Abstract
Gamma interferon (IFN-γ)-mediated indoleamine-2,3-dioxygenase (IDO) activity in human astrocytoma cells and in native astrocytes was found to be responsible for the inhibition of herpes simplex virus replication. The effect is abolished in the presence of excess amounts of l-tryptophan. Both IFN-α and IFN-β restricted herpes simplex virus replication in both cell types, but (in contrast to the results seen with IFN-γ) the addition of an excess amount of l-tryptophan did not inhibit the induced antiviral effect.
Human herpes simplex virus (HSV) type 1 (HSV-1) and HSV-2, along with varicella-zoster virus, are classified in the alpha-herpesvirus subfamily of herpesviruses whose presence leads to an establishment of latency in neural ganglia (43). The most serious infection caused by HSV-1 is sporadic encephalitis, with an untreated mortality rate of approximately 70% (24, 42). Generally, in immunocompromised patients HSV-1 infections are often severe. A locally invasive infection may result in mucocutaneous necrosis and spread to contiguous organs, resulting in (for example) esophagitis or leading to viremia, with subsequent distant organ manifestations such as meningoencephalitis, pneumonitis, and hepatitis (25, 26, 31, 35, 44).
Studies conducted using animal models of HSV-1 infections and animal and human cells have shown that control of the primary infection and reactivation depends on the host immune system (1, 14, 34), where the production of interferons (IFNs) plays a key role (23).
Two distinct but functionally overlapping types of IFNs are known: alpha/beta IFNs (IFN-α/β), which include some species of IFN-α and a single IFN-β, and IFN-γ (36). Within the last decades several IFN-induced effector mechanisms for the control of viruses have been described. These include the expression of double-stranded RNA-dependent protein kinase (22), Mx proteins (19), 2′-5′ oligoadenylate synthase, and RNase L (15). The most prominent IFN-inducible antimicrobial effector mechanism for the control of parasitic, bacterial, and viral growth in murine cells is NO production by the inducible isoform of nitric oxide synthase (iNOS) (4). However, there are only few studies showing an antimicrobial effect mediated by the induction of NO in human cells. In contrast there are abundant published data showing antibacterial and antiparasitic effects mediated by the induction of indoleamine-2,3-dioxygenase (IDO) in human cells (3, 7, 27, 33).
MacKenzie et al. and Pfefferkorn have indicated that after stimulation with IFN-γ, human astrocytes and astrocytoma cells are capable of inhibiting the growth of the parasite Toxoplasma gondii and of group B streptococci (27, 33). In both cases, the activation of IDO and the subsequent degradation of the essential amino acid l-tryptophan were found to comprise the effector mechanism involved. In 1999 Bodaghi et al. (3) showed that IDO activation is also responsible for the inhibition of the growth of human cytomegalovirus in retinal pigment epithelial cells. In this report we show that IFN-γ-induced IDO activity is a potent antiviral effector mechanism for the control of HSV in astrocytes, which are considered to play a key role in HSV-encephalitis. In contrast, IDO-mediated tryptophan depletion is not involved in the antiviral effects mediated by IFN-α/β.
IFNs mediate antiviral effects in astrocytoma cells.
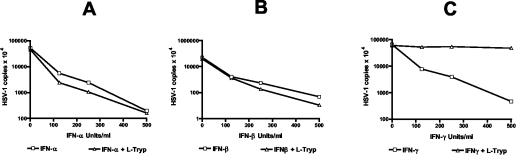
To determine whether IFN-α/β and IFN-γ induce an antiviral state in different astrocytoma cells, we stimulated 86HG39 cells (2) (kindly provided by T. Bilzer, Institute of Neuropathology, Dusseldorf, Germany) for 3 days with various doses of IFN-α, IFN-β (both obtained from R&D Systems GmbH [Wiesbaden-Nordenstadt, Germany]), or IFN-γ (a gift of M. Augst [Karl Thomae GmbH, Bieberach an der Riss, Germany]). Cells were held in Iscove's modified Dulbecco's medium and RPMI 1640 (Gibco, Grand Island, N.Y.) (with and without l-tryptophan) supplemented with 2 mM l-glutamine and 5% heat-inactivated fetal calf serum. Thereafter, cells were infected in quadruplicates (with a 50% tissue culture infective dose) or duplicates (with quantification by PCR) with HSV-1 (strain HSV-F1), (kindly provided by K.E. Schneweis, University of Bonn, Bonn, Germany); virus growth levels were determined (either microscopically or by the use of real-time PCR) 3 days after infection. The 50% infective dose for all groups was calculated on the basis of estimations performed using the Spearman and Kärber method with the support of ID-50 software (V 5.0) (J. L. Spouge, National Institutes of Health, Bethesda, Md.). For PCR analysis, cultures were frozen at −20°C. After being thawed, the content of the wells was resuspended and 200 μl from each duplicate well was harvested, mixed, and centrifuged. The supernatant was removed, and the DNA from the pellet was digested with proteinase K. A total of 5 μl of each DNA sample was mixed with 25 μl of qPCRTM Mastermix (Eurogentec), a 0.3 μM concentration of each primer (HSV-1 forward, 5′-ACC ATG ACC AAG TGG CAG GA; HSV-1 reverse, 5′-AGA A[GT]C GGA AGG AGC CGC), 0.2 μM fluorescence-labeled probe (5′-[carboxyfluorescein]-CGG AGC GCA GCA TCT CGT CCA-[6-carboxy-tetramethyl-rhodamine]), and aqua bidest (total volume, 50 μl) and a plasmid encompassing the amplified region as a standard. The amplification was carried out in an ABI Prism 5700 sequence detector (Applied Biosystems) with the following cycling program: 50°C for 2 min, 95°C for 10 min, 95° for 15 s, and 60°C for 1 min. A standard graph of the threshold cycle (CT) values obtained from serial dilutions of the standard was constructed using the appropriate software, the CT values of the unknown samples were plotted on standard curves, and the number of HSV-1 genomes was calculated. The data shown in Fig. 1 and 2 indicate that HSV-1 is able to replicate in astrocytoma cell cultures and that pretreatment of the cells with IFNs results in a strong and dose-dependent reduction of virus replication. The results of experiments using both techniques showed an approximately 99% inhibition of virus growth in IFN-treated cells. Furthermore, comparable data were obtained with two different batches of human native astrocytes (NHA4631 and NHA5889) (Clonetics, BioWhittaker, Walkersville, Md.) (Fig. 2).
FIG. 1.
Antiviral effect of IFN-α/β and IFN-γ: 3 × 104 86HG39 cells were stimulated with different IFNs (0 to 500 U/ml) for 3 days. Thereafter, the cells were infected with HSV-1 (with or without supplemental l-tryptophan [l-Tryp]) and virus growth was quantified using real-time PCR after 3 days of culture growth. Data are given as the means of the results seen with duplicate cultures.
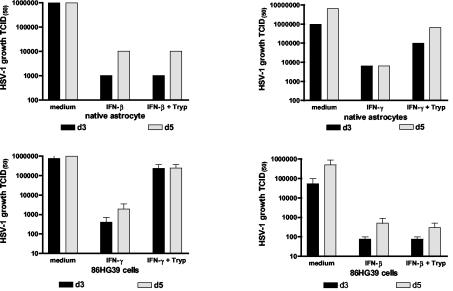
FIG. 2.
Antiviral effect of IFNs: 3 × 104 native astrocytes (upper panels) were stimulated for 3 days with IFN-β (upper left panel) or IFN-γ (upper right panel) (500 U/ml) or 86HG39 cells (lower panels) were stimulated for 3 days with IFN-β (lower right panel) or IFN-γ (lower left panel) (500 U/ml). Thereafter, cells were infected with HSV-1 (with or without supplemental l-tryptophan [l-Tryp]) and virus growth was monitored by estimation of the 50% tissue culture infective dose (TCID(50)). Data obtained with human astrocytes are given as the means of the results seen with duplicate cultures. Data obtained with astrocytoma cells are given as means ± standard deviations of the results of three independent experiments.
Antiviral effects of IFN-γ are mediated by IDO and can be blocked by supplementation of l-tryptophan.
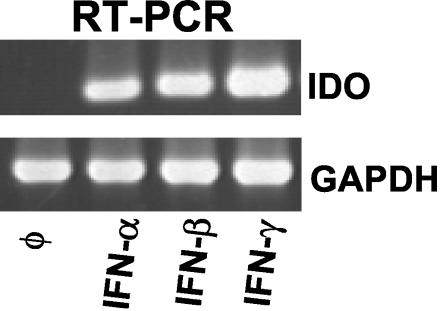
We analyzed IDO mRNA expression induced by IFNs in astrocytoma cells. Total RNA from unstimulated and stimulated cells was extracted with guanidinium thiocyanate. Total RNA (1 μg) for first-strand synthesis with an Advantage RT-for-PCR kit (Clontech, Palo Alto, Calif.) was used according to the instructions of the manufacturer. PCR was carried out with specific IDO primers (downstream primer, 5′-GCA AAT GCA AGA ACG GGA CAC T-3′; upstream primer, 5′-TCA GGG AGA CCA GAG CTT TCA CAC-3′) and GAPDH primers (downstream primer, 5′-ATG GGG AAG GTG AAG GTC GGA GTC-3′; upstream primer, 5′-CAG CGT CAA AGG TGG AGG AGT GG-3′). The annealing time was 45 s at 62°C (and for GAPDH was 45 s at 60°C). The synthesis time for all reverse transcription-PCR procedures was 1 min at 72°C for 30 cycles and was followed by a further incubation for 4 min at 72°C at the end of the last cycle. As indicated in Fig. 3, IFN-α/β and IFN-γ induce IDO mRNA production. In the next experiments we therefore analyzed whether or not the antiviral effect mediated by IFNs could be blocked by supplementation of the cultures with excess amounts of l-tryptophan. Cells were stimulated (as described above) in culture medium supplemented with 50 to 100 μg of l-tryptophan/ml. Figures 1 and 2 show the results of typical experiments. As mentioned above, all three IFNs were able to reduce HSV-1 growth in 86HG39 cells and in human native astrocytes. The supplementation of the cultures with l-tryptophan nearly completely abolished the antiviral effect mediated by IFN-γ, however, while tryptophan supplementation did not influence the antiviral effect mediated by IFN-γ. Comparable results were also obtained with the astrocytoma cell line U373 MG (data not shown). We therefore conclude that IDO induction by IFN-γ is the main antiviral effector mechanism in astrocytoma cells and in human native astrocytes and that IDO induction by IFN-γ is not responsible for the antiviral effect mediated by IFN-α and IFN-β.
FIG. 3.
Detection of IDO mRNA in IFN-stimulated astrocytoma cells. 86HG 9 cells were stimulated with IFN-α, IFN-β, or IFN-γ (500 U/ml) for 8 h, and reverse transcription-PCR (RT-PCR) was performed as described in the text. As a control, GPDH mRNA was analyzed in parallel.
Quantitiative analysis of IFN-induced IDO activity.
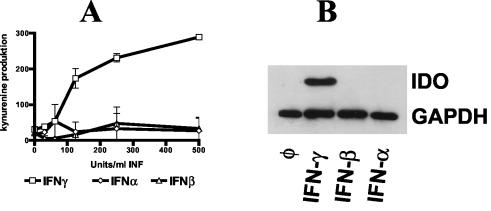
In further experiments we analyzed the induction of IDO by the different IFNs in more detail. First we compared it to the iNOS activity. Nitrite (NO2−) accumulation in the supernatant of culture-grown cells 3 days after stimulation with different cytokines was used as an indicator of NO production and was measured by a Griess reaction (detection limit, 1 μM), with sodium nitrite as a standard (13). We are aware that this method is not a direct measurement of NO and underestimates total NO synthesis. We found that astrocytes and astrocytoma cells do not produce NO in detectable amounts (detection limit, 1 μM) after stimulation with IFNs at up to 1,000 U/ml (data not shown). Thereafter we measured IDO activity via the determination of the level of kynurenine content in the supernatant of the activated cells as previously described by Däubener et al. (12). As shown in Fig. 4A, only IFN-γ stimulation resulted in strong kynurenine production and the kynurenine level in the supernatant of IFN-α- and IFN-β-stimulated cells was below or near the detection limit. In addition IDO protein was detected (using an IDO-specific mouse monoclonal antibody [a gift from Osamu Takikawa] and an anti-GAPDH monoclonal antibody [HyTest, Turku, Finland] as a control) in a Western blot analysis of the cell lysates of astrocytoma cells stimulated with the IFNs (Fig. 4B). Once again IDO protein was found only in IFN-γ-stimulated cells and was undetectable in IFN-α- or IFN-β-stimulated cells.
FIG. 4.
(A) Detection of IDO activity in IFN-stimulated 86HG39 cells. 86HG39 cells (3 × 104) were stimulated with different IFNs (0 to 500 U/ml) for 3 days. Thereafter, supernatants were harvested and the level of kynurenine content was determined by the use of Ehrlich's reagent. Data are given as values of mean optical density at 492 nm (± standard errors) of triplicate cultures. (B) Detection of IDO protein in IFN-stimulated astrocytoma cells. 86HG39 cells were stimulated with different IFNs (500 U/ml). After 3 days, cells were harvested and subjected to Western blot analysis. As a control, GAPDH was analyzed in parallel.
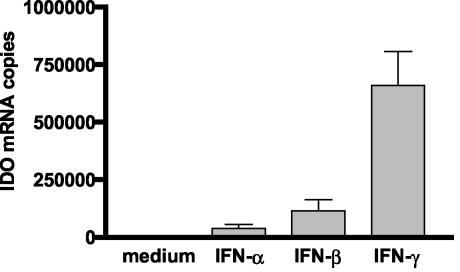
The failure to detect IDO activity and IDO protein in IFN-α- and IFN-β-activated cells correlates well with the results shown in Fig. 1 and 2 and confirms that viral replication is not abolished by l-tryptophan supplementation. This leads to the conclusion that the antiviral effect induced by these two IFNs is not mediated via IDO induction. Because mRNA was detected after stimulation of cells with IFN-γ (Fig. 3), we analyzed IDO mRNA induction in astrocytoma cells in more detail. Astrocytoma cells were stimulated with IFN-α/β and IFN-γ, and IDO mRNA levels were determined quantitatively using a real-time PCR-technique. Total RNA (1 μg) and an Advantage RT-for PCR kit (BD Biosciences) were used for cDNA synthesis, following the manufacturer's instructions. The cDNA strands were used for quantification of IDO mRNA by real-time PCR (TaqMan technique) in 96-well optical plates (Eurogentec, Seraing, Belgium). As a standard, solutions with known molecule numbers of a construct consisting of full-length IDO cDNA cloned into the plasmid pMEP4 were used; the copy number was calculated as described for the HSV-1 PCR (see above). For amplification, the following oligonucleotides were used as primers: IDO forward primer, 5′-CGC CTT GCA CGT CTA GTT CTG; IDO reverse primer, 5′-CGG ACA TCT CCA TGA CCT TTG. For the IDO probe, 5′-(carboxyfluorescein) ATG CAT CAC CAT GGC ATA TGT GTG GG-(6-carboxy-tetramethyl-rhodamine) was used. The primer pair creates an amplicon comprising bases 868 to 938 of IDO mRNA (GenBank accession no. M34455). The data shown in Fig. 5 are representative of the results of three different experiments and indicate that IFN-γ stimulates a IDO mRNA expression level in astrocytoma cells more than 10-fold higher than that seen with IFN-α/β.
FIG. 5.
Quantification of IDO mRNA in IFN-stimulated astrocytoma cells. 86HG39 cells were stimulated with different IFNs (500 U/ml), and RNA was prepared 8 h after stimulation. IDO and GAPDH mRNAs were detected using real-time PCR. The copy numbers of IDO mRNA were normalized to the copy number of GAPDH mRNA in the corresponding sample. Data are given as the means of the results of four independent experiments. Bars indicate standard deviations.
In summary, we have shown that IFN-γ-induced IDO activation is an antiviral effector mechanism for the control of herpes simplex virus. This seems to exist in the absence of detectable NO production. The effect of the presence of NO on inhibition of viral replication has been controversial and is dependent on the in vitro or in vivo experimental conditions. Taking these results together, NO seems to play a pivotal role in the antimicrobial defense mechanism of murine cells (16, 28) whereas this is not true for human cells (5, 9, 17). Additionally, we have recently shown that human astrocytoma cells exhibit strong IDO activation after stimulation with IFN-γ (12). There is an increasing body of evidence indicating that IDO activation in human cells is a potent antiparasitic and antibacterial effector mechanism (7, 9, 10, 11, 27, 33, 39). That viruses are also controlled by IDO activation in human cells was first indicated by Bogdahi et al. in 1999 (3). This group showed that IFN-γ-induced IDO activity was capable of inhibiting the replication of cytomegalovirus in human retinal pigment epithelial cells and that supplementation of l-tryptophan completely blocked the antiviral effect. In this paper we describe the same effect of IDO on viral growth as determined through the use of native astrocytes and astrocytoma cells and found that IFN-α and IFN-β are less potent than IFN-γ in inducing IDO activity and IDO mRNA accumulation. Comparable data were found by (for example) Schmitz et al. (38), who showed that IFN-β-induced IDO activity in human macrophages was not sufficient to mediate an inhibition of the intracellular parasite T. gondii.
There are some possible explanations of how IDO-mediated tryptophan degradation reduces virus replication. First, proteins necessary for virus replication might contain more tryptophan than do the host proteins and therefore a reduction of available tryptophan might preferentially affect synthesis of viral proteins. For example, human immunodeficiency virus type 1 has a tryptophan-rich region which induces helices in protein secondary structures (37). Alternatively, a reduced amount of available tryptophan or an enhanced concentration of tryptophan metabolites might constitute a danger signal in the cells and influence host cell metabolism (29, 30). Moreover, an influence of the degradation products of tryptophan on cell growth (40), as well as a stabilization of several mRNAs by IDO-mediated tryptophan degradation (41), has been shown.
Transfer of this in vitro data to the in vivo situation is difficult. First, it is known that viruses of the herpes virus group interfere with IFN signaling in both a positive and a negative fashion (8, 32, 32). Second, it is known that in in vivo IFN-γ therapy or in humans with infections, a strong IDO induction occurs (6, 18). However, it has also been indicated that IDO induction in vivo might result in an inhibition of T-cell activation and proliferation (20, 29, 30) (which could be interpreted as a negative effect on the antiviral defense). In contrast, some data in the literature indirectly argue for a protective effect of IDO for the control of viral infections. Using IFN-γ-treated iNOS-deficient mice, Karupiah et al. (21) found that IFN-γ induces a strong antiviral effect against influenza virus that was inhibited in the presence of NO. Given the fact that NO is able to inhibit IDO activity, it is possible that the IFN-γ-induced NO-sensitive effector mechanism described by these authors and demonstrated with their in vivo model is that of induction of IDO.
REFERENCES
- 1.Becker, Y. 2002. Herpes simplex virus evolved to use the human defense mechanisms to establish a lifelong infection in neurons—a review and hypothesis. Virus Genes 24:187-196. [DOI] [PubMed] [Google Scholar]
- 2.Bilzer, T., D. Stavrou, E. Dahme, E. Keiditsch, K. F. Burrig, A. P. Anzil, and W. Wechsler. 1991. Morphological, immunocytochemical and growth characteristics of three human glioblastomas established in vitro. Virchows Arch. A Pathol. Anat. Histopathol. 418:281-293. [DOI] [PubMed] [Google Scholar]
- 3.Bodaghi, B., O. Goureau, D. Zipeto, L. Laurent, J. L. Virelizier, and S. Michelson. 1999. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J. Immunol. 162:957-964. [PubMed] [Google Scholar]
- 4.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 5.Bukrinsky, M., H. Schmidtmayerova, G. Zybarth, L. Dubrovsky, B. Sherry, and G. Enikolopov. 1996. A critical role of nitric oxide in human immunodeficiency virus type 1-induced hyperresponsiveness of cultured monocytes. Mol. Med. 2:460-468. [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne, G. I., L. K. Lehmann, J. G. Kirschbaum, E. C. Borden, C. M. Lee, and R. R. Brown. 1986. Induction of tryptophan degradation in vitro and in vivo: a gamma-interferon-stimulated activity. J. Interferon Res. 6:389-396. [DOI] [PubMed] [Google Scholar]
- 7.Byrne, G. I., L. K. Lehmann, and G. J. Landry. 1986. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect. Immun. 53:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Däubener, W., K. Pilz, Z. S. Seghrouchni, T. Bilzer, H. G. Fischer, and U. Hadding. 1993. Induction of toxoplasmostasis in a human glioblastoma by interferon gamma. J. Neuroimmunol. 43:31-38. [DOI] [PubMed] [Google Scholar]
- 10.Däubener, W., V. Posdziech, U. Hadding, and C. R. MacKenzie. 1999. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: tryptophan degradation vs. NO production. Med. Microbiol. Immunol. (Berlin) 187:143-147. [DOI] [PubMed] [Google Scholar]
- 11.Däubener, W., B. Spors, C. Hucke, R. Adam, M. Stins, K. S. Kim, and H. Schroten. 2001. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 69:6527-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Däubener, W., N. Wanagat, K. Pilz, S. Seghrouchni, H. G. Fischer, and U. Hadding. 1994. A new, simple, bioassay for human IFN-gamma. J. Immunol. Methods 168:39-47. [DOI] [PubMed] [Google Scholar]
- 13.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 14.Ellison, A. R., L. Yang, C. Voytek, and T. P. Margolis. 2000. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 268:17-28. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh, S. K., J. Kusari, S. K. Bandyopadhyay, H. Samanta, R. Kumar, and G. C. Sen. 1991. Cloning, sequencing, and expression of two murine 2′-5′-oligoadenylate synthetases. Structure-function relationships. J. Biol. Chem. 266:15293-15299. [PubMed] [Google Scholar]
- 16.Guidotti, L. G., H. McClary, J. M. Loudis, and F. V. Chisari. 2000. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J. Exp. Med. 191:1247-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann, E., T. Idziorek, J. P. Kusnierz, Y. Mouton, A. Capron, and G. M. Bahr. 1997. Role of nitric oxide in the regulation of lymphocyte apoptosis and HIV-1 replication. Int. J. Immunopharmacol. 19:387-397. [DOI] [PubMed] [Google Scholar]
- 18.Heyes, M. P., B. J. Brew, K. Saito, B. J. Quearry, R. W. Price, K. Lee, R. B. Bhalla, M. Der, and S. P. Markey. 2003. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J. Neuroimmunol. 40:71-80. [DOI] [PubMed] [Google Scholar]
- 19.Huang, T., J. Pavlovic, P. Staeheli, and M. Krystal. 1992. Overexpression of the influenza virus polymerase can titrate out inhibition by the murine Mx1 protein. J. Virol. 66:4154-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwu, P., M. X. Du, R. Lapointe, M. Do, M. W. Taylor, and H. A. Young. 2000. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164:3596-3599. [DOI] [PubMed] [Google Scholar]
- 21.Karupiah, G., J. H. Chen, S. Mahalingam, C. F. Nathan, and J. D. MacMicking. 1998. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katze, M. G. 1995. Regulation of the interferon-induced PKR: can viruses cope? Trends Microbiol. 3:75-78. [DOI] [PubMed] [Google Scholar]
- 23.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 24.Kleinschmidt-DeMasters, B. K., and D. H. Gilden. 2001. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 11:440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebau, P., E. Kuse, M. Winkler, H. J. Schlitt, K. Oldhafer, W. Verhagen, J. Flik, and R. Pichlmayr. 1996. Management of herpes simplex virus type 1 pneumonia following liver transplantation. Infection 24:130-135. [DOI] [PubMed] [Google Scholar]
- 26.Ljungman, P. 1993. Herpes virus infections in immunocompromised patients: problems and therapeutic interventions. Ann. Med. 25:329-333. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie, C. R., U. Hadding, and W. Däubener. 1998. Interferon-gamma-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J. Infect. Dis. 178:875-878. [DOI] [PubMed] [Google Scholar]
- 28.MacLean, A., X. Q. Wei, F. P. Huang, U. A. Al Alem, W. L. Chan, and F. Y. Liew. 1998. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 79(Pt. 4):825-830. [DOI] [PubMed] [Google Scholar]
- 29.Munn, D. H., E. Shafizadeh, J. T. Attwood, I. Bondarev, A. Pashine, and A. L. Mellor. 1999. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189:1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn, D. H., M. Zhou, J. T. Attwood, I. Bondarev, S. J. Conway, B. Marshall, C. Brown, and A. L. Mellor. 1998. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191-1193. [DOI] [PubMed] [Google Scholar]
- 31.Naik, H. R., and P. H. Chandrasekar. 1996. Herpes simplex virus (HSV) colitis in a bone marrow transplant recipient. Bone Marrow Transplant. 17:285-286. [PubMed] [Google Scholar]
- 32.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 33.Pfefferkorn, E. R. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldo, C. R., Jr., and D. J. Torpey III. 1993. Cell-mediated immunity and immunosuppression in herpes simplex virus infection. Immunodeficiency 5:33-90. [PubMed] [Google Scholar]
- 35.Roubalova, K., A. Suchankova, A. Vitek, and J. Sajdova. 2000. Presence of herpes simplex virus (HSV) in peripheral leukocytes of patient who developed active HSV infection after bone marrow transplantation. J. Clin. Virol. 17:37-42. [DOI] [PubMed] [Google Scholar]
- 36.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schibli, D. J., R. C. Montelaro, and H. J. Vogel. 2001. The membrane-proximal tryptophan-rich region of the HIV glycoprotein, gp41, forms a well-defined helix in dodecylphosphocholine micelles. Biochemistry 40:9570-9578. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz, J. L., J. M. Carlin, E. C. Borden, and G. I. Byrne. 1989. Beta interferon inhibits Toxoplasma gondii growth in human monocyte-derived macrophages. Infect. Immun. 57:3254-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroten, H., B. Spors, C. Hucke, M. Stins, K. S. Kim, R. Adam, and W. Daubener. 2001. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3-dioxygenase. Neuropediatrics 32:206-210. [DOI] [PubMed] [Google Scholar]
- 40.Terness, P., T. M. Bauer, L. Rose, C. Dufter, A. Watzlik, H. Simon, and G. Opelz. 2002. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Wissen, M., M. Snoek, B. Smids, H. M. Jansen, and R. Lutter. 2002. IFN-gamma amplifies IL-6 and IL-8 responses by airway epithelial-like cells via indoleamine 2,3-dioxygenase. J. Immunol. 169:7039-7044. [DOI] [PubMed] [Google Scholar]
- 42.Whitley, R. 1981. Diagnosis and treatment of herpes simplex encephalitis. Annu. Rev. Med. 32:335-340. [DOI] [PubMed] [Google Scholar]
- 43.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 44.Wingard, J. R. 1993. Viral infections in leukemia and bone marrow transplant patients. Leuk. Lymphoma 11(Suppl. 2):115-125. [DOI] [PubMed] [Google Scholar]