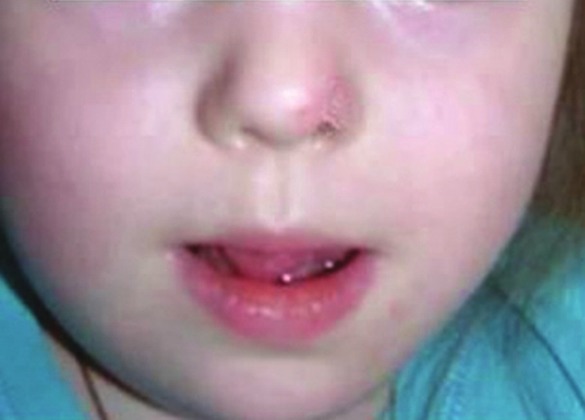
The patient 1 year after the injection of Picibanil
As to postinjection complications, all children had fever (38.5-39°C), treated with paracetamol (10-15 mg/kg, 3-4 times/day) and resolved after 1-3 days. Five children presented signs of inflammation (pain, redness, and induration) in the site of injection, which were treated with nonsteroidal antiinflammatory drug (ibuprofen 15 mg/kg 3 times/day) and resolved after 3-5 days. One patient presented anemia (Hb = 6 g/dl) 7 days after the injection; he was treated with concentrated red blood cells transfusion and did not show any further blood alteration. In six cases we noticed a transitory increase of platelets’ concentration, with spontaneous resolution within 1 month [Table 1]. No electrolyte alteration was recorded.
DISCUSSION
The main goal in the management of head and neck LMs is the restoration or preservation of functional and aesthetic integrity.[4,5,6] Surgery is traditionally considered the first choice treatment of LMs, however, such approach can have anesthetic and functional consequences. Furthermore, the impossibility to perform extensive resections in the head and neck region, may prevent radical tumor excision, with consequent high rates of recurrence, complications, and persistent symptomatic disease.[4,5,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] In the attempt to avoid such complications, in 1987 Ogita et al.[3] described the use of OK-432 (Picibanil) as a sclerosing agent in the management of LMs. Despite the positive results reported in the literature, the use of OK-432 in children with LMs is still controversial.
Our experience confirms the efficacy and safety of OK-432 intralesional injection in the treatment of macrocystic LMs in children. After 1-3 injections 8/15 patients showed clinical complete disappearance of the lesion, with a recurrence rate (12.5%) comparable to that reported in the literature (11%).[20] A marked to moderate response was noticed in the other 7 kids and all children presented an improvement in their quality of life.
The efficacy of OK-432 LMs intralesional injection seems to be related to its capacity to induce a local inflammatory reaction with activation of macrophages and production of cytokines (i.e., tumor necrosis factor, TNF), which increase the permeability of endothelial cells and promote lymph excretion. These events cause a decrease in the size of lymphatic vascular lumen and cause cyst sclerosis. Since this reaction seems too limited to the LM lumen, no perilesional fibrosis is produced, which will not increase the difficulty of surgical excision in case of OK-432 unsuccessful.[21,26,27] Another advantage of Picibanil is represented by the possibility to perform multiple subsequent injections with additional shrinkage responses. In the medical literature, a dose not exceeding 0.2mg of OK-432 per single injection is recommended. However, as a general rule, the dosage should equal the amount of aspirated fluid from LMs, so the dose may be adjusted depending on the patient's age and clinical conditions.[8,21]
Since size reduction of LMs may take weeks to months, we suggest waiting 6 months before assessing lesion shrinkage and performing a subsequent injection.[4,5,6]
Even though several OK-432 injection side effects have been reported (erythema, discomfort at the injection site, swelling and pyrexia, and anaphylaxis in patients with allergy to penicillin) in the literature, in our experience, Picibanil side effects were limited: The only relevant side effect was represented by a single case of anemia resolved with concentrated red blood cells transfusion.[8,21]
CONCLUSION
Our results suggest that OK-432 intralesional injections can be considered a feasible alternative to surgical treatment in children with LMs. The absence of cervical/facial incisions results in a better overall functional outcome and prevention of cervical scars with improved aesthetic results in comparison to traditional surgical approach. Even though multiple injections of OK-432 require successive episodes of sedation, 6-7 Picibanil shrinking effect on LMs and low complication rate suggest introducing LMs treatment among the indications of OK-432 also outside Japan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kennedy TL. Cystic hygroma-lymphangioma: a rare and still unclear entity. Laryngoscope. 1989;99:1–10. doi: 10.1288/00005537-198910001-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy TL, Whitaker M, Pellitteri P, Wood WE. Cystic hygroma/lymphangioma: A rational approach to management. Laryngoscope. 2001;111:1929–37. doi: 10.1097/00005537-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ogita S, Tsuto T, Tokiwa K, Takahashi T. Intracystic injection of OK-432: A new sclerosing therapy for cystic hygroma in children. Br J Surg. 1987;74:690–1. doi: 10.1002/bjs.1800740812. [DOI] [PubMed] [Google Scholar]
- 4.Oosthuizen JC, Burns P, Russell JD. Lymphatic malformations: A proposed management algorithm. Int J Pediatr Otorhinolaryngol. 2010;74:398–403. doi: 10.1016/j.ijporl.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Poldervaart MT, Breugem CC, Speleman L, Pasmans S. Treatment of lymphatic malformations with OK-432 (Picibanil): Review of the literature. J Craniofac Surg. 2009;20:1159–62. doi: 10.1097/SCS.0b013e3181abb249. [DOI] [PubMed] [Google Scholar]
- 6.Kim KH, Sung MW, Roh JL, Han MH. Sclerotherapy for congenital lesions in the head and neck. Otolaryngol Head Neck Surg. 2004;131:307–16. doi: 10.1016/j.otohns.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Fung K, Poenaru D, Soboleski DA, Kamal IM. Impact of magnetic resonance imaging on the surgical management of cystic hygromas. J Pediatr Surg. 1998;33:839–41. doi: 10.1016/s0022-3468(98)90654-6. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki T, Iwatani S, Yanai T, Kobayashi H, Kato Y, Marusasa T, et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg. 2007;42:386–9. doi: 10.1016/j.jpedsurg.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years’ experience with lymphangiomas in children. J Pediatr Surg. 1999;34:1164–8. doi: 10.1016/s0022-3468(99)90590-0. [DOI] [PubMed] [Google Scholar]
- 10.Claesson G, Kuylenstierna R. OK-432 therapy for lymphatic malformation in 32 patients (28 children) Int J Pediatr Otorhinolaryngol. 2002;65:1–6. doi: 10.1016/s0165-5876(02)00117-9. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo JL, Shah RK, Brietzke SE. Nonsurgical therapies for lymphangiomas: A systematic review. Otolaryngol Head Neck Surg. 2008;138:418–24. doi: 10.1016/j.otohns.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Hall N, Ade-Ajayi N, Brewis C, Roebuck DJ, Kiely EM, Drake DP, et al. Is intralesional injection of OK-432 effective in the treatment of lymphangioma in children? Surgery. 2003;133:238–42. doi: 10.1067/msy.2003.62. [DOI] [PubMed] [Google Scholar]
- 13.Greinwald JH, Jr, Burke DK, Sato Y, Poust RI, Kimura K, Bauman NM, et al. Treatment of lymphangiomas in children: an update of Picibanil (OK-432) sclerotherapy. Otolaryngol Head Neck Surg. 1999;121:381–7. doi: 10.1016/S0194-5998(99)70225-1. [DOI] [PubMed] [Google Scholar]
- 14.Peters DA, Courtemanche DJ, Heran MK, Ludemann JP, Prendiville JS. Treatment of cystic lymphatic vascular malformations with OK-432 sclerotherapy. Plast Reconstr Surg. 2006;118:1441–6. doi: 10.1097/01.prs.0000239503.10964.11. [DOI] [PubMed] [Google Scholar]
- 15.Sanlialp I, Karnak I, Tanyel FC, Senocak ME, Buyukpamukcu N. Sclerotherapy for lymphangioma in children. Int J Pediatr Otorhinolaryngol. 2003;67:795–800. doi: 10.1016/s0165-5876(03)00123-x. [DOI] [PubMed] [Google Scholar]
- 16.Giguere CM, Bauman NM, Smith RJ. New treatment options for lymphangioma in infants and children. Ann Otol Rhinol Laryngol. 2002;111:1066–75. doi: 10.1177/000348940211101202. [DOI] [PubMed] [Google Scholar]
- 17.Yoo JC, Ahn Y, Lim YS, Hah JH, Kwon TK, Sung MW, et al. OK-432 sclerotherapy in head and neck lymphangiomas: long-term follow-up result. Otolaryngol Head Neck Surg. 2009;140:120–3. doi: 10.1016/j.otohns.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Rautio R, Keski-Nisula L, Laranne J, Laasonen E. Treatment of lymphangiomas with OK-432 (Picibanil) Cardiovasc Intervent Radiol. 2003;26:31–6. doi: 10.1007/s00270-002-1980-3. [DOI] [PubMed] [Google Scholar]
- 19.Baskota DK, Singh BB, Sinha BK. OK-432: An effective sclerosing agent for the treatment of lymphangiomas of head and neck. Kathmandu Univ Med J. 2007;5:312–7. [PubMed] [Google Scholar]
- 20.Luzzatto C, Midrio P, Tchaprassian Z, Guglielmi M. Sclerosing treatment of lymphangiomas with OK-432. Arch Dis Child. 2000;82:316–8. doi: 10.1136/adc.82.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narkio-Makela M, Makela T, Saarinen P, Salminen P, Julkunen I, Pitkaranta A. Treatment of lymphatic malformations of head and neck with OK-432 sclerotherapy induce systemic inflammatory response. Eur Arch Otorhinolaryngol. 2011;268:123–9. doi: 10.1007/s00405-010-1332-x. [DOI] [PubMed] [Google Scholar]
- 22.Lucchese A, Carinci F, Brunelli G, Monguzzi R. Everstick ® and Ribbond ® fiber reinforced composities: Scanning electron microscope (sem) comparative analysis. Eur J Inflamm. 2011;9:73–80. [Google Scholar]
- 23.Lucchese A, Carinci F, Brunelli G, Monguzzi R. An in vitro study of resistance to corrosion in brazed and laser welded orthodontic. Eur J Inflamm. 2011;9:67–72. [Google Scholar]
- 24.Palmieri A, Zollino I, Clauser L, Lucchese A, Girardi A, Farinella F, et al. Biological effects of resorbable plates on normal osteoblasts and osteoblasts derived from Pfeiffer syndrome. J Craniofac Surg. 2011;22:1–4. doi: 10.1097/SCS.0b013e31820f7d34. [DOI] [PubMed] [Google Scholar]
- 25.Sollazzo V, Pezzetti F, Massari L, Palmieri A, Brunelli G, Zollino I, et al. Evaluation of gene expression in MG63 human osteoblastlike cells exposed to tantalum powder by microarray technology. Int J Periodontics Restorative Dent. 2011;31:e17–28. [PubMed] [Google Scholar]
- 26.Ogita S, Tsuto T, Nakamura K, Deguchi E, Tokiwa K, Iwai N. OK-432 therapy for lymphangioma in children: Why and how does it work? J Pediatr Surg. 1996;31:477–80. doi: 10.1016/s0022-3468(96)90478-9. [DOI] [PubMed] [Google Scholar]
- 27.Perkins JA, Manning SC, Tempero RM, Cunningham MJ, Edmonds JL, Jr, Hoffer FA, et al. Lymphatic malformations: Current cellular and clinical investigations. Otolaryngol Head Neck Surg. 2010;142:789–94. doi: 10.1016/j.otohns.2010.02.025. [DOI] [PubMed] [Google Scholar]


