Abstract
Background
To evaluate the feasibility of an implantable subconjunctival glucose monitoring system (SGMS) for long-term glucose monitoring, we investigated the in vivo performance of the system.
Method
The SGMS consists of an implantable ocular mini implant (OMI) and a handheld fluorescence photometer. A clinical study was performed on 47 diabetes patients split into two cohorts. Two different types of OMI were used, with and without a biocompatible surface coating. Duration of the study was 1 year. Correlation between capillary blood glucose and SGMS-derived interstitial fluid glucose was investigated during the first 6 months of the study.
Results
Both OMI types were tolerated well in the eyes of the patients. At the beginning of the study, the SGMS of both cohorts revealed a high accuracy with mean absolute relative difference (MARD) values of 7–12%. The performance of the uncoated OMIs deteriorated within 3 months of wearing time, exhibiting a MARD value of 20%. The performance of the surface-coated OMIs was preserved longer. Glucose correlation measurement with reasonable results (MARD of 14%) could be performed for up to 6 months of wear.
Conclusions
The biocompatible surface coating on the OMIs enabled a longer duration of action of up to 6 months compared with 3 months for uncoated implants in a clinical trial.
Keywords: concanavalin A, diabetes, fluorescence, glucose monitoring, glucose sensor, long-term sensor
Introduction
Self-monitoring of blood glucose (BG) is an essential component of diabetes therapy1,2 that is usually done with in vitro test strips utilizing capillary blood taken by finger pricking. The known disadvantages of this method are the unpleasant and painful finger-pricking procedure, the high costs of the disposable measurement strips, and the limited number of measured values. Therefore, a long-term implantable glucose sensor that allows frequent, accurate measurements is a desirable alternative to the currently available in vitro BG self-monitoring test strips. This is especially true if the yearly total costs of the new system were comparable to the average yearly finger-pricking therapy costs based on 4–6 measurements per day but allowed more frequent measurements at no additional cost per measurement.
Several attempts to develop long-term sensors have been made, utilizing the concepts of enzyme-based glucose sensors, noninvasive spectroscopic techniques, boronic acid sensors,3–4 or sensors with the competitive binding assay between the plant lectin concanavalin A (Con A) from Canavalia ensiformis5–7 and dextran, which was first introduced in the 1980s.8–11 Since then, the Con A competitive assay has been used in viscosimetric affinity assays,12–13 glucose sensitive hydrogels,14–15 and in various fluorescence-based assays.16–25
Our group has developed a long-term BG monitoring system based on the fluorescence resonance energy transfer competitive binding assay of Con A and dextran. The system consists of an ocular mini implant (OMI) placed under the bulbar conjunctiva of the patient’s eye and a handheld fluorescence photometer that reads out the sensor signal from the implant and translates it to a BG reading. The bulbar conjunctiva of the eye provides several advantages for noninvasive optical measurements, such as its optical transparency, high blood perfusion, and good wound healing. The layer structure of the conjunctiva allows a reproducible placement of the OMI. First clinical evaluations of the subconjunctival glucose measurement system have already proven a high correlation between the sensor data and corresponding BG values,26 but the performance of the system decreased with longer implantation times.27 This is most likely caused by fibrotic encapsulation of the OMI due to the foreign body response (FBR) initiated after implantation. Foreign body response is a common cause of failure of implanted medical devices, especially implanted glucose sensors. Several attempts to overcome FBR are discussed in the literature, e.g., specially designed surface architectures and biocompatible or drug-eluting surface coatings.28–35
This article provides in vivo data from a clinical trial of the EyeSense subconjunctival glucose monitoring system (SGMS) with a biocompatible surface coating, designed to minimize protein–surface interaction, to prolong the duration of action of the system.
Methods
Production of Ocular Mini Implants
The production of OMIs is described in detail elsewhere.26 Briefly, fluorescently labeled Con A (Zedira GmbH, Darmstadt, Germany) and dextran (Life Technologies GmbH, Darmstadt, Germany) were loaded to previously formed alginate microbeads by incubation of the beads in a solution of 4 mg/ml Con A and 7 mg/ml dextran. The exact nature of the fluorescent dyes used cannot be disclosed because of a patent in preparation.
Alginate beads containing the sensor chemistry were mixed with Nelfilcon™ A polymer (kindly provided by CIBA Vision GmbH, Grosswallstadt, Germany), an ultraviolet-polymerizable, derivatized poly(vinyl alcohol). The highly viscous solution was molded into poly(methyl methacrylate) molds and cured under ultraviolet light for 1 s.
Implants for cohort 2 were afterward coated with a proprietary hydrophilic biocompatible surface coating (BioInteractions Ltd., Reading, UK).
Implants were sterilized by beta radiation at a contractor (BGS Beta-Gamma-Service GmbH, Bruchsal, Germany).
Fluorescence Detection by the Handheld Photometer
To determine the glucose concentration, the OMI is excited by an orange light-emitting diode of a computer-driven handheld fluorescence photometer specially developed by Qiagen Lake Constance (Qiagen Lake Constance GmbH, Stockach, Germany). The setup of the photometer is described in detail elsewhere.26 The fluorescence photometer measures the intensity of fluorescent light emitted back by the OMI at two wavelengths, one of which correlates with glucose concentration while the other acts as a reference and independent of glucose concentration. The photometer contains a target light for the patient to look at, and its front shape is ergonomically designed to mechanically guide the patient in correctly placing the photometer in front of the face and the eye.
Figure 1 depicts a schematic view of the photometer in front of a face.
Figure 1.
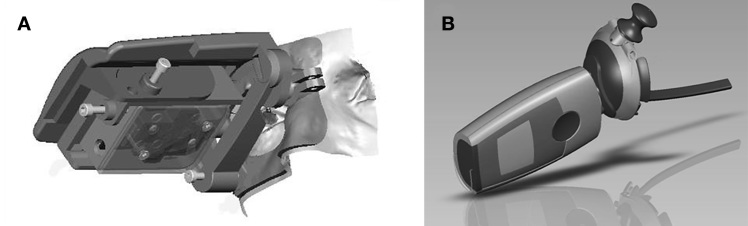
Schematic view of the photometer. (A) Prototype photometer and ergonomically designed front shape as used in the clinical study. (B) Model of the final photometer to be marketed. The face support is formed like half a pair of eyeglasses.
In Vitro Sensor Test System
For in vitro measurements of the fluorescence response to glucose, the OMI was stepwise submerged in solutions of 0 to 500 mg/dl glucose, as previously described.26 Fluorescence readings were taken after equilibration. For determination of the in vitro stability of the OMI response, OMIs were stored at 37 °C in artificial interstitial fluid buffer,26 and the response to glucose was measured at 0, 1, 14, 30, 60, 120, 180, 270, and 360 days of storage. The response between 50 and 250 mg/dl glucose concentration was calculated using linear least squares regression analysis and was compared over storage time.
Ocular Mini Implant Implantation in Human Eyes
As previously described,26 the OMI was inserted under sterile conditions and after topical anesthesia under the temporal conjunctiva of the right eye of the patients. The edges of the incision were sutured. A topical antibiotic (Floxal Augentropfen, Dr. Mann Pharma, Berlin, Germany) was instilled and administration was continued as required by the ophthalmologist. Additionally, corticosteroid-containing eye drops (Inflanefran forte, Allergan, Ettlingen, Germany) were administered in the first week after implantation.
Measurement and Analysis of In Vivo Subconjunctival Glucose Monitoring System Response
After implantation of the OMI, the handheld fluorescence photometer was adjusted to the patient by individually aligning the position of the target light relative to the measurement light and afterward setting the individual reference intensity threshold. The correct threshold setting guarantees that the excitation beam illuminates the OMI at the center and at the distance of the focal length of the collecting lens during the measurement.
The patients self-triggered the OMI measurements by positioning the photometer’s front shape to their face and looking into the direction of the target light. Valid measurements were recorded after reaching the individually set reference threshold, ensuring a reproducible positioning. Typically, 20 readings were collected and averaged. The software calculated the ratio of donor to reference fluorescence. The system was blinded to the patients, as the measurement software did not directly calculate any BG value from the fluorescence readings.
The correlation between capillary glucose measured by a standard laboratory system (Hitado Super GL easy, Hitado GmbH, Moehnesee, Germany) and interstitial fluid glucose measured by the OMI was investigated by inducing an increase and decrease of BG values by oral intake of a standardized carbohydrate-rich meal or subcutaneous insulin injection (patient-specific insulin) in a range of 50–400 mg/dl. The decision on initial carbohydrate uptake or insulin administration was individually taken by the clinical investigator and depended on the level of the patient’s BG value at the beginning of the measurement visit.
Insulin-dependent diabetes patients requiring daily finger pricking to monitor BG were included in the study. The main exclusion criteria were pregnant or lactating females, known hypersensitivity to any of the products to be used in the study, malignancies or other severe chronic diseases, poorly controlled diabetes (hemoglobin A1c >10%), hypertension (>180/100 mm Hg), any ocular disease requiring topical medication, or other conditions that increase the risk of ocular surgery.
During the first 6 months of wearing the OMI, the patients were called into the study center every 2 to 4 weeks for a total of 9–10 correlation measurement visits. Capillary blood was sampled every 10 min. Patients performed fluorescence measurements with the handheld photometer at the same time intervals; each fluorescence measurement consisted of three averaged consecutive readings. One measurement session took 4–6 h.
For data analysis after the measurement sessions, the EyeSense glucose monitoring system was individually calibrated against BG at each measurement day using two capillary BG readings taken at different BG concentrations within a time interval of approximately 1 h to determine the slope of the response function.
Time lag was empirically determined by shifting the fluorescence sensor data and BG values to each other in 5 min intervals.
Ocular Mini Implant Removal from Human Eyes
The OMI was removed from the patient’s eye after a wearing time of 1 year.
After administration of topical anesthesia, a small incision was made adjacent to the OMI position, and the OMI as well as the adherent tissue capsule was explanted using suitable forceps and scissors. The edges of the incision were sutured. A topical antibiotic (Floxal Augentropfen, Dr. Mann Pharma, Berlin, Germany) was instilled and administration was continued as required by the ophthalmologist. A follow-up visit was performed 1 week after OMI explantation.
The study was conducted in accordance with the Declaration of Helsinki and International Organization for Standardization (ISO 14155) and was approved by local ethics committees. All patients provided written informed consent.
Glucose Response Measurements after Explantation
After removal of the OMI from the eye, the in vitro fluorescence response to glucose was measured as described earlier. Measurements of the explanted OMI with intact fibrous tissue capsule were compared with measurements after removal of the capsule.
Histological Analysis of the Ocular Mini Implant and Surrounding Tissue
The explanted fibrous tissue capsule either with or without the OMI was fixed in Bouin solution (Morphisto GmbH, Frankfurt, Germany), infiltrated and embedded in paraffin, cut in 6 µm sections, stained with hematoxylin/eosin or Movat Pentachrome (Verhöff), and analyzed by light microscopy.
Results
Safety Evaluation of the Clinical Study
In total, 47 diabetes patients were included in the study, separated in two cohorts. The demographics are shown in Table 1. The 28 patients of cohort 1 received an uncoated OMI, while the 19 patients of cohort 2 wore a surface-coated OMI.
Table 1.
Demographics
| Cohort 1 | Cohort 2 | |
| Sex Female Male |
12 16 |
10 9 |
| Age | 50 ± 10 | 59 ± 10 |
| Diabetes Type 1 Type 2 |
20 8 |
5 14 |
| Average hemoglobin A1c | 7.5 ± 1.3 | 7.6 ± 1.1 |
Both kinds of OMI were inserted under the temporal conjunctiva of the right eye without any complications. Postoperatively, most patients showed a small subconjunctival hemorrhage, which disappeared within 7–14 days, as reported previously.27 All patients reported a “foreign body feeling,” which lasted approximately 5–10 days after implantation and was attributed to the suture. Both uncoated and surface-coated OMIs were well tolerated by the patients. Figure 2 shows the eye of a patient wearing a surface-coated OMI. Eight patients from cohort 1 (29%) and 11 patients from cohort 2 (58%) were reported to develop a very mild conjunctivitis due to a FBR to the OMI, which could be successfully treated with prednisolone (10 mg/ml) and ofloxacine (3 mg/ml) eye drops. The median duration of the conjunctivitis was 24 days (48 days interquartile range).
Figure 2.
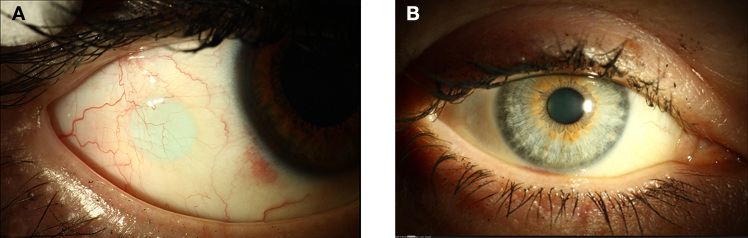
Patient wearing an OMI. (A) The patient looks to the left; the OMI is visible as a light blue disk under the conjunctiva. (B) When the patient looks straight ahead, the OMI is not visible.
Analysis of the In Vivo Measurement Response of the Subconjunctival Glucose Monitoring System
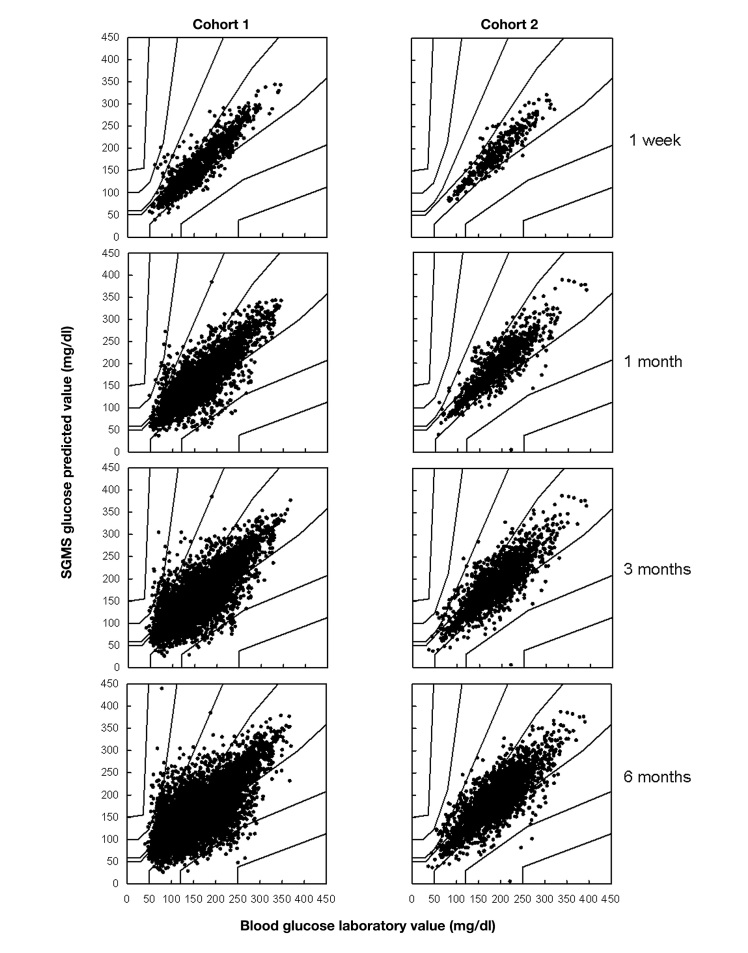
All patients performed 9–10 measurement sessions within time intervals of 2–4 weeks throughout the first 6 months of wearing the OMI. At the beginning of the study, the SGMS of both cohorts yielded very precise measurements, with mean absolute relative difference (MARD) values of 12% for cohort 1 and 7% for cohort 2 and 99% and 100%, respectively, in zones A+B of the consensus error grid (see Table 2 and Figure 3) 36 The performance of both types of OMI deteriorated with increased wearing time of the OMI, but the speed of the deterioration differed between the cohorts. For cohort 1 with the uncoated OMI, the MARD value increased from 12% after week 1 to more than 20% after 6 months of wearing time. The percentage of data pairs in zone A decreased in the same time frame from 86% to 62%. In contrast, cohort 2 with the surface-coated OMI still yielded a MARD of 14% and 80% of the data pairs in zone A of the error grid after 6 months of wearing time.
Table 2.
Consensus Error Grid Analysis of the Two Cohorts Cumulated up to Different Time Points
| Data up to | 1 week | 1 month | 3 months | 6 months | ||||
| Cohort | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| n | 1166 | 368 | 3511 | 1009 | 5833 | 1914 | 8894 | 2799 |
| Error grid analysis | ||||||||
| Zone A | 86.2% | 94.6% | 75.2% | 87.9% | 66.9% | 82.2% | 61.6% | 79.6% |
| Zone B | 12.8% | 5.4% | 22.6% | 11.7% | 29.9% | 17.0% | 33.9% | 19.3% |
| Zone A+B | 99.0% | 100% | 97.8% | 99.6% | 96.8% | 99.2% | 95.5% | 98.9% |
| Zones C, D, E | 1.0% | 0.0% | 2.2% | 0.4% | 3.2% | 0.8% | 4.5% | 1.1% |
| Mathematical parameters | ||||||||
| MARD | 12.5% | 6.9% | 16.5% | 10.5% | 19.8% | 12.8% | 22.5% | 14.0% |
| Median absolute relative difference | 8.7% | 4.8% | 11.1% | 6.8% | 13.4% | 8.5% | 15.2% | 9.5% |
| 25th percentile | 3.8% | 1.9% | 4.7% | 3.0% | 5.9% | 3.6% | 6.7% | 4.0% |
| 75th percentile | 15.9% | 9.2% | 21.1% | 14.2% | 26.0% | 17.3% | 29.4% | 18.9% |
Figure 3.
Consensus error grid analysis of cohort 1 (left) and cohort 2 (right) up to time points of 1 week, 1 month, 3 months, and 6 months (cumulative data).
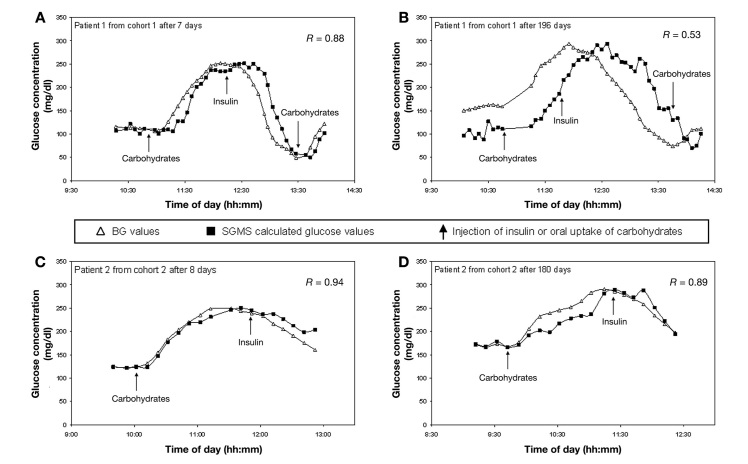
Figure 4 depicts typical individual time courses from one patient of each cohort. At a short wearing time of 1 week, BG is predicted precisely by the EyeSense SGMS in patients from both cohorts. At longer wearing times of 6 months, the result of the patient from cohort 1 is corrupted by a pronounced lag time of up to 50 min. In contrast, the patient of cohort 2, even after 6 months of wearing time, exhibits only a moderate lag time of 10–20 min.
Figure 4.
Individual time courses of patients from cohort 1 (A) at 1 week and (B) 6 months of wearing time; and from cohort 2 (C) at 1 week and (D) 6 months of wearing time.
Explantation and In Vitro Analysis of the Measurement Response of the Ocular Mini Implant after Wear
In accordance with the study schedule, OMIs were removed from the eyes of patients in both cohorts after a wearing time of 1 year. The procedure was quick and well tolerated by the patients. As after implantation, most patients showed a small subconjunctival hemorrhage, which disappeared within 7–14 days. The conjunctiva of all patients healed well and without excessive scarring. At explantation, it was discovered that all OMIs were surrounded by a tight fibrous capsule that was only loosely connected to the conjunctival tissue. These fibrous capsules were removed together with the OMIs.
Directly after explanting the OMIs with their surrounding fibrous capsule, their in vitro response to changing glucose concentrations was determined. Afterward, the fibrous capsule was removed and the OMI response was measured again. Figure 5 shows that the glucose response of the explanted OMI after 1 year of wear in a patient’s eye is comparable with the glucose response of a nonimplanted OMI measured after 1 year of storage under physiological conditions in an in vitro physiological stability assay. However, with the fibrous capsule around the OMI, sensor performance was heavily diminished for OMIs in both cohorts.
Figure 5.
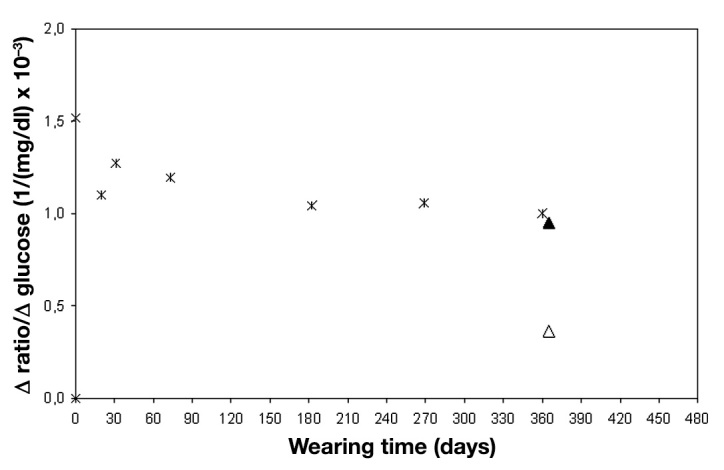
Comparison of in vitro physiological stability test data of a surface-coated OMI (cross signs) and in vitro measurements of the corresponding explanted OMI with (hollow triangle) and without (solid triangle) fibrous capsule after 1 year in vivo
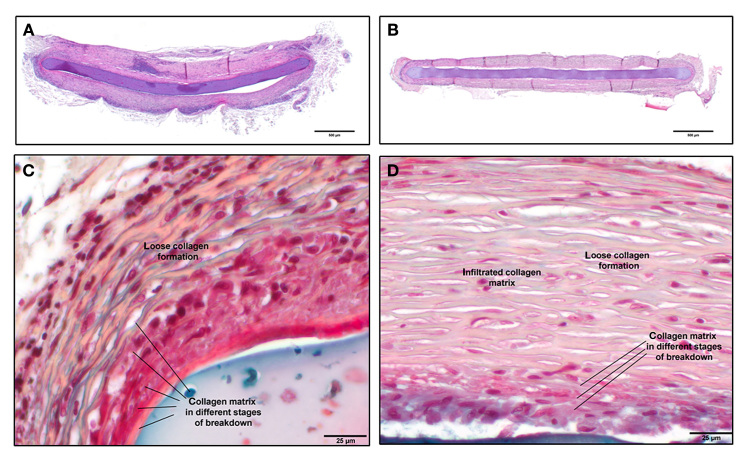
The explanted fibrous capsules were analyzed by histology. Figure 6A and 6B show an overview of a typical capsule from each cohort, and Figure 6C and 6D depict detailed images. The overall structure of the capsules at 1 year of wear time does not differ between the cohorts. The capsules mainly consist of layers of collagen with intermediate fibroblast cells. Nevertheless, in all capsules, fields or bands of other types of cells are present. Adjacent to the OMI, there are areas of collagen necrosis visible in all specimens. Nearly no blood vessels are visible in the histopathology specimens; the capsule tissue is mostly avascular. The thickness of the collagen capsules is very heterogeneous, ranging from 100 to 450 µm per side, with a mean thickness of 290 ± 105 µm for the uncoated OMI and 250 ± 100 µm for the surface-coated OMI. Hence, there is no statistical difference in thickness of the fibrous capsules in between the cohorts.
Figure 6.
Histopathological staining of OMIs in the surrounding fibrous capsule from (A,C) cohort 1 and (B,D) cohort 2.
Discussion
The motivation behind this research was to show the feasibility of the SGMS as a long-term glucose monitoring device. Therefore, at this stage of development, we have chosen to use a partly retrospective calibration method for analysis. The data sets of the measurement sessions were afterward analyzed with an individual two-point calibration received within the corresponding data set. The calibration points were not included in the analysis. We are aware that this kind of calibration is not applicable for a final device, as it does not lead to glucose values displayed in real time, but it is well suited to compare the longevity of the different SGMS versions used. A suitable calibration method and algorithm for real-time display of glucose values has to be developed in the future.
Previous work by our group has shown the general feasibility of using subconjunctival interstitial fluid for glucose monitoring purposes,26 but the performance of the previously tested SGMS was impaired after 1–3 months of wear.27 This is also shown in the results of cohort 1 in this article and is most likely caused by the FBR to the subconjunctival implant, leading to the formation of a fibrous capsule around the OMI. Histology analysis has revealed that the capsule is highly avascular and therefore adds approximately 250 µm to the diffusion distance of the glucose from the blood microvessels to the implant. Hence, in cohort 1, the essentially prolonged in vivo lag time of approximately 50 min at longer OMI wearing times seems to be caused by the extended diffusion distance of glucose.
To reduce the extent of FBR to the OMI for glucose sensing, we applied a specially designed proprietary biocompatible surface coating to the OMI. The OMIs with this coating, which were implanted in patients of cohort 2, exhibited a prolonged duration of action when compared with the uncoated OMIs of cohort 1. After 6 months of wearing time in vivo, the empirically determined lag time increased only to approximately 20 min, compared with 50 min in cohort 1 with uncoated OMIs. This coincides with the increased clinical accuracy of the results compared with cohort 1 at month 6. However, the clinical accuracy of the surface-coated OMIs also deteriorated over time, from 95% in zone A of the consensus error grid after 1 week to 80% in zone A after 6 months and an increase in MARD value from 7% to 14%. Moreover, a lag time of approximately 20 min is still not sufficient to give adequately accurate results in periods of rapid glucose changes. Hence, even the performance of the surface-coated OMI is not sufficient for a long-term glucose monitoring device with a duration of action longer than 6 months and needs to be improved further.
Glucose correlation measurements were stopped after 6 months of wear, but according to the study schedule, the OMIs of both cohorts were explanted after 1 year in vivo. The sensor response to glucose was measured directly after OMI removal from the eyes. With the surrounding fibrous capsule, the in vitro sensor response was reduced heavily, which was due to the retarded diffusion of glucose through the fibrous capsule. After removal of the capsule, the response was comparable to the one of an in vitro stability test at the same time interval. This implies that the glucose sensor chemistry itself is stable for 1 year and the in vivo sensing failure is indeed caused by the formation of the fibrous capsule around the sensor. Analysis of the capsules from cohorts 1 and 2 after one year of wear did not reveal any difference in capsule thickness or composition. However, no capsule was analyzed at the end of the measurement phase after 6 months of wear or at 3 months of wear, when the measurements of cohort 1 worsened while the measurements of cohort 2 were still precise. We assume that the buildup of the fibrous capsule is slowed down for the surface-coated OMI compared with the uncoated OMI, resulting in a slower decrease of measurement performance and that a difference between the capsules of the two cohorts may have been visible at earlier wearing times. To test this, optical coherence tomography (OCT) of the OMI and surrounding conjunctival tissue was performed throughout the wearing time (data not shown). Unfortunately, the resolution of the OCT device originally designed for examinations of the cornea was not sufficient to reveal detailed images of the OMI and surrounding tissue. Hence, the assumed differences in buildup time of the fibrous capsules remain unresolved.
Conclusion
We have evaluated the long-term performance of a SGMS comprising an OMI and a prototype handheld fluorescence photometer. In a clinical trial, we compared the accuracy of surface-coated OMIs for enhanced biocompatibility with uncoated OMIs. In contrast to the uncoated devices, the OMIs with a biocompatible surface coating yield relatively accurate readings even after 6 months in vivo. With further optimization of the biocompatible coating, the use of the SGMS for long-term glucose monitoring seems possible.
Acknowledgments
The authors gratefully acknowledge the kind cooperation and conscientious work of all those who participated in this study.
Glossary
- (BG)
blood glucose
- (Con A)
concanavalin A
- (FBR)
foreign body response
- (MARD)
mean absolute relative difference
- (OCT)
optical coherence tomography
- (OMI)
ocular mini implant
- (SGMS)
subconjunctival glucose monitoring system
Funding
This work was funded by EyeSense GmbH, Grossostheim, Germany.
Disclosure
Achim J. Müller, Monika Knuth, Katharina S. Nikolaus, Frank Küster, and Roland Krivánek are employees of EyeSense GmbH. Achim J. Müller, Monika Knuth, and Katharina S. Nikolaus own stock from EyeSense AG.
References
- 1.International Diabetes Federation. 4th ed. Brussels: International Diabetes Federation; 2009. IDF diabetes atlas. [PubMed] [Google Scholar]
- 2.Klonoff DC. The increasing incidence of diabetes in the 21st century. J Diabetes Sci Technol. 2009;3(1):1–2. doi: 10.1177/193229680900300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver NS, Toumazou C, Cass AE, Johnston DG. Glucose sensors: a review of current and emerging technology. Diabet Med. 2009;26(3):197–210. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 4.Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: Current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540–62. doi: 10.1177/193229681000400632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumner JB, Howell SF. Identification of hemagglutinin of jack bean with concanavalin A. J Bacteriol. 1936;32(2):227–37. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein IJ, Hollerman CE, Smith EE. Protein-carbohydrate interaction. II. Inhibition studies on the interaction of concanavalin a with polysaccharides. Biochemistry. 1965;4:876–83. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein IJ, Reichert CM, Misaki A. Interaction of Concanavalin A with model substrates. Ann N Y Acad Sci. 1974;234(0):283–96. doi: 10.1111/j.1749-6632.1974.tb53040.x. [DOI] [PubMed] [Google Scholar]
- 8.Schultz JS, Mansouri S, Goldstein IJ. Affinity sensor: a new technique for developing implantable sensors for glucose and other metabolites. Diabetes Care. 1982;5(3):245–53. doi: 10.2337/diacare.5.3.245. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri S, Schultz JS. A miniature optical glucose sensor based on affinity binding. Biotechnology. 1984;2:885–90. [Google Scholar]
- 10.Meadows D, Schultz JS. Fiber-optic biosensors based on fluorescence energy transfer. Talanta. 1988;35(2):145–50. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 11.Meadows DL, Schultz JS. Design, manufacture and characterization of an optical fiber glucose affinity sensor based on an homogenous fluorescence energy transfer assay system. Anal Chim Acta. 1993;280(1):21–30. [Google Scholar]
- 12.Ballerstadt R, Ehwald R. Suitability of aqueous dispersions of dextran and concanavalin A for glucose sensing in different variants of the affinity sensor. Biosens Bioelectron. 1994;9(8):557–67. [Google Scholar]
- 13.Beyer U, Schäfer D, Thomas A, Aulich H, Haueter U, Reihl B, Ehwald R. Recording of subcutaneous glucose dynamics by a viscometric affinity sensor. Diabetologia. 2001;44(4):416–23. doi: 10.1007/s001250051637. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Park K. Synthesis and characterization of sol-gel phase-reversible hydrogels sensitive to glucose. J Mol Recognit. 1996;9(5-6):549–57. doi: 10.1002/(sici)1099-1352(199634/12)9:5/6<549::aid-jmr299>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Obaidat AA, Park K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials. 1997;18(11):801–6. doi: 10.1016/s0142-9612(96)00198-6. [DOI] [PubMed] [Google Scholar]
- 16.Ballerstadt R, Schultz JS. Competitive-binding assay method based on fluorescence quenching of ligands held in close proximity by a multivalent receptor. Anal Chim Acta. 1997;345(1):203–12. [Google Scholar]
- 17.Russell RJ, Pishko MV, Gefrides CC, McShane MJ, Coté GL. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly(ethylene glycol) hydrogel. Anal Chem. 1999;71(15):3126–32. doi: 10.1021/ac990060r. [DOI] [PubMed] [Google Scholar]
- 18.Ballerstadt R, Schultz JS. A fluorescence affinity hollow fiber sensor for continuous transdermal glucose monitoring. Anal Chem. 2000;72(17):4185–92. doi: 10.1021/ac000215r. [DOI] [PubMed] [Google Scholar]
- 19.Ballerstadt R, Polak A, Beuhler A, Frye J. In vitro long-term performance study of a near-infrared fluorescence affinity sensor for glucose monitoring. Biosens Bioelectron. 2004;19(8):905–14. doi: 10.1016/j.bios.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Ballerstadt R, Evans C, Gowda A, McNichols R. In vivo performance evaluation of a transdermal near-infrared fluorescence resonance energy transfer affinity sensor for continuous glucose monitoring. Diabetes Technol Ther. 2006;8(3):296–311. doi: 10.1089/dia.2006.8.296. [DOI] [PubMed] [Google Scholar]
- 21.Ballerstadt R, Evans C, Gowda A, McNichols R. Fiber-coupled fluorescence affinity sensor for 3-day in vivo glucose sensing. J Diabetes Sci Technol. 2007;1(3):384–93. doi: 10.1177/193229680700100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20:2555–65. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Dutt-Ballerstadt R, Evans C, Pillai AP, Orzeck E, Drabek R, Gowda A, McNichols R. A human pilot study of the fluorescence affinity sensor for continuous glucose monitoring in diabetes. J Diabetes Sci Technol. 2012;6(2):362–70. doi: 10.1177/193229681200600222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci U S A. 2011;108(33):13399–403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao KC, Hogen-Esch T, Richmond FJ, Marcu L, Clifton W, Loeb GE. Percutaneous fiber-optic sensor for chronic glucose monitoring in vivo. Biosens Bioelectron. 2008;23(10):1458–65. doi: 10.1016/j.bios.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Müller AJ, Knuth M, Nikolaus KS, Herbrechtsmeier P. First clinical evaluation of a new long-term subconjunctival glucose sensor. J Diabetes Sci Technol. 2012;6(4):875–83. doi: 10.1177/193229681200600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasslacher C, Auffarth G, Platten I, Rabsilber T, Smith B, Kulozik F, Knuth M, Nikolaus K, Müller A. Safety and accuracy of a new long-term subconjunctival glucose sensor. J Diabetes. 2012;4(3):291–6. doi: 10.1111/j.1753-0407.2012.00192.x. [DOI] [PubMed] [Google Scholar]
- 28.Morais JM, Papadimitrakopoulos F, Burgess DJ. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010;12(2):188–96. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2(6):1003–15. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayant RD, McShane MJ, Srivastava R. Polyelectrolyte-coated alginate microspheres as drug delivery carriers for dexamethasone release. Drug Deliv. 2009;16(6):331–40. doi: 10.1080/10717540903031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ju YM, Yu B, West L, Moussy Y, Moussy F. A dexamethasone-loaded PLGA microspheres/collagen scaffold composite for implantable glucose sensors. J Biomed Mater Res A. 2010;93(1):200–10. doi: 10.1002/jbm.a.32512. [DOI] [PubMed] [Google Scholar]
- 32.Galeska I, Kim TK, Patil SD, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess DJ. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005;7(1):E231–40. doi: 10.1208/aapsj070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401–12. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586–97. doi: 10.1002/(sici)1097-4636(19980615)40:4<586::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 35.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598–605. doi: 10.1002/(sici)1097-4636(19980615)40:4<598::aid-jbm11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 36.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–8. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]