Abstract
Background:
To assess the effect of smoking on lip and gingival pigmentation and also to assess the relationship of pigmentation with periodontal parameters.
Materials and Methods:
109 smokers and an equal number of control subjects who were nonsmokers in the age range of 35 – 44 years comprised the study sample. All the participants were assessed for pigmentation on lip and gingiva and a total periodontal status examination was done with measurements on gingival bleeding, probing depth and loss of attachment at six points in each tooth.
Results:
Melanin pigmentation on lips and gingiva was observed in all the smokers except for one who did not exhibit gingival pigmentation. Significantly greater number of smokers exhibited pigmentation than nonsmokers. Gingival bleeding on probing, probing depth and loss of attachment differed significantly in relation to gingival and lip pigmentation.
Conclusions:
Oral pigmentation was widespread and more commonly observed in smokers than nonsmokers and there was a relationship between pigmentation and periodontal deterioration.
Keywords: Oral mucosa, pigmentation, smoking, periodontium
INTRODUCTION
Smoking is seen to have many adverse effects on the body. It is evident beyond doubt from past literature that cigarette smoking is a significant risk factor for periodontal disease.[1] The current epidemiological evidence demonstrates that cigarette smoking is a stronger risk factor for periodontitis in comparison to other potential periodontal pathogens.[2]
In addition to periodontal destruction, one of the adverse effects smoking could exert on the oral cavity is the pigmentation of the oral mucosa. However, smoking is not the sole contributor for oral pigmentation but it has also been associated with various etiologic factors.[3,4] Axel and Hedin[5] first described oral pigmentation including lip pigmentation in 1982; since then, there were no reports except for a recent study by Haresaku, et al.,[6] who observed the association of oral pigmentation (lip and gingiva) with smoking status.
However, the relationship of pigmentation with periodontal parameters has not been studied earlier. Thus, the present study was initiated to assess the effect of smoking on lip and gingival pigmentation and also to assess the relationship of pigmentation with periodontal parameters.
MATERIALS AND METHODS
The present study was conducted on 218 patients attending Darshan Dental College and Hospital, Udaipur, India during the period September to November 2008. Consequently, 109 smokers and an equal number of control subjects (pair matched) who were nonsmokers in the age range of 35–44 years were selected as the final sample. Mean age of the subjects was 39.5 years. Individuals suffering from nutritional deficiencies, systemic disorders, and oral mucosal disorders that would cause oral pigmentation were excluded from the study. Inclusion criteria for control subjects constituted those who were not using any form of tobacco and had not undergone any form of periodontal therapy.
The methodology for the present study has been adopted from a previous report;[6] clinical examination was conducted by a single examiner and each subject was assessed for lip[6] and gingival pigmentation[7] (Score 0: No pigmentation; score 1: 1 or 2 solitary unit (s) of pigmentation on papillary gingiva; Score 2: >3 units of pigmentation on papillary gingiva; Score 3: One or more short continuous ribbons of pigmentation on papillary gingiva; Score 4: One continuous ribbon including the entire area between canines) besides periodontal assessment (gingival bleeding, probing depth, and loss of attachment in each tooth at six sites). Gingival bleeding was assessed as the presence or absence of bleeding, and thus the mean percentage of sites with bleeding was calculated for each individual. Statistical differences in periodontal parameters in relation to grades of lip and gingival pigmentation in maxillary and mandibular gingiva were assessed using the Mann–Whitney U and Kruskal–Wallis tests, respectively.
RESULTS
Melanin pigmentation on lips and gingiva was observed in all the smokers except for one who did not exhibit gingival pigmentation. Significantly, a greater number of smokers exhibited pigmentation than nonsmokers which could be attributed to nicotine and benzopyrene in the tobacco smoke that stimulate the production of melanin from the melanocytes.[8] Nearly two-thirds (31.2%) and 73.4% nonsmokers exhibited lip and gingival pigmentation, respectively (not presented in tables), which might be due to ethnic pigmentation. This is in accordance with the fact that oral pigmentation was reported among 96% of the Indian population, whereas it was reported to be 15% among Europeans.[9] However, among the population of Turkey (Eurasia), it was found to be 37%.[10]
DISCUSSION
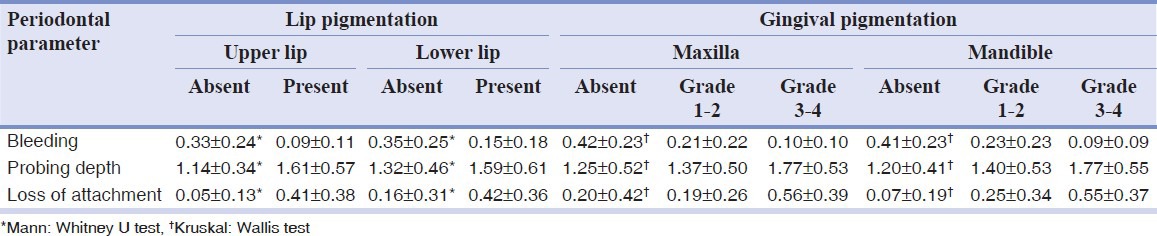
Table 1 depicts that gingival bleeding on probing, probing depth, and loss of attachment differed significantly in relation to gingival and lip pigmentation. Gingival bleeding was inversely related to lip and gingival pigmentation, in accordance to an earlier study in Turkey.[10] On the other hand, measurements of periodontal probing depth and loss of gingival attachment were seen highest in subjects with pigmented lips and in those who had grade 3–4 of gingival pigmentation. The observed relationship between pigmentation and periodontal deterioration could be attributed to the indirect relationship between these two entities which have a common etiological agent, smoking.
Table 1.
Mean scores of periodontal parameters according to levels of lip and gingival pigmentation
CONCLUSION
Oral pigmentation was widespread and more commonly observed in smokers than nonsmokers and there was a relationship between pigmentation and periodontal deterioration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Tanner AC, Kent RJ, Van Dyke T, Sonis ST, Murray LA. Clinical and other risk indicators for early periodontitis in adults. J Periodontol. 2005;76:573–81. doi: 10.1902/jop.2005.76.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darby IB, Hodge PJ, Riggio MP, Kinane DF. Clinical and microbiological effect of scaling and root planning in smoker and non-smoker chronic aggressive periodontitis patients. J Clin Periodontol. 2005;32:200. doi: 10.1111/j.1600-051X.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 3.Meyerson MA, Cohen PR, Hymes SR. Lingual hyperpigmentation associated with minocycline therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:180–4. doi: 10.1016/s1079-2104(05)80279-3. [DOI] [PubMed] [Google Scholar]
- 4.Amir E, Gorsky M, Buchner A, Sarnat H, Gat H. Physiologic pigmentation of the oral mucosa in Israeli children. Oral Surg Oral Med Oral Pathol. 1991;71:396–8. doi: 10.1016/0030-4220(91)90325-7. [DOI] [PubMed] [Google Scholar]
- 5.Axell T, Hedin CA. Epidemiologic study of excessive oral melanin pigmentation with special refrence to the influence of tobacco habits. Scand. J Dent Res. 1982;90:434–42. doi: 10.1111/j.1600-0722.1982.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 6.Haresaku S, Hanioka T, Tsutsui A, Watanabe T. Association of lip pigmentation with smoking and gingival melanin pigmentation. Oral Dis. 2007;13:71–6. doi: 10.1111/j.1601-0825.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 7.Hedin CA. Smokers melanosis. Occurence and localization in the attached gingiva. Arch Dermatol. 1977;113:1533–8. doi: 10.1001/archderm.113.11.1533. [DOI] [PubMed] [Google Scholar]
- 8.Araki S, Murata K, Ushio K, Sakai R. Dose response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch Environm Health. 1983;38:375–8. doi: 10.1080/00039896.1983.10545823. [DOI] [PubMed] [Google Scholar]
- 9.Hedin CA, Axell T. Oral melanin pigmentation in 467 Thai and Malaysian people with special emphasis on smoker's melanosis. J Oral Pathol Med. 1991;20:8–12. doi: 10.1111/j.1600-0714.1991.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 10.Unsal E, Paksoy C, Soykan E, Elhan AH, Sahin M. Oral melanin pigmentation related to smoking in a Turkish population. Community Dent Oral Epidemiol. 2001;29:272–7. doi: 10.1034/j.1600-0528.2001.290406.x. [DOI] [PubMed] [Google Scholar]


