Abstract
Background:
Odontogenic keratocyst (OKC) is an aggressive cyst and its recurrence is higher than other odontogenic cysts, orthokeratinized odontogenic cyst (OOC) is a cyst with moderate biological behavior in comparison with OKC, but with the probability of carcinomatous changes. The present study aims to evaluate the quantity and intensity of the expression of P53 protein and transforming growth factor alpha (TGF-alpha) in OKC and OOC in order to compare the biologic behavior of these two cysts.
Materials and Methods:
This is a cross-sectional study. The samples include 30 cysts (15 OKC and 15 OOC), all stained immunohistochemically for P53 protein and TGF-alpha by the Novolinke polymer method. Then, all the cases were examined with an optical microscope with Χ400 magnification and the stained cells were counted in the basal and parabasal layers. Finally the results were analyzed by the Mann–and Wilcoxon tests (P value < 0.05).
Results:
The difference between the expression of P53 protein in the basal layer in OKC and OOC was not statistically significant (P value = 0.076). The difference between the expression of P53 protein in the parabasal layer in OKC and OOC was statistically significant (P value = 0.003); moreover, the difference between the expression of TGF-alpha in the basal layer in OKC and OOC was not statistically significant (P value = 0.284). The difference between the expression of TGF-alpha in the parabasal layer in OKC and OOC was statistically significant (P value = 0.015).
Conclusion:
Since there was a higher expression of P53 protein and TGF-alpha in OKC compared to those in OOC, the probability of carcinomatous changes was at least theoretically higher in OKC than in OOC.
Keywords: Odontogenic cysts, P53 protein, TGF-alpha
INTRODUCTION
The term odontogenic keratocyst (OKC) was used for the first time by Philipsen in 1956 in defining an odontogenic cyst with parakeratinized epithelial surface.[1]
Despite its bland histology, OKC shows more aggressive behavior and higher expression rate which make it different from other odontogenic cysts that produce keratin. It has been a long time since many researchers supported that the cyst has a neoplastic nature due to its biological behaviors and based on a number of molecular and genetic evidences, they found. This is a reason why, in its recent classification, WHO has called this lesion a benign neoplasm entitled keratocystic odontogenic tumor (KCOT), because the epithelial surface of OKC shows a higher mitotic activity than other odontogenic cysts.[2,3,4,5]
OKC may be found at any age, but the outbreak of this type of cyst is more common in the second and third decades of life and also it is common more in males than females. It happens in the mandible in 60% to 80% of the cases and it has special tendency toward mandibular angle and ascending ramus.[2,6,7]
Radiographically, OKC appears in the form of unilocular or multilocular radiolucency with specific borders and usually with sclerotic smooth margins.[8,9]
The histopathologic characteristics of OKC are pathognomonic and include uniform thickness, rete ridgeless stratified squamous epithelium, corrugated superficial parakeratinization, basal cell hyperchromatism, reverse polarization, and palisading arrangement.[1,10,11]
In the past, OKCs were categorized into two types: parakeratinized and orthokeratinized. However, it was revealed that the orthokeratinized type not only lacks the typical characteristics of the parakeratinized type and composed of orthokeratinized stratified squamous epithelium with a prominent granular layer, but also shows different biological or clinical behaviors in the way that its recurrence is very much lower than the parakeratinized type. This is the reason why nowadays the orthokeratinized type is considered a different cyst with different histopathological and clinical characteristics.[12,13,14]
Therefore, in this study, we were going to compare the OKCs and OOCs through investigating the extent of P53 protein expression which is one of the tumor suppressors and TGF-alpha expression which is a member of the growth factors family. Note that both markers can be found in the head and neck cancers higher than normal tissues. By doing so, we may find out the cause of different biological behaviors of these cysts.
MATERIALS AND METHODS
This study is a descriptive analytic and cross-sectional study without direction. A total of 30 cases including those of 15 OKC and 15 OOC were used in this study.
3-4 μm sections from paraffin-embedded specimens were mounted on poly-L-lysine-coated glass slides.
After deparaffinization and rehydration with five descending alcohol, the sections were incubated in citrate buffer in a microwave oven for 15 min for antigen retrieval and incubated in 0.5% H2O2 in methanol for 10 min to block endogenous peroxidase activity and then rinsed with phosphate-buffered saline (PBS). Specimens were incubated for 1 h with the lyophilized monoclonal anti-P53 (NCL-P53-DO1, Novacastra, Germany) at a dilution of 1:50 and the lyophilized monoclonal anti-TGF-alpha (NCL-TGFα R1, Novacastra, Germany) at a dilution of 1:100. Immunocomplexes were subsequently treated with post-primary block and then detected by Novolink polymer (Novacastra, Germany) for 30 min, both incubated for 30 min at room temperature. After rinsing with PBS, the immunoreactivity was visualized by diaminobenzidine (DABO, DAKO, Denmark).
Sections were finally counterstained with hematoxylin, cleared and mounted with PV mount, and slides were blindly viewed independently by two oral pathologists by light microscopy (Olympus BX41TF, Tokyo, Japan).
Positive controls consisted of tissue specimen sections of breast carcinoma with known antigenic reactivity. A negative control was stained by omitting the primary antibody.
Specimen evaluation
The specimens were examined at ×400 magnification in a light microscope. The percentage of positive epithelial cells in ten high-power fields of a microscope was determined and with regard to cytoplasmic positivity for TGF-alpha and nuclear positivity for P53 antigen, classified at a scale 1-4: (+1) 0−25% positive cells; (+2) 26-50% positive cells; (+3) 51-75% positive cells; (+4) 76−100% positive cells.
Also intensity of staining with P53 and TGF-alpha antigen was evaluated on the following method: (0) when cells had not been staining and (+1), (+2), (+3), (+4) for very low, low, moderate, and high staining.
Finally the SID (staining intensity distribution) score was calculated by multiplication of these two scores for each specimen.
The data were analyzed by means of statistical software SPSS 10 with the Mann–Whitney and Wilcoxon statistical tests at a significant level of 0.05 for comparison of data between P53 protein and TGF-alpha.
RESULTS
The expression with p53 protein leads to definite bright brown staining in the nucleus of the epithelial cells.
According to Wilcoxon's statistical test, the expression in basal and parabasal layers of OKC for P53 protein was not statistically significant (P value = 0.356) but was statistically significant for TGF-alpha (P value = 0.041).
The expression in the basal and parabasal layers of OOC for P53 protein was not statistically significant but was near to significant (P value = 0.053) and also was statistically significant for TGF-alpha (P value = 0.012).
According to Mann–Whitney's statistical test, the following results for P53 protein were obtained.
The average expression for P53 protein in the basal layer in OKC was higher than OOC but this difference was not statistically significant (P value = 0.076) [Table 1] [Figure 1a–b].
Table 1.
P53 protein expression in OKC and OOC
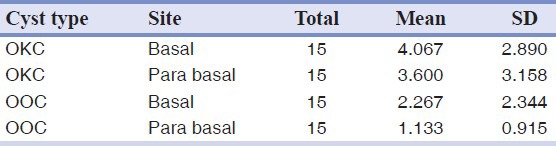
Figure 1.
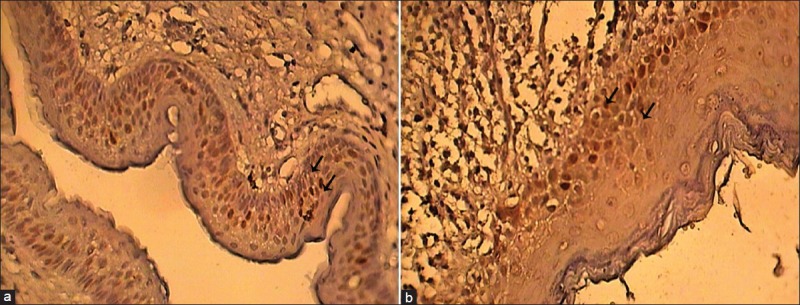
(a) P53 protein expression in the basal and parabasal layers of OKC (×400); (b) P53 protein expression in the basal and parabasal layers of OOC (×400)
The average expression for P53 protein in the parabasal layer in OKC was higher than OOC and this difference was statistically significant (P value = 0.003) [Table 1] [Figure 1a–b].
The expression for TGF-alpha was localized to the cytoplasm.
According to Mann–Whitney statistical test, the following results for TGF-alpha were obtained.
The average expression for TGF-alpha in the basal layer in OKC was higher than OOC but this difference was not statistically significant (P value = 0.284) [Table 2] [Figure 2a–b].
Table 2.
TGF-alpha expression in OKC and OOC
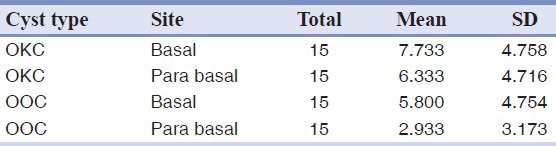
Figure 2.
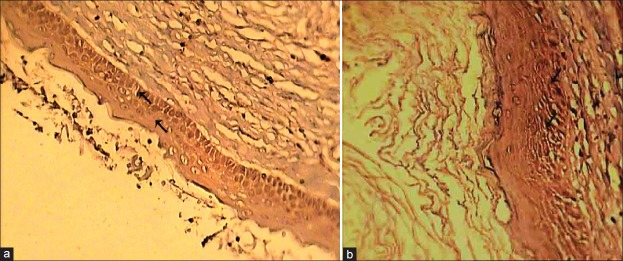
(a) TGF-alpha expression in the basal and parabasal layers of OKC (×400); (b) TGF-alpha expression in the basal and parabasal layers of OOC (×400)
The average expression for TGF-alpha in the parabasal layer in OKC was higher than OOC and this difference was statistically significant (P value = 0.015) [Table 2] [Figure 2a–b].
Figure 3 compares the expression of P53 protein and TGF-alpha in OKC and OOC.
Figure 3.
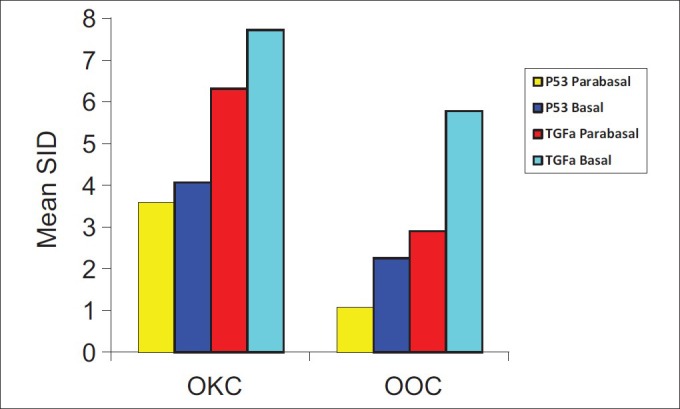
P53 protein and TGF-alpha expression in the basal and parabasal layers of OKC and OOC
DISCUSSION
The growth mechanism and biologic behavior of OKC is different from the more prevalent radicular and dentigerous cysts, in which, contrary to other cysts, the main factor in the growth of OKC is proliferation of epithelial lining of the cyst under the induced effect of ectomesenchyme. Etiology and pathogenesis of OOC are not clear. Some of the researchers proposed that OOC be considered as metapelastic orthokeratinized dentigerous cysts because of the common relationship between OCC and the unerupted teeth, and its less aggressive biological behavior compared to OKC.[1]
The findings of the present study indicate that the average of cell expression of P53 protein in the parabasal layer of OKC is more than that of OOC. This difference is statistically significant. Such findings support Li which indicates that the number of positive P53 protein cells is the highest in OKC and it is mainly in the parabasal layer.[15]
Slootweg believes that the increase in proliferation is not necessarily related to the mutation of the P53 gene. This phenomenon cannot be determined through immunohistochemical studies, because stabilization of P53 protein can occur due to the increase in protein production or protection of P53 protein against damage by bonding to viral or other cell proteins.[16]
Lo Muzio investigated the expression of P63 that is from the p53 family in several cysts. It was indicated that OKCs, especially the parakeratinized ones, had the highest number of positive P63 cells and the higher dissemination of P63 in parakeratinized OKCs in comparison with orthokeratinized types can account for the clinical and pathological characteristics of OKC and OOC.[17]
Also the expression of P63 in the orthokeratinized type was limited to basal and parabasal layers and only in 50% of the cases, the cells of the middle layer were stained, while the cells had expression in all layers (basal, parabasal, and surface basal) in the parakeratinized type. This finding is in accordance with those of the present study which indicate that OKC has significantly higher expression in the parabasal layer for P53 protein in comparison with OOC.[17]
Findings of the present study also accord with those of Dong in which the markers Ki67 and P63 were significantly in lower amount in orthokeratinized type than in OKC. Also, in orthokeratinized type, P63 is observed merely in the basal layer and some parts of the suprabasal layer, while in parakeratinized type, the expression was observed in all layers except for the superficial parakeratinized layer.[18]
In contrast, Moghaddam et al. compared ameloblastoma with OKC, and found that only 5.12% of OKCs had positive areas for P53 protein. Accordingly, it was concluded that among OKCs, some were P53 positive and some were P53 negative. These findings indicate that OKCs can show different reactions. It was also probable that because the nature of the lesion was benign, OKC was not positive for P53 protein. Another reason may be due to the technical problems in the adopted immunohistochemical method.[19]
Furthermore, Baghaei et al. by using Ki67 and P53 markers between OKCs and OOCs demonstrated that the expression of OKC for Ki67 marker was significantly higher than OOC and deduced that is a reason for the aggressive behavior of OKC and its tendency to recur more. Also P53 protein expression was higher in OOC than in OKC. But this difference was not statistically significantly. They attributed this finding, in contrast with the findings of the present study, to the neoplastic changes in the epithelial lining of OOC.[20]
The possible reason for such a contrast is the use of two different techniques which may cause different levels of accuracy. Another point is that the difference found in Baghaei et al. was not statistically significant and therefore not enough for rejecting the obtained findings of this study.
Another finding of the present study was the presence of a higher average of expression for TGF-alpha in the basal layer of OKC than in OOC. While this difference is not statistically significant, the difference in the parabasal layer is statistically significant.
Li's findings support the findings of this study. Li indicates that the epithelial tissues of all types of odontogenic cysts have TGF-alpha gene manifestation despite the fact that the stainability of various cysts is different and its amount is higher in OKCs in comparison to radicular cysts. This feature accords with higher layers of EGF-R gene expression and with Ki67 nuclear antigene in OKC epithelium. This demonstrates that these cysts have a unique growth characteristic which is not observed in other odontogenic cysts. It has been proved that the simultaneous gene expression of TGF-alpha and TGF-R in neoplastic cells results in the growth feature of these cells compared to the normal cells. The strong reaction of TGF-alpha in accordance with high levels of gene expression of TGF-R in OKC demonstrates that TGF-alpha can act as a growth factor to stimulate cell proliferation and differentiation in this cyst type.[21]
Moghaddam et al.'s study demonstrated that TGF- alpha was stained +2 and +3 and in a patchy form for OKC in all cases, while the expression for most of the ameloblastomas was high. It is reasoned that this protein is secreted as endogenic by tumor cells and this can reinforce proliferation of ameloblastoma in affected area.[19]
The last finding of this study indicates that the expression of TGF-alpha and P53 protein is higher in the basal layer of both OKC and OOC than in their parabasal layer. This relation is statistically significant for TGF-alpha and not significant for P53 protein.
In previous studies conducted on Ki67 protein, the expression amount was higher in the parabasal layer than in the basal layer. The proposed reason was that parabasal layer cells are in a stage of cell cycle which has higher expression of Ki67. One possible justification for this finding is that basal layer cells are mostly in the form of stem cells with slow cell cycle and long the G1phase.[22]
Our hypothesis was that the probability of TGF-alpha and P53 expressions in the parabasal layer will be higher than in the basal layer. This hypothesis was not confirmed by the obtained findings. This may be due to be expression of P53 protein and TGF-alpha is not directly related to mitosis. Therefore, it is not possible to generalize the findings for Ki67 to P53 protein and TGF-alpha.
CONCLUSION
Considering the higher expression of P53 protein and TGF-alpha in OKCs in comparison with OOC, the possibility of carcinomatous changes of OKC is, at least theoretically, higher than that of OOC cysts. However, the finding of long-term follow-ups of OKC does not confirm this hypothesis and it is rare if any.
Footnotes
Source of Support: This report is based on a thesis which was submitted to the School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran, in partial fulfillment of the requirements for the MSc degree in Oral and Maxillofacial Pathology (#390276). The study was approved by the Medical Ethics and Research Office at the Isfahan University of Medical Sciences and financially supported by this University
Conflict of Interest: The authors declare no conflicts of interest, real or perceived, financial or nonfinancial
REFERENCES
- 1.Shear M. The aggressive nature of odontogenic keratocyst is it a benign cystic or neoplasm? Part 2. Prolifration and genetic studies. Oral Oncol. 2002;38:323–31. doi: 10.1016/s1368-8375(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 2.Neville BW, Dam DD, Allen CM, Bouqyot JE. 3rd ed. Philadelphia: WB Saunders; 2009. Oral and maxillofacial pathology; pp. 683–91. [Google Scholar]
- 3.Philipsen HP. Keratocystic odontogenic tumor. In: Barnes L, Eveson JW, Reichart PA, Sidransky D, editors. World Health Organization Classification of Tumours: Pathology and Genetics Head and Neck Tumours. Lyon, France: IARC Press; 2005. pp. 306–7. [Google Scholar]
- 4.Madras J, Lapointe H. Keratocystic odontogenic tumour: Reclassification of the odontogenic keratocyst from cyst to tumour. J Can Dent Assoc. 2008;74:165–165h. [PubMed] [Google Scholar]
- 5.Artese L, Iezzi G, Piattelli A, Rubini C, Goteri G, Pernotti V, et al. p16 Expression in odontogenic Cysts. Dent Res J. 2008;5:61–4. doi: 10.1177/030089160809400513. [DOI] [PubMed] [Google Scholar]
- 6.Hyun HK, Hong SD, Kim JW. Recurrent keratocystic odontogenic tumor in the mandible: A case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:7–10. doi: 10.1016/j.tripleo.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Eryilmaz T, Ozmen S, Findikcioglu K, Kandal S, Aral M. Odontogenic keratocyst: An unusual location and review of the literature. Ann Plast Surg. 2009;62:210–2. doi: 10.1097/SAP.0b013e31817dad9c. [DOI] [PubMed] [Google Scholar]
- 8.Chirapathomsakul D, Sastravaha P, Jansisyanont P. A review of odontogenic keratocysts and the behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:5–10. doi: 10.1016/j.tripleo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.White SC, Pharoah MJ. Cysts of the jaws. In: White SC, Pharoah J, editors. Oral Radiology: Principles and Interpretation. 5th ed. St. Louis: Mosby; 2004. pp. 384–409. [Google Scholar]
- 10.Shafer W, Hine M, Levy B. 4th ed. Philadelphia: W.B. Suanders; 1983. A textbook of oral pathology; pp. 258–76. [Google Scholar]
- 11.Pindborg JJ, Philipsen HP, Henriksen J. Studies on odontogenic cyst epithelium. In: Sognnaes RF, editor. Fundamentals of Keratinization. Washington, DC: American Association of the Advancement of Science; 1962. pp. 151–60. [Google Scholar]
- 12.Wright JM. The odontogenic keratocyst: Orthokeratinized variant. Oral Surg Oral Med Oral Pathol. 1981;51:609–18. doi: 10.1016/s0030-4220(81)80011-4. [DOI] [PubMed] [Google Scholar]
- 13.Deyhimi P. 1st ed. Isfahan: Isfahan University of Medical Science; 2006. Pathology of tooth and odontogenic lesions; pp. 417–47. [Google Scholar]
- 14.Simarpreet V, Sudesh K, Ramandeep S, Tushar K. Orthokeratinized odontogenic cyst of the mandible: A case report. Int J Oral Maxillofac Pathol. 2012;3:69–73. [Google Scholar]
- 15.Li TJ, Browne RM, Prime SS, Paterson IC, Matthews JB. p53 expression in odontogenic keratocyst epithelium. J Oral Pathol Med. 1996;25:249–55. doi: 10.1111/j.1600-0714.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 16.Slootweg PJ. P53 protein and Ki-67 reactivity in epithelial odontogenic lesions. An immunohistochemical study. J Oral Pathol Med. 1995;24:393–7. doi: 10.1111/j.1600-0714.1995.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 17.Muzio LO, Santarelli A, Caltabiano R, Rubini C. P63 Expression in odontogenic cysts. Int Assoc Oral Maxillofac Surg. 2005;34:668–73. doi: 10.1016/j.ijom.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Dong Q, Pan S, Sun Li, Jun Li S. Orthokeratinized odontogenic cyst: A clinicopathologic study of 61 cases. Arch Pathol Med. 2010;134:271–5. doi: 10.5858/134.2.271. [DOI] [PubMed] [Google Scholar]
- 19.Moghadam BK, Yazdi I, Barker B, Cob C, Cumming CG. Immunohistocemical determination of tumor-Associated Antigens in Ameloblastoma and odontogenic cyst. Acta Media Iranica. 1997;35:1–7. [Google Scholar]
- 20.Baghaei F, Eslami M, Sadri D. Evaluation of ki-67 Antigen and Protein P53 Expression in orthokratinized and Parakratinized odontogenic keratocyst. J Dent Tehran Univ Med Sci. 2004;2:53–8. [Google Scholar]
- 21.Li TJ, Browne RM, Matthews JB. Immunocytochemical expression of growth factors by odontogenic jaw cysts. J Clin Pathol Mol Pathol. 1997;50:21–7. doi: 10.1136/mp.50.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan S, Chandran GJ, Piall KR. Expression of p53 in leukoplakia and squamous cell carcinoma of the oral mucosa. Correlation with expression of ki-67. Mol Pathol. 1996;46:1705. doi: 10.1136/mp.49.3.m170. [DOI] [PMC free article] [PubMed] [Google Scholar]


