Abstract
Whereas several recent AIDS vaccine strategies have protected rhesus macaques against a pathogenic simian/human immunodeficiency virus (SHIV)89.6P challenge, similar approaches have provided only modest, transient reductions in viral burden after challenge with virulent, pathogenic SIV, which is more representative of HIV infection of people. We show here that priming with replicating adenovirus recombinants encoding SIV env/rev, gag, and/or nef genes, followed by boosting with SIV gp120 or an SIV polypeptide mimicking the CD4 binding region of the envelope, protects rhesus macaques from intrarectal infection with the highly pathogenic SIVmac251. Using trend analysis, significant reductions in acute-phase and set point viremia were correlated with anti-gp120 antibody and cellular immune responses, respectively. Within immunization groups exhibiting significant protection, a subset (39%) of macaques have exhibited either no viremia, cleared viremia, or controlled viremia at the threshold of detection, now more than 40 weeks postchallenge. This combination prime-boost strategy, utilizing replication competent adenovirus, is a promising alternative for HIV vaccine development.
Human and simian immunodeficiency virus (HIV and SIV) vaccines capable of inducing broad immunity and strong protection from experimental challenge have remained elusive. The more promising vaccine strategies currently in development have induced strong cellular immunity by incorporating DNA priming and boosting with recombinant vectors, including modified virus Ankara (1), vesicular stomatitis virus (37), and replication-defective adenovirus (Ad) (41). To date, these approaches have provided the best protective efficacy in rhesus macaques challenged with the chimeric virus SHIV89.6P, the utility of which has been questioned as a model relevant to human infection with HIV (11). SHIV89.6P, although it induces a rapid depletion of CD4+ T cells within a few weeks after exposure, may be more easily contained if there is a marginal preservation of the immune system. Unlike HIV transmission, where CCR5-using strains predominate, SHIV89.6P uses CXCR4 and is highly sensitive to neutralization with autologous antibodies. With the exception of live attenuated SIV vaccines able to confer complete protection (7) but with associated safety concerns (38), current strategies have not provided equivalent protection against more vigorous SIV strains with greater relevance to HIV infection, including uncloned SIVmac251 (4, 19, 28, 30), cloned SIVmac239 (10, 15), SIVsmE660 (8, 9, 29) and SIVsmDeltaB670 (12). Strong protection against SIVmac251 intrarectal challenge was reported in one study (2), but similar protective efficacy was not achieved subsequently (13). The significance of protection against SIVmac251 elicited in two of seven cynomolgus macaques by poliovirus-recombinant vaccination (6) is unclear since other studies have used rhesus monkeys. Herpesvirus recombinants protected two of seven macaques against intrarectal SIVmac239 challenge (27); however, the result has not been extended to the heterogeneous SIVmac251.
We have been developing replication-competent Ad HIV and SIV recombinant vectors. Ad-HIVenv/rev recombinant priming with HIV gp120 protein boosting successfully elicited mucosal, cellular, and humoral immunity in chimpanzees and protection from homologous and heterologous HIV challenges (23, 24, 33, 46). Immunizing with E3-deleted replicating Ad type 5 host range mutant (Ad5hr) recombinants expressing SIV env/rev and/or SIV gag prior to boosting with SIV gp120 subunit protein elicited potent immunity in rhesus macaques (3, 4, 44) and reduced viral burdens after a pathogenic mucosal SIVmac251 challenge (4, 45). Strong persistent control of viral replication was not achieved, however. We modified the immunization regimen and added Ad5hr-SIV nefΔ1-13 to the vaccine strategy. Priming with these Ad5hr-SIV recombinants elicited potent cellular immunity to all four encoded SIV genes: env, rev, gag, and nef. The immunity was persistent, extending to 30 weeks after the last Ad immunization (25, 32).
Here we present the results of a rectal challenge with pathogenic SIVmac251 of these macaques. We included a comparison of macaques boosted with gp120 versus a novel SIV peptide polymer (peptomer) analogous to a conformationally constrained, α-helical, 18-mer derived from the C4 domain of HIV gp120 that mimics the CD4 binding site (35). An HIV peptomer was previously shown to bind CD4 and compete with gp120 for binding to CD4 (34). The rationale for SIV peptomer immunization is compelling. Since the polypeptide mimics the highly conserved and functionally important CD4 binding site of gp120, escape mutants arising might be noninfectious. Further, it represents a conformational B-cell epitope and contains a T-helper-cell epitope (39). CTL epitopes overlapping this region have been mapped in SIVmac239-infected sooty mangabeys (16). We achieved significant protection in macaques primed with either three or more SIV genes or boosted with SIV peptomer. A subset of animals (39%) exhibited exceptionally strong protection, being completely aviremic or clearing or controlling viremia at the threshold of detection. This solid, sustained protection from a pathogenic SIVmac251 challenge elicited by a vaccine other than live attenuated virus provides further evidence of the potential of replication competent Ad vaccines and support for their continued development.
MATERIALS AND METHODS
Animals, immunization, and sample collection.
In order to have sufficient statistical power, 28 male and 20 female juvenile rhesus macaques (Macaca mulatta) were studied. One macaque in group II died of renal failure unrelated to the vaccine protocol during the course of immunization and was not replaced. All macaques tested negative for prior exposure to SIV, simian retrovirus type D, and simian T-cell leukemia virus. Animal maintenance and experimental procedures were conducted according to National Institutes of Health (NIH) guidelines. Mamu A*01-positive animals were identified by using previously published PCR primers and protocols (17) and evenly distributed among groups.
As outlined in Table 1, five groups of seven to eight macaques each were primed at week 0 intranasally and orally and at week 12 intratracheally with Ad5hr-SIVsmH4 env/rev alone, with the Ad5hr-SIVenv/rev recombinant plus Ad5hr-SIVmac239 gag or Ad5hr-SIVmac239 nefΔ1-13, or with all three recombinants (5 × 108 PFU/recombinant). A sixth control group was given empty E3-deleted Ad5hr vector. Macaques in all groups received 1.5 × 109 total infectious Ad, with Ad5hr vector added as necessary. Each group included one or two Mamu-A*01-positive macaques. At 12 and 24 weeks after the second priming immunization, SIVmac251 gp120 in monophosphoryl A-stable emulsion (MPL-SE) adjuvant or the SIVmac251 peptomer in phosphate-buffered saline (PBS) was given as a subunit boosting immunogen. All macaques were challenged intrarectally at week 42, 6 weeks after the final boost, with high-dose, pathogenic SIVmac251.
TABLE 1.
Immunization regimen and challengea
| No. of macaques | Group | Prime (wk 0, oral + intranasal; wk 12, intratracheal) | Boost (wk 24, intramuscular; wk 36, intramuscular) |
|---|---|---|---|
| 8 | I | Ad5hr-SIV env/rev | SIV gp120 in MPL-SE (100 μg) |
| 7 | II | Ad5hr-SIV env/rev + Ad5hr-SIV gag | SIV gp120 in MPL-SE (100 μg) |
| 8 | III | Ad5hr-SIV env/rev + Ad5hr-SIV nef | SIV gp120 in MPL-SE (100 μg) |
| 8 | IV | Ad5hr-SIV env/rev + Ad5hr-SIV gag + Ad5hr-SIV nef | SIV gp120 in MPL-SE (100 μg) |
| 8 | V | Ad5hr-SIV env/rev | SIV peptomer in PBS (100 μg) |
| 8 | VI | Ad5hr vector | MPL-SE |
The intrarectal challenge at week 42 for all immunization groups was SIVmac251 at a 1:10 dilution in PBS.
Peripheral blood mononuclear cells (PBMC) were obtained throughout the immunization course and postchallenge by using lymphocyte separation medium (ICN Pharmaceutical, Inc.) and used fresh or viably frozen in fetal bovine serum with 7% dimethyl sulfoxide. Serum was collected and stored at −20°C until use.
Challenge stock.
The SIVmac251 challenge stock was obtained from Nancy Miller, Division of AIDS, NIH, and originally prepared by Ronald Desrosiers by expanding virus from macaque 251 in rhesus PBMC. The titer of the stock was determined intrarectally at 1:2, 1:10, and 1:50 dilutions, with two macaques/dose. All macaques became infected, with mean peak acute phase viral loads of 1.24 × 109, 6.11 × 108, and 2.3 × 108 SIV RNA copies/ml of plasma, respectively. Macaques infected with the 1:50 dilution exhibited a slight delay in peak viremia. In a further titration, only one of two macaques given a 1:100 dilution intrarectally and one of two given a 1:500 dilution became infected. A 1:10 dilution was used for the challenge, containing an estimated 10 monkey infectious doses.
Viral RNA and DNA measurements.
Viral RNA in plasma was detected by using the nucleic acid sequence-based amplification technique as described previously (36). Samples were measured in a quantitative assay with a sensitivity threshold of <2,000 copies/input volume of 100 μl. Negative samples were recorded as 104 copies/ml of plasma and retested by using a qualitative assay with an increased sensitivity of <200 copies/input volume. Samples negative in the qualitative assay were recorded as 103 copies/ml of plasma. SIV proviral DNA was detected by using a nested PCR assay for the SIV gag gene (4).
ELISPOT assays.
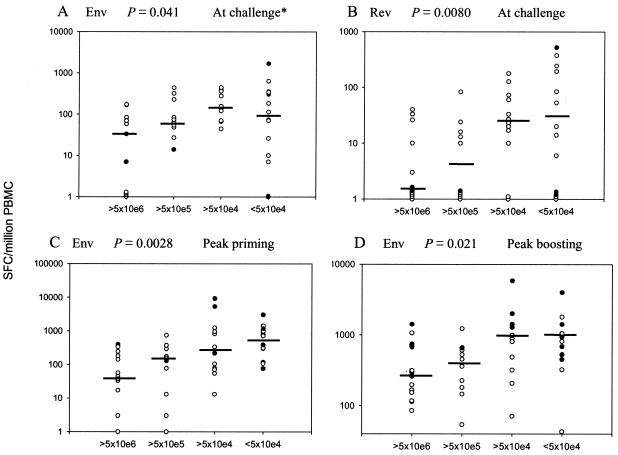
SIV-specific gamma interferon (IFN-γ) secretion by fresh PBMC was measured in response to stimulation with overlapping peptide pools representing all four SIV genes as previously described in detail (32). Briefly, twofold dilutions of PBMC beginning with 105 cells/well were plated onto 96-well plates previously coated with anti-IFN-γ monoclonal MD-1 (U-Cytech ELISPOT kit; U-Cytech, Utrecht, The Netherlands). Peptide pools were added at 1 μg/ml, incubated overnight at 37°C, and then washed and developed according to the manufacturer's protocol. Assays were carried out in triplicate and, after subtraction of background spots seen in medium-only wells, the numbers of mean spot-forming cells (SFC) per million PBMC were recorded. The results for <2% of PBMC samples that failed to respond to concanavalin A were discarded. Enzyme-linked immunospot (ELISPOT) assays for some macaques exhibited higher backgrounds (>250 spots/106 PBMC) in the medium-only wells. The results of such assays, representing 20% of the datum points in Fig. 2, are identified as dark circles and discussed below.
FIG. 2.
Trends of cellular immune responses with set point viremia. Median IFN-γ secretion (SFC/106 PBMC) for the indicated antigens at the time of challenge and peak responses during priming and boosting periods of immunization are indicated on the scatter plots, with macaques grouped by median set point viremia over weeks 8 to 24 postchallenge. In some cases not all datum points are visible due to clustering of similar values. Dark circles represent macaque PBMC that gave background counts (medium-only wells) of >250 SFC/106 PBMC. (A) The asterisk denotes that this trend analysis becomes nonsignificant if the high background points are eliminated (P = 0.19). The trend analyses in panels B, C, and D remain significant when high background values are excluded (P = 0.0092 for all three).
T-cell proliferation assay.
Viably frozen PBMC were used to measure T-cell proliferation to native SIVmac251 p27, SIVmac251 Nef (Advanced Bioscience Laboratories, Inc., Kensington, Md.), SIVmac251 gp120, or aldrithiol-2 inactivated SIVmac239 as described previously (32). The stimulation index for each assay condition was obtained by dividing the mean experimental count by the mean medium or aldrithiol-2-inactivated Supt-T1 microvesicle control count.
CD8+ antiviral activity suppression assay.
The endogenous suppression assay of SIV-specific CD8+ antiviral activity in macaque PBMC has been described (45). Briefly, CD8+ effector (E) T cells, separated by 0.2-μm-pore-size Anopore semipermeable membranes (Nunc) from target (T) allogeneic CD4+ T cells from an SIV-infected macaque, were cultured at effector/target ratios ranging from 4:1 to 0.25:1. Culture supernatants were removed periodically, and SIV replication was assessed by p27 antigen capture assay. The percent suppression was calculated relative to p27 production by control CD4+ targets cultured in the absence of CD8+ effectors.
Antibody assays.
Serum-binding antibodies to SIVmac251 gp120 were determined by enzyme-linked immunosorbent assay (4). The binding titer was defined as the reciprocal of the serum dilution at which the absorbance of the test serum was twice that of the negative control serum diluted 1:50. Neutralizing antibodies against lab-adapted SIVmac251 were evaluated in macaque sera as described previously (45). Endpoint titers of 50% are reported. Sera from macaques immunized with the SIV peptomer were screened for anti-peptomer antibodies by using an enzyme-linked immunosorbent assay as previously described (31).
Statistical analysis.
Analysis of viral burdens made use of median viremia levels during acute-phase infection (weeks 1.5 to 3) and at the set point (weeks 8 to 24). Differences between groups were analyzed by using the Wei-Johnson test (43) corrected for multiple comparisons by the method of Hochberg (14). Comparisons of Mamu-A*01 and non-Mamu-A*01 macaques made use of the stratified Wilcoxon rank sum test with immunization group as the stratification factor. The proportions of Mamu-A*01 macaques within set point viremia groups were examined by using the Cochran-Armitage trend test. Differences in immune responses between immunization groups were analyzed by using the Wilcoxon rank sum test corrected for multiple comparisons. Correlations of immune responses with acute-phase and set point viremia were determined by using the Jonckheere-Terpstra trend test across viremia groups, corrected for multiple comparisons at each time or interval. The trend analysis of ELISPOT values was conducted both with and without inclusion of datum points with a high background level.
RESULTS
Study design and challenge outcome.
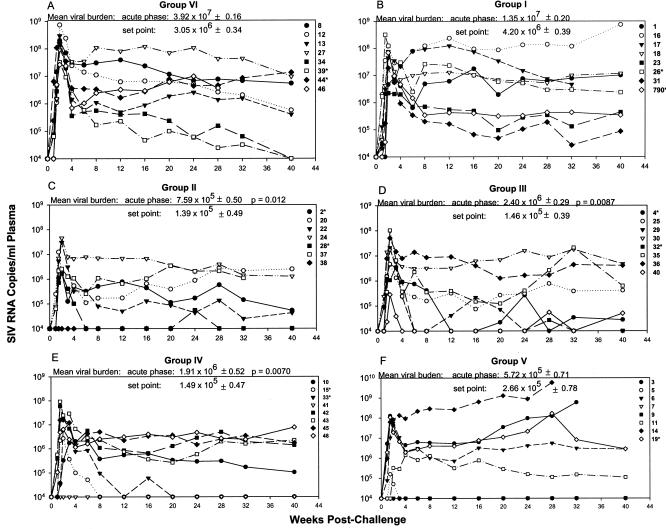
We compared the relative contributions of three different Ad5hr-SIV recombinant priming immunogens in combination with envelope boosting to protection against a pathogenic SIV mucosal challenge (groups I to IV; Table 1). A separate arm (group V) evaluated boosting with the SIV peptomer in comparison to gp120 (group I), following priming with the Ad5hr-SIVenv/rev recombinant only. After intrarectal challenge with SIVmac251, all control (group VI) macaques became infected, exhibiting high acute-phase viral burdens (the geometric mean of median value over weeks 1.5 to 3 was 3.92 × 107 SIV RNA copies/ml of plasma; Fig. 1A). Set point viremia (median value over weeks 8 to 24) was characteristically lower, with a geometric mean of 3.05 × 106 RNA copies/ml of plasma. Mamu-A*01 macaques have exhibited resistance to infection by an SIVmac251(561) challenge stock prepared by culturing PBMC of infected Mamu-A*01-positive macaque 561L (30) and to SIVmac239 infection (26). Here, with a different SIVmac251 stock, no significant difference in set point viremia was observed between the 11 Mamu-A*01 macaques and the non-Mamu-A*01 animals (P = 0.14). Therefore, no adjustments were made to the data set with regard to major histocompatibility complex type.
FIG. 1.
Viral load outcomes for all macaques by immunization group post SIVmac251 rectal challenge. An asterisk denotes a Mamu-A*01-positive macaque. For each group shown (A to F), the geometric mean of the median SIV RNA copies/ml of plasma during acute infection (weeks 1.5 to 3) and of the median SIV RNA copies/ml of plasma at the set point of infection (weeks 8 to 24) is shown ± the standard error of the mean expressed as a log value. Statistical significance in comparison to control values is indicated by P values.
Priming with Ad5hr-SIVenv/rev plus SIVgp120 boosting (group I; Fig. 1B) conferred no protection. Viral loads at acute phase and set point were not significantly different from controls. However, after a priming step with Ad5hr recombinants encoding three or more SIV genes, env/rev plus gag or nef or env/rev plus gag and nef, followed by SIV gp120 boosting, a significant reduction in viremia during the acute phase was observed for groups II (52-fold reduction; Fig. 1C), III (16-fold reduction; Fig. 1D), and IV (21-fold reduction; Fig. 1E) versus controls (P = 0.012, 0.0087, and 0.0070, respectively). Viral loads at set point varied within these groups. Some animals completely suppressed viral replication to undetectable levels (e.g., 15, 33, and 41 from group IV), whereas others showed modest control of viremia, accounting for a lack of significant differences at set point when each immunization group was separately compared to controls. However, a significant reduction in viremia of 20- to 22-fold versus controls was seen at set point for these three groups when taken together (P = 0.0097).
Group V animals (Fig. 1F) primed with Ad5hr-SIVenv/rev but boosted with SIV peptomer showed reductions in geometric mean viral burdens at acute phase (69-fold) and set point (11-fold) compared to controls, but these differences were not statistically significant due to widely diverse viremia outcomes among the macaques. Four of eight macaques became highly viremic. However, macaques 7 and 9 completely resisted infection, and macaque 5 cleared the virus to undetectable levels. The eighth macaque, number 11, showed a blunting of acute viremia but did not control viremia at set point. The peptomer boost, although giving a variable outcome, was highly effective in inducing strong protection in three of eight group V macaques.
SIV-specific IFN-γ-secreting cells correlate with control of viremia.
We have previously reported that potent, persistent cellular immune responses were elicited in the macaques in the present study after immunization according to the regimens outlined in Table 1. When IFN-γ-secreting cells were enumerated in response to SIV Env, Gag, Nef, and Rev peptide pools as a measure of cellular immunity, positive responses were detected in 90, 67, 44, and 39% of the macaques, respectively (32). The response levels were high, with mean peak responses during both the priming and boosting periods of more than 900 and 1,000 SFC/million PBMC for Env and Gag, respectively, and of ca. 300 SFC/million PBMC for both of the smaller proteins, Nef and Rev. Among the Mamu-A*01-positive macaques, strong, persistent cellular immunity was confirmed by tetramer staining (25). CD8+ T cells specific for the dominant p11C Gag epitope ranged from 0.4 to 1.6% for fresh cells and from 40 to 80% for stimulated cells. Mean peak tetramer-positive cells for the subdominant Env p15m and p54m epitopes were 0.5 and 0.6%, respectively, for fresh cells and 3.5 and 5%, respectively, for stimulated cells. Detection of responses to subdominant epitopes indicated that the cellular immunity induced was both potent and broad.
Here we investigated whether cellular immune responses correlated with postchallenge viremia by using all 47 immunized and control macaques. SIV-specific IFN-γ-secreting cells at the time of challenge did not correlate with acute-phase viremia. However, by using the Jonckheere-Terpstra trend test across viremia groups, significantly higher median cellular immune responses to Env and Rev at the time of challenge did correlate with decreased set point viral loads (P = 0.041 and 0.0080, respectively; Fig. 2A and B). The statistical analysis used median values as more representative of each group, as opposed to mean values that can be skewed by very high or low values. If the macaques that gave a higher background response (>250 SFC) (Fig. 2) are removed from the analysis, the correlation of Env-specific IFN-γ-secreting cells at the time of challenge with set point viremia is not significant (P = 0.19); however, the Rev-specific cellular response remains significant (P = 0.0092). ELISPOT data resulting from assays with high backgrounds may point to greater assay variability. Alternatively, high backgrounds may reflect the presence of cells already activated in vivo. Our vaccine regimen uses replicating Ad recombinants that may elicit more persistent acquired immunity and stimulate innate immunity, resulting in a higher level of background cellular activation in comparison to nonreplicating vectors. High backgrounds were observed repeatedly for certain macaques (see, e.g., Table 2 below), further implying that rather than assay variability, high background values may accurately reflect the in vivo situation. Therefore, the trend analyses shown were conducted by using data sets with and without high background values, and both resulting P values are reported. Note that in this trend analysis, P value calculations are based on the overall trend across all four viral load groups and not between any two individual groups. Given the small group sizes, slight deviations from the expected median value are not unexpected and are reflected in the associated P value. This can be seen, for example, in the lower P value for the trend in Env responses at the time of challenge depicted in Fig. 2A compared to that for the Rev responses in Fig. 2B.
TABLE 2.
Immune response profile in subset of highly protected macaquesa
| Viremia level and macaque no. (group)b | Peak responses prechallenge (IFN-γ secretion [SFC/106 PBMC])
|
Responses at challenge
|
Rectal antibodyc
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (IFN-γ secretion [SFC/106 PBMC])
|
Binding antibody titer
|
CD8AA (% suppression) | Proliferation (SI)
|
|||||||||||||
| Env | Rev | Gag | Nef | Env | Rev | Gag | Nef | α-SIVgp120 | α-peptomer | gp120 | ALD-SIV | p27 | IgG | IgA | ||
| Undetectable viremia | ||||||||||||||||
| 7 (V) | 324 | 174 | 73 | 53 | 10 | 150 | 47 | 0 | 0 | 7 | − | |||||
| 9 (V) | 351 | 374 | 351 | 374 | 100 | 16 | 89 | 0 | 0 | 9 | − | |||||
| 38 (II) | 146 | 36 | 720 | 10 | 14 | 166 | 125,000 | 0 | 24 | 6.2 | ||||||
| 41 (IV) | 712 | 336 | 847 | 60 | 7 | 0 | 34 | 0 | 270,000 | 95 | 0 | 4.7 | 0 | − | 4.4 | |
| Clearance or strong control of viremia | ||||||||||||||||
| 5 (V) | HB | HB | HB | HB | 180 | 1,350 | 22 | 0 | 0 | − | − | |||||
| 15* (IV) | 814 | 87 | HB | 154 | 26 | 6 | HB | 0 | 85,000 | 1 | 3.1 | 4.5 | 2.1 | − | 2 | |
| 28* (II) | 1,198 | 190 | 440 | 70 | 20 | 143 | 82,000 | 0 | 0 | 2.6 | 4.6 | 118 | 17.6 | |||
| 32* (III) | 946 | 967 | 607 | 183 | 83 | 33 | 25,600 | 0 | 0 | 0 | − | − | ||||
| Control at threshold of viremia | ||||||||||||||||
| 4* (III) | 774 | 407 | 170 | 113 | 193 | 170 | 92,000 | 28 | 7.1 | 0 | − | − | ||||
| 29 (III) | 1,800 | 296 | 450 | HB | HB | HB | 145,000 | 92 | 0 | 0 | 25 | − | ||||
| 33* (IV) | 70 | HB | 194 | HB | HB | HB | HB | HB | 25,600 | 19 | 0 | 0 | 2.4 | 7 | − | |
| 40 (III) | 1,054 | 280 | 310 | 632 | 243 | 310 | 63,000 | 68 | 3.3 | 0 | − | 2 | ||||
ELISPOT responses with medium backgrounds of >250 SFC are marked “HB” (high background). The resulting mean background levels for data shown are 121 SFC for Env, Rev, and Nef and 138 SFC for Gag.
An asterisk denotes MamuA*01. CD8AA, CD8+-T-cell antiviral activity; SI, stimulation index; ALD-SIV, aldrithiol-treated SIV.
Rectal anti-SIV gp120 IgG and IgA antibody levels are reported as the fold increase in the OD of vaccinated macaques relative to the controls at week 38 postimmunization. −, an increase of <2 compared to controls.
The Gag cellular immune response in group II and IV macaques and the Nef response in group III animals were elevated compared to control levels at the time of challenge (P = 0.016 and 0.020, respectively), a finding indicative of persistent acquired immunity. Nevertheless, Gag and Nef responses at the time of challenge were not significantly correlated with set point viremia (data not shown). Thirty-nine macaques were immunized with the Ad5hr-SIVenv/rev recombinant, whereas 15 and 16 received the Ad5hr-SIVgag and Ad5hr-SIVnef recombinants, respectively, with a corresponding reduction in statistical power. The unequal numbers of macaques primed with different recombinants precluded analysis for correlation of total ELISPOT responses with protective efficacy.
Some SIV-specific cells might have been memory cells at the time of challenge and not detected by the ELISPOT assay. Therefore, we investigated whether peak cellular immune responses observed prechallenge were correlated with protective outcome. Cellular immunity to Env was correlated with set point viremia for both the priming and boosting periods of immunization (P = 0.0028 and 0.021, respectively; Fig. 2C and D), even after elimination of datum points with higher backgrounds (P = 0.0092 for both periods). Rev responses were not, although responses during the boosting period were marginally nonsignificant (P = 0.068; data not shown). Similarly, examination of Gag and Nef peak cellular immune responses during the boosting period showed marginally nonsignificant trends of higher values with lower set point viremia (P = 0.068 for both; data not shown). The latter analyses also had lower statistical power.
Surprisingly, IFN-γ secretion measured 1, 2, 4, and 8 weeks postchallenge did not correlate with viremia outcome (data not shown). Group VI control monkeys quickly developed IFN-γ-secreting cells to all antigens after the first week postchallenge, obscuring differences between the control and vaccinated macaques. The latter monkeys did exhibit anamnestic responses but not necessarily to each antigen. Further, since the vaccinated macaques were not all immunized with each Ad-SIV recombinant, analysis of total postchallenge ELISPOT responses was not feasible. The rapid appearance of IFN-γ-secreting cells in the control macaques was unexpected; however, the challenge stock was highly virulent and induced high acute-phase viremia (Fig. 1A), which could have contributed to the rapid appearance of a cellular immune response. Notably, whereas two of the control macaques exhibited viremia control by 40 weeks postchallenge (Fig. 1A), this could not be attributed to strong postchallenge cellular immunity as previously reported by others (22, 40). In fact, ELISPOT responses in these two macaques were not higher than responses seen in the other control animals (data not shown).
Correlation of antibody responses with challenge outcome.
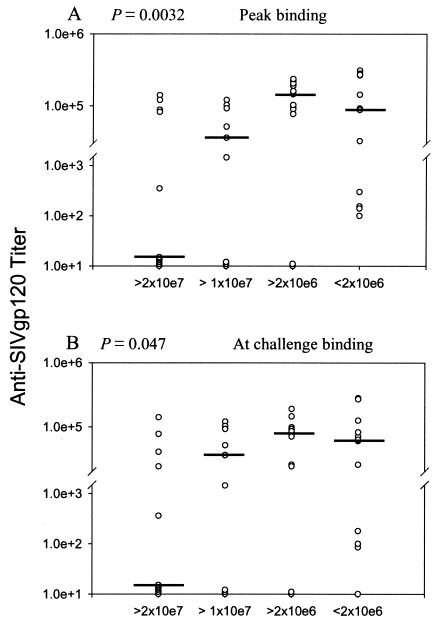
All group I to IV macaques developed serum antibodies able to bind gp120 and neutralize T-cell-line-adapted, but not primary SIVmac251, with mean titers at the time of challenge of 84,052 and 6,338, respectively. In contrast, group V macaques boosted with the SIV peptomer exhibited only low-level binding antibodies at challenge (mean titer 276), and no neutralizing antibodies. In spite of these low antibody levels, the titers of group V macaques were included in a trend analysis across animals grouped according to their acute-phase viremia. Analysis of all 47 macaques showed binding antibodies were significantly correlated with acute-phase viremia (Fig. 3), both peak titers prechallenge (P = 0.0032) and titers at the time of challenge (P = 0.047). Note that Fig. 3 illustrates log antibody titers on a split y axis, which overemphasizes low values. Further, deviations of the median values of the most highly protected macaques in Fig. 3 from expected higher values can be attributed in part to inclusion of the group V macaques, four of which exhibited acute viral burdens in the lowest range and yet lacked high titer binding antibody to envelope. Neutralizing antibodies exhibited a similar trend (data not shown), but peak titers and titers at the time of challenge were marginally nonsignificant (P = 0.067 for both). No significant correlations between either binding or neutralizing antibodies and set point viremia were observed.
FIG. 3.
Trends of peak anti-gp120 binding antibody with acute-phase viremia. Median peak binding antibody titers prechallenge and titers at the time of challenge are shown on the scatter plots with macaques grouped by median acute phase viremia over weeks 1.5 to 3 postchallenge.
Analysis of a subset of highly protected macaques.
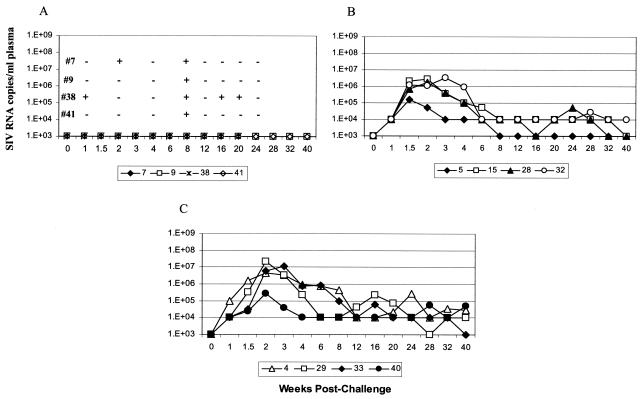
The significant reductions in acute-phase and set point viremia in group II, III, and IV macaques were encouraging advances for our replicating Ad recombinant vaccine approach. The most striking result, however, was the extent of viremia control seen in a subset of 12 (39%) of the 31 immunized, protected macaques in groups II through V. The strong reduction in viral burden of these 12 macaques was validated by reassessing SIV RNA levels in plasma by using a qualitative assay with a 10-fold-lower sensitivity level. Four macaques again had undetectable viremia over the 40-week postchallenge observation period (Fig. 4A). These four were exposed to virus, however, and did not have “sterilizing immunity,” since they were positive for proviral SIV gag DNA at one or more time points (Fig. 4A). Four additional macaques either cleared viremia (macaque 5) or strongly controlled viremia at or below the 104 threshold (macaques 15, 28, and 32; Fig. 4B). A final four macaques showed a blunting of viremia during the acute phase and continued to exhibit strong viremia control thereafter, repeatedly bringing viral loads back to the 104 threshold (Fig. 4C).
FIG. 4.
Viral loads among highly protected macaques with undetectable viremia (A), clearance or strong control of viremia (B), or control of viremia at the threshold of detection (C). For the macaques in panel A, SIVgag proviral DNA was detected at the time points indicated. In panel A it should also be noted that macaque 38 died at week 21 postchallenge of surgical complications after intestinal endoscopy for the collection of samples.
Additional measures of immunity.
Immune parameters contributing to the exceptional control of viremia in this subset are of interest. Both humoral and cellular immune responses, summarized in Table 2, were correlated with their protection. In general, high levels of IFN-γ-secreting cells were not observed at the time of challenge, reflecting a diminution in the primary immune response. Peak prechallenge cellular immune responses were more indicative of the level of cellular immunity elicited (Table 2). Some macaques consistently gave higher background counts, and these data are not shown. Overall, the majority of these highly protected macaques developed strong responses to Env and Rev peptides, and most of those primed with Ad5hr-SIVgag and -SIVnef recombinants responded strongly to corresponding peptide pools. High-titer binding antibodies were induced in each macaque except those boosted with peptomer (Table 2). Among the latter three macaques, peptomer binding antibodies were seen in sera of two: macaques 7 and 5. The protective mechanism afforded by this anti-peptomer response is under investigation.
Additional immune responses seen in this subset might have contributed to their exceptional control of viremia (Table 2). High levels of CD8+-T-cell antiviral activity (CD8AA) able to suppress endogenous SIV infection of rhesus CD4+ T cells were exhibited by macaques 9 and 41 with no detectable viremia and macaque 29 that controlled viremia at the threshold of detection. CD8AA has previously been associated with vaccine-induced protection (18, 20, 21, 42). Only modest proliferative responses to envelope or Gag antigens were exhibited, although measurements on cryopreserved cells might have underestimated the responses. Several macaques exhibited anti-gp120 immunoglobulin G and/or immunoglobulin A antibodies in rectal secretions obtained 4 weeks prior to challenge so as not to damage the mucosal surface. A protective mechanism associated with these responses is unknown. Overall, high-level, broadly generated immune responses may be key to exceptional control of viremia. However, other than cellular immunity, the mechanisms by which antibody and ancillary immune responses contribute to protection require further study.
Unidentified host factors may have contributed to the exceptional protection of these 12 macaques. Importantly, the four macaques exhibiting undetectable viremia (Fig. 4A) are not Mamu-A*01 positive. Although other groups have reported a correlation of the Mamu-A*01-positive allele with control of viremia (26, 30), it was not observed here. Although there was a tendency for a higher proportion of Mamu-A*01 macaques among those with lower set point viremia, this trend was not significant (P = 0.10).
DISCUSSION
Our results show clearly that priming with Ad5hr-SIVenv/rev and boosting with gp120 was not sufficient to protect against SIVmac251 rectal challenge but that additional priming with either Ad5hr-SIVgag and/or Ad5hr-SIV nefΔ1-13 resulted in significant and similar extents of viremia control. Protective effects seen in group II, III, and IV macaques were not restricted to viremia reduction, but extended to survival times. To date, 53 weeks postchallenge, 1 macaque has died of AIDS in each of Groups II, III, and IV, while 4 group I, 4 group V, and 3 controls have succumbed. Moreover, a similar percentage of macaques from each of these three groups comprised the subset of highly protected animals: 2 of 7 (29%) for group II, 4 of 8 (50%) for group III, and 3 of 8 (38%) for group IV.
The similarity in challenge outcome for groups II, III, and IV was unexpected. A similar prime-boost protocol with the env/rev and gag recombinants, followed by rectal SIVmac251 challenge, gave only modest protection (45). We anticipated that priming with the additional recombinant would improve protective outcome. Surprisingly, group II macaques primed with the env/rev and gag recombinants had significantly greater viremia reductions than similarly primed macaques in the earlier experiment (J. Pinczewski et al., unpublished data), perhaps due to a different immunization route and adjuvant. Further, priming with the nef recombinant was as effective as the gag recombinant in eliciting protection. Although Gag has been considered exceedingly potent in eliciting cellular immune responses, cellular immunity to Nef, expressed early in the viral replication cycle, may provide an extra measure of protection. Alternatively, gag- and nef-recombinant immunizations may have increased protective efficacy by enhancing cellular responses to Env. We have reported that Env responses were significantly elevated in group II compared to group I macaques (P = 0.0056) and higher, but not significantly so, in group III animals (32). Here, cellular immunity to Env was significantly correlated with reduced set point viremia throughout the immunization regimen and at challenge (Fig. 2A, C, and D).
Unexpectedly, priming with all three recombinants encoding four SIV gene products did not elicit greater protection, suggesting that extensive multigenic approaches may not be necessary for vaccine efficacy. If true, vaccine designs could perhaps accommodate genes of multiple clades to address viral heterogeneity rather than multiple genes within a clade. Downmodulation of cellular immune responses was observed during priming of group IV macaques with all three Ad5hr recombinants, but this effect did not extend into the boosting period of the immunization regimen (32). Here, we did not see lesser protection of group IV macaques in comparison to group II or III macaques as a result of the downmodulation. However, the possibility that greater protection was not seen in group IV macaques because of this effect should be further explored.
The SIV peptomer boost after Ad5hr-SIVenv/rev priming was previously shown to be immunogenic in a pilot study but did not confer protection against SIVmac251 rectal challenge (31). Because peptomer administration in Ribi's adjuvant may have altered its α-helical structure (35), we reassessed peptomer boosting in group V macaques by using the peptomer in PBS. A dichotomous outcome was observed after challenge of group V macaques. Four exhibited high viral burdens and by week 53 postchallenge had died of AIDS. In contrast, three of the remaining four have exhibited remarkably strong protection, two being completely aviremic throughout the postchallenge period and one clearing viremia to undetectable levels (Fig. 4A and B). The protection of these macaques was associated with cellular immune responses to Env and Rev, but other immune responses may have also contributed (Table 2). Since no protection was observed in group I macaques boosted with gp120, peptomer immunization had a clear effect. Further characterization of immune responses elicited after its administration is warranted. Comparison of responses between the protected and unprotected macaques within group V should be informative.
The significant correlations of humoral and cellular immune responses with different phases of the postchallenge infectious cycle were not surprising. Antibodies at the time of challenge would be expected to decrease the initial level of infecting virus, and binding antibody responses were correlated with reductions in acute-phase viremia. Cellular immunity would be expected to play a role later in the infectious cycle, and cellular immune responses were correlated with reductions in set point viremia. These results lend support to continued use of a vector prime-protein boost vaccine strategy.
On the other hand, the fact that the antibodies elicited did not neutralize a SIVmac251 primary isolate calls into question their protective mechanism. Possibilities under investigation include antibody-dependent cellular cytotoxicity and physical trapping of virus at the mucosal surface. With regard to cellular immunity, the strong correlation with Env and Rev cellular immune responses is of special interest. The genes in the Ad5hr-SIVenv/rev recombinant were from the SIVsmH4 isolate (5) and thus heterologous to the challenge virus, SIVmac251. The implication is that strong immunity to conserved epitopes was elicited by the vaccine regimen. Epitope-mapping studies could confirm this and identify important protective epitopes.
An immune response that is narrow in scope or limited in functionality will probably not translate into control of infection. Here we achieved significant protection correlated with both humoral and cellular immune responses. Protein boosting clearly impacted initial viral exposure. Further, induction of broad, potent, and importantly persistent cellular immunity elicited by priming with replicating Ad recombinants was critical in controlling the virulent SIVmac251 infection after initial reduction of the infectious dose. Given the call for a solid test of a vaccine with an SIV strain that more closely resembles human infection with HIV, these promising results point us in the right direction for more detailed characterization of the correlates of immune protection and continued development of an AIDS vaccine.
Acknowledgments
We thank Marsha Sowers, Steve Harbaugh, and Marisa St. Claire at Bioqual, Inc., for their expert advice and performance of all animal procedures; Ranajit Pal for conducting the in vivo titration of the challenge stock; Roxanne Shurtliff and Sonia Grebogi for technical support; and Sharon Orndorff for sample coordination. The challenge stock was generously provided by Ronald Desrosiers (New England National Primate Center) and made available by Nancy Miller (DAIDS, NIAID, NIH) under contract N01-AI15430 with Advanced BioScience Laboratories, Inc. The aldrithiol-2-inactivated SIV and control microvesicles were provided by the AIDS Vaccine Program, SAIC Frederick; the MPL-SE adjuvant was provided by Wyeth Lederle-Vaccines, and overlapping SIV Gag peptides were provided by the NIAID AIDS Research and Reference Reagent Program.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buge, S. L., L. Murty, K. Arora, V. S. Kalyanaraman, P. D. Markham, E. S. Richardson, K. Aldrich, L. J. Patterson, C. J. Miller, S. Cheng, and M. Robert-Guroff. 1999. Factors associated with slow disease progression in macaques immunized with an adenovirus-simian immunodeficiency virus (SIV) envelope priming-gp120 boosting regimen and challenged vaginally with SIVmac251. J. Virol. 73:7430-7440. (Erratum, 73:9692). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buge, S. L., E. Richardson, S. Alipanah, P. Markham, S. Cheng, N. Kalyan, C. J. Miller, M. Lubeck, S. Udem, J. Eldridge, and M. Robert-Guroff. 1997. An adenovirus-SIV env vaccine elicits humoral, cellular and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 71:8531-8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, S. M., S. G. Lee, M. Ronchetti-Blume, K. P. Virk, S. Mizutani, J. W. Eichberg, A. Davis, P. P. Hung, V. M. Hirsch, and R. M. Chanock. 1992. Coexpression of the simian immunodeficiency virus Env and Rev proteins by a recombinant human adenovirus host range mutant. J. Virol. 66:6721-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel, M. D, F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 8.Davis, N. L., I. J. Caley, K. W. Brown, M. R. Betts, D. M. Irlbeck, K. M. McGrath, M. J. Connell, D. C. Montefiori, J. A. Frelinger, R. Swanstrom, P. R. Johnson, and R. E. Johnston. 2000. Vaccination of macaques against pathogenic simian immunodeficiency virus with venezuelan equine encephalitis virus replicon particles. J. Virol. 74:371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, D. T., L.-M. Chen, J. Gillis, K.-C. Lin, B. Harty, G. P. Mazzara, R. O. Donis, K. G. Mansfield, J. D. Lifson, R. C. Desrosiers, J. E. Galan, and R. P. Johnson. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 77:2400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 12.Fuller, D. H., P. A. Rajakumar, L. A. Wilson, A. M. Trichel, J. T. Fuller, T. Shipley, M. S. Wu, K. Weis, C. R. Rinaldo, J. R. Haynes, and M. Murphey-Corb. 2002. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J. Virol. 76:3309-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hel, Z., J. Nacsa, E. Tryniszewska, W.-P. Tsai, R. Washington-Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and post-challenge CD4+ and CD8+ T cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg, Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800-802. [Google Scholar]
- 15.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur, A., J. Yang, D. Hempel, L. Gritz, G. P. Mazzara, H. McClure, and R. P. Johnson. 2000. Identification of multiple simian immunodeficiency virus (SIV)-specific CTL epitopes in sooty mangabeys with natural and experimentally acquired SIV infection. J. Immunol. 164:934-943. [DOI] [PubMed] [Google Scholar]
- 17.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in rhesus macaques detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed]
- 18.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Ward. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2:767-775. [DOI] [PubMed] [Google Scholar]
- 19.Lena, P., F. Villinger, L. Giavedoni, C. J. Miller, G. Rhodes, and P. Luciw. 2002. Coimmunization of rhesus macaques with plasmid vectors expressing IFN-γ, GM-CSF, and SIV antigens enhances antiviral humoral immunity but does not affect viremia after challenge with highly pathogenic virus. Vaccine 20:A69-A79. [DOI] [PubMed] [Google Scholar]
- 20.Leno, M., L. Carter, D. J. Venzon, J. Romano, P. D. Markham, K. Limbach, J. Tartaglia, E. Paoletti, J. Benson, G. Franchini, and M. Robert-Guroff. 1999. CD8+ lymphocyte antiviral activity in monkeys immunized with SIV recombinant poxvirus vaccines: potential role in vaccine efficacy. AIDS Res. Hum. Retrovir. 15:461-470. [DOI] [PubMed] [Google Scholar]
- 21.Leno, M., M. Kowalski, and M. Robert-Guroff. 2000. CD8 T-cell anti-HIV activity as a complementary protective mechanism in vaccinated chimpanzees. AIDS 14:893-894. [DOI] [PubMed] [Google Scholar]
- 22.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubeck, M. D., R. J. Natuk, M. Chengalvala, P. K. Chanda, K. K. Murthy, S. Murthy, S. Mizutani, S.-G. Lee, M. S. Wade, B. M. Bhat, R. Bhat, S. K. Dheer, J. W. Eichberg, A. R. Davis, and P. P. Hung. 1994. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res. Hum. Retrovir. 10:1443-1449. [DOI] [PubMed] [Google Scholar]
- 24.Lubeck, M. D., R. Natuk, M. Myagkykh, N. Kalyan, K. Aldrich, F. sinangil, S. Alipahan, S. C. S. Murthy, P. K. Chanda, S. M. Nigida, Jr., P. D. Markham, S. Zolla-Pazner, K. Steimr, M. Wade, M. S. Reitz, Jr., L. O. Arthur, S. Mizutani, A. Davis, PlP. Hung, R. C. Gallo, J. Eichberg, and M. Robert-Guroff. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651-658. [DOI] [PubMed] [Google Scholar]
- 25.Malkevitch, N., L. J. Patterson, K. Aldrich, E. Richardson, W. G. Alvord, and M. Robert-Guroff. 2003. A replication-competent Ad5hr-SIV recombinant priming/subunit boosting vaccine regimen induces broad, persistent SIV-specific cellular immunity in Mamu-A*01 rhesus macaques. J. Immunol. 170:4281-4289. [DOI] [PubMed] [Google Scholar]
- 26.Mothe, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthumani, K., M. Bagarazzi, D. Conway, D. S. Hwang, K. Manson, R. Ciccarelli, Z. Israel, D. C. Montefiori, K. Ugen, N. Miller, J. Kim, and D. B. Weiner. 2003. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduces viral loads after pathogenic intrarectal SIVmac251 challenge in rhesus macaques. Vaccine 21:629-637. [DOI] [PubMed] [Google Scholar]
- 29.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nasca, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson, L. J., F. Robey, A. Muck, K. VanRemoortere, K. Aldrich, E. Richardson, W. G. Alvord, P. D. Markham, M. Cranage, and M. Robert-Guroff. 2001. A conformational C4 peptide polymer vaccine coupled with live recombinant vector priming is immunogenic but does not protect against rectal SIV challenge. AIDS Res. Hum. Retrovir. 17:837-849. [DOI] [PubMed] [Google Scholar]
- 32.Patterson, L. J., N. Malkevitch, J. Pinczewski, D. Venzon, Y. Lou, B. Peng, C. Munch, M. Leonard, E. Richardson, K. Aldrich, V. S. Kalyanaraman, G. N. Pavlakis, and M. Robert-Guroff. 2003. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J. Virol. 77:8607-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robert-Guroff, M., H. Kaur, L. J. Patterson, M. Leno, A. J. Conley, P. M. McKenna, P. D. Markham, E. Richardson, K. Aldrich, K. Arora, L. Murty, L. Carter, S. Zolla-Pazner, and F. Sinangil. 1998. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J. Virol. 72:10275-10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robey, F. A., T. Harris-Kelson, M. Robert-Guroff, D. Batinic, B. Ivanov, M. Lewis, and P. P. Roller. 1996. A synthetic conformational epitope from the C4 domain of HIV gp120 that binds CD4. J. Biol. Chem. 271:17990-17995. [DOI] [PubMed] [Google Scholar]
- 35.Robey, F. A., T. Kelson-Harris, P. P. Roller, and M. Robert-Guroff. 1995. A helical epitope in the C4 domain of HIV glycoprotein 120. J. Biol. Chem. 270:23918-23921. [DOI] [PubMed] [Google Scholar]
- 36.Romano, J. W., B. van Gemen, and T. Kievits. 1996. A novel, isothermal detection technology for qualitative and quantitative HIV-1 RNA measurements. Clin. Lab. Med. 16:89-103. [PubMed] [Google Scholar]
- 37.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. C. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 38.Ruprecht, R. M. 1999. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol. Rev. 170:135-149. [DOI] [PubMed] [Google Scholar]
- 39.Sarkar, S., V. Kalia, M. Murphey-Corb, and R. C. Montelaro. 2002. Characterization of CD4+ T helper cell fine specificity to the envelope glycoprotiens of simian immunodeficiency virus. J. Med. Primatol. 31:194-204. [DOI] [PubMed] [Google Scholar]
- 40.Shacklett, B. L., B. Ling, R. S. Veazey, A. Luckay, W. J. Moretto, D. T. Wilkens, J. Hu, Z. R. Israel, D. F. Nixon, and P. A. Marx. 2002. Boosting of SIV-specific T-cell responses in rhesus macaques that resist repeated intravaginal challenge with SIVmac251. AIDS Res. Hum. Retrovir. 18:1081-1088. [DOI] [PubMed] [Google Scholar]
- 41.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M.-E. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. E. Emini. 2002. Replication-imcompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 42.Wang, Y., L. Tao, E. Mitchell, W. M. J. M. Bogers, C. Doyle, C. A. Bravery, L. A. Bergmeier, C. G. Kelly, J. L. Heeney, and T. Lehner. 1998. Generation of CD8 suppressor factor and β chemokines, induced by xenogenic immunization, in the prevention of simian immunodeficiency virus infection in macaques. Proc. Natl. Acad. Sci. USA 95:5223-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei, L. J., and W. E. Johnson. 1985. Combining dependent tests with incomplete repeated measurements. Biometrika 72:359-364. [Google Scholar]
- 44.Zhao, J., Y. Lou, J. Pinczewski, N. Malkevitch, K. Aldrich, V. S. Kalyanaraman, B. Peng, L. J. Patterson, J. Mattapallil, M. Roederer, Y. Edghill-Smith, R. Woodward, G. N. Pavlakis, and M. Robert-Guroff. 2003. Boosting of SIV-specific cellular immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and -SIVgag recombinants. Vaccine 21:4022-4035. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, J., J. Pinczweski, V. R. Gomez-Roman, D. Venzon, V. S. Kalyanaraman, P. D. Markham, K. Aldrich, M. Moake, D. C. Montefiori, Y. Lou, G. N. Pavlakis, and M. Robert-Guroff. 2003. Improved protection of rhesus macaques against intrarectal SIVmac251 challenge by a replication competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen. J. Virol. 77:8354-8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zolla-Pazner, S., M. Lubeck, S. Xu, S. Burda, R. J. Natuk, F. Sinangil, K. Steimer, R. C. Gallo, J. W. Eichberg, T. Matthews, and M. Robert-Guroff. 1998. Induction of neutralizing antibodies in chimpanzees to T-cell line-adapted and primary human immunodeficiency virus type 1 isolates with a prime/boost vaccine regimen in chimpanzees. J. Virol. 72:1052-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]