Abstract
We review progress in our laboratories toward developing in vivo glucose sensors for diabetes that are based on fluorescence labeling of glucose/galactose-binding protein. Measurement strategies have included both monitoring glucose-induced changes in fluorescence resonance energy transfer and labeling with the environmentally sensitive fluorophore, badan. Measuring fluorescence lifetime rather than intensity has particular potential advantages for in vivo sensing. A prototype fiber-optic-based glucose sensor using this technology is being tested.Fluorescence technique is one of the major solutions for achieving the continuous and noninvasive glucose sensor for diabetes. In this article, a highly sensitive nanostructured sensor is developed to detect extremely small amounts of aqueous glucose by applying fluorescence energy transfer (FRET). A one-pot method is applied to produce the dextran-fluorescein isothiocyanate (FITC)-conjugating mesoporous silica nanoparticles (MSNs), which afterward interact with the tetramethylrhodamine isothiocyanate (TRITC)-labeled concanavalin A (Con A) to form the FRET nanoparticles (FITC-dextran-Con A-TRITC@MSNs). The nanostructured glucose sensor is then formed via the self-assembly of the FRET nanoparticles on a transparent, flexible, and biocompatible substrate, e.g., poly(dimethylsiloxane). Our results indicate the diameter of the MSNs is 60 ± 5 nm. The difference in the images before and after adding 20 μl of glucose (0.10 mmol/liter) on the FRET sensor can be detected in less than 2 min by the laser confocal laser scanning microscope. The correlation between the ratio of fluorescence intensity, I(donor)/I(acceptor), of the FRET sensor and the concentration of aqueous glucose in the range of 0.04–4 mmol/liter has been investigated; a linear relationship is found. Furthermore, the durability of the nanostructured FRET sensor is evaluated for 5 days. In addition, the recorded images can be converted to digital images by obtaining the pixels from the resulting matrix using Matlab image processing functions. We have also studied the in vitro cytotoxicity of the device. The nanostructured FRET sensor may provide an alternative method to help patients manage the disease continuously.
Keywords: continuous glucose monitoring, diabetes, fluorescence, glucose/galactose binding protein, glucose sensor
Introduction
Continuous glucose monitoring (CGM) entered clinical practice in 1999. Although randomized controlled trial (RCT) evidence for clinical efficacy compared with self-monitoring of blood glucose was relatively slow to accumulate, a series of RCTs and meta-analyses now provide robust evidence for a reduction in hemoglobin A1c and exposure to hypoglycemia when CGM is used frequently by patients with type 1 diabetes.1–4 Current CGM technology is based on subcutaneously implanted amperometric enzyme electrodes or microdialysis probes, and the accuracy of these sensors is improving as changes in design and manufacture occur. Nevertheless, the performance of glucose sensors is widely thought to be a major bottleneck in the development of a closed-loop insulin delivery system (artificial pancreas),5 where the clinical and regulatory requirements are for optimal accuracy and reliability of the CGM component.
Apart from next-generation reimplantable but minimally invasive sensors, there has been a long-standing quest for completely noninvasive glucose monitoring. Many technologies are being investigated, including near-infrared (NIR), Raman, impedance, and photoacoustic spectroscopy, as well as optical coherence tomography, polarimetry, and reverse iontophoresis, but none have yet reached routine clinical application.6
Fluorescence has been researched for a number of years as an alternative glucose-sensing methodology,7 partly because it has the flexibility to be developed both as a reimplantable CGM probe and for noninvasive blood glucose monitoring (discussed later) but also because it offers notable advantages for both sensing strategies. Fluorescence intensity measurements are very sensitive and are not subject to interference by electroactive substances in the tissues, which may contribute to the underperformance of some amperometric enzyme electrodes. However, arguably the most important advantageous feature of fluorescence for in vivo sensing is that the fluorescence lifetime can be measured as well as the intensity.7 The decay lifetime is the average time the fluorophore remains in the excited state and is an intrinsic property of the fluorophore, largely independent of fluorophore concentration, illumination intensity, light path length, scattering, or photobleaching.8 Thus a fluorescence-based sensor implanted in the tissues may become coated by protein or cells or the illuminating or emitted light may be scattered, which may diminish the fluorescent light intensity, but the decay lifetime of the emitted fluorescence will remain unaltered. One may therefore expect that the performance of fluorescence lifetime-based glucose sensors would be especially stable and thus suitable for in vivo use.
As with other biosensors, fluorescent glucose sensors involve binding of glucose to a receptor, which is thereby associated with a change in fluorescence that is proportional to the glucose concentration. Several glucose receptors have been studied by investigators, including the plant lectin, concanavalin A,9,10 enzymes such as glucose oxidase (or its apo derivative with the prosthetic group removed)11 or hexokinase,12 and boronic acid derivatives, which bind to 1,2 and 1,3 diol groups and thus recognize glucose.13,14 We have developed several fluorescent glucose monitoring systems based on an alternative receptor—bacterial glucose/galactose-binding protein (GBP)—which we believe is particularly suitable for in vivo use, especially when decay lifetimes are used for sensing.15–18
Glucose/Galactose-Binding Protein and Glucose Sensing
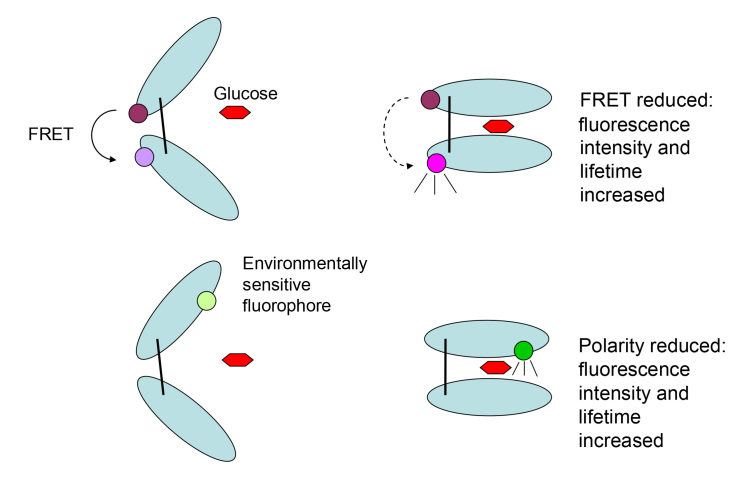
Periplasmic binding proteins are a family of nonenzymatic binding proteins present in the periplasmic space of gram-negative bacteria.19 They are involved in nutrient transport into the bacterium and bind with high affinity small molecular weight substances such as sugars, amino acids, ions, and vitamins. Glucose/galactose-binding protein, like the other ligand-binding bacterial proteins, has a single polypeptide chain that folds into two lobular domains separated by a hinge region where glucose binds20 (Figure 1). Binding is accompanied by a marked conformational change in the protein, with the two lobules closing around the glucose molecule to form a “closed-form” of GBP. Fluorophores covalently attached to specific sites on GBP can therefore be used to transduce the glucose-induced three-dimensional change in GBP into a fluorescence change that is related to glucose concentration.
Figure 1.
Diagrammatic illustration of the structure of GBP, its conformational change on binding glucose, and fluorescence sensing strategies. The top of the figure shows FRET sensing. At the top left, GBP consists of two lobular domains separated by a hinge region where glucose binds. The protein can be labeled by a fluorophore donor and acceptor at different sites such that FRET occurs between the two (open conformation). At the top right, glucose binds and the lobules close around the glucose, increasing the distance between donor and acceptor, decreasing FRET, and thereby increasing fluorescence intensity and lifetime. The bottom of the figure shows glucose sensing using environmentally sensitive fluorophore covalently linked near the binding site. At the bottom left, with the open conformation of GBP, the fluorophore is in a polar environment and fluorescence is suppressed. At the bottom right, glucose binds and the lobules close around the glucose, changing the fluorophore environment in the binding site to a hydrophobic one, thereby increasing fluorescence intensity and lifetime.
A number of researchers have developed glucose-sensing systems based on fluorophore-labeled GBP, with fluorescence intensity as the signal and with two main strategies. With fluorescence resonance energy transfer (FRET) assays, a fluorescent donor and an acceptor are attached at two different sites on the protein; glucose binding and conformational change of GBP alters the molecular distance between donor and acceptor—closer together increases FRET and decreases fluorescence whereas separation decreases FRET and increases fluorescence15,21–25 (Figure 1). Hence, labeling the lobes of GBP with a donor and acceptor can produce the less favorable measurement situation of decreasing fluorescence with increasing glucose concentration. The alternative strategy is to covalently link an environmentally sensitive fluorophore at a site near the binding site. Environmentally sensitive or solvatochromic fluorophores have a fluorescence that is dependent on the solvent polarity—low in a polar/hydrophilic solvent or environment but high in a hydrophobic/water-excluded environment.15,21,22,26–30 In our research, we have used the solvatochromic fluorophore, badan (6-bromo-acetyl-2-dimethylaminonaphthalene; Figure 2), which we covalently attached to cysteine mutations near the GBP binding site (Figure 1). Native GBP does not contain any thiol groups, so that a site-directed mutagenesis can be used to substitute the thiol-containing amino acid cysteine at a chosen site, allowing covalent linkage of a thiol-reactive fluorophore at only this position. We found that, when badan is attached to position H152C (i.e., a mutant where histidine is substituted by cysteine at the 152 position of the polypeptide chain near the glucose-binding site), addition of glucose resulted in a marked (300%) increase in fluorescence intensity (Figure 3). Control measurements on GBP using the thiol-reactive dye Texas red, which is not environmentally sensitive, produced negligible change in fluorescence intensity over the glucose concentration range of 0–100 mM.
Figure 2.
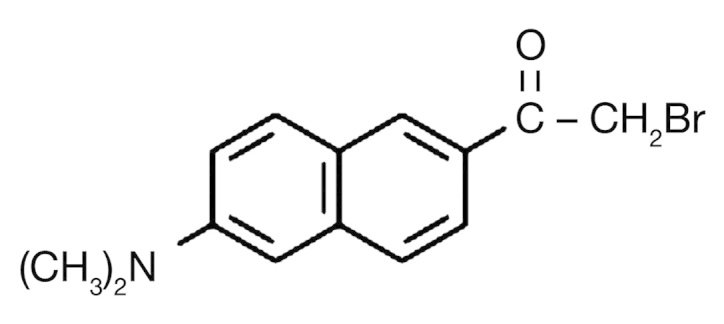
The molecular structure of badan.
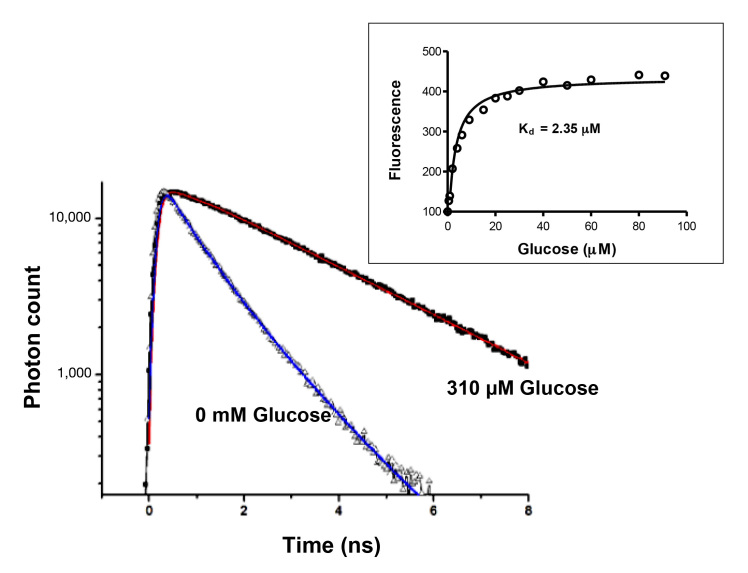
Figure 3.
Fluorescence lifetime decay of GBP (H152C)–badan, measured by TCSPC. Addition of glucose increases lifetime. The inset shows that fluorescence intensity of GBP (H152C)–badan increases with glucose addition, but the Kd is in the micromolar range (representative data).
In our hands, FRET-based systems produce a much lower signal change in response to glucose.15 Thus we attached the donor fluorophore Alexa Fluor 488/555 at the N terminus of GBP by transglutaminase-catalyzed conjugation of Alexa Fluor-cadaverine to a glutamine-containing tag that we introduced at the N terminus by recombinant deoxyribonucleic acid techniques. A non-fluorescent acceptor (QSY7) was attached at one of two cysteine mutations near the binding site. We found significant glucose concentration-dependent increases in fluorescence for QSY7 at both position M182C and position H152C, but the increase was relatively small (<20%). Other investigators have also reported FRET-mediated fluorescence changes of the same order when two different variants of green fluorescent protein were used as donor–acceptor pairs, linked at the N and C termini.23,24 The increase in the fluorescence we observed with glucose indicates a decrease in FRET, so that the donor at the N terminus and the acceptor at the binding site have moved farther apart; others have also noted a glucose-induced fluorescence increase when the two termini are labeled with fluorophores.24
Glucose/Galactose-Binding Protein and Fluorescence Lifetime-Based Glucose Sensing
Using time-correlated single-photon counting (TCSPC) to measure fluorescence decay of GBP–badan, we found that, at zero glucose concentration, the decay could be best fitted by a biexponential model with decay constants of 1.3 nsand 0.5 ns and a mean lifetime of 0.8 ns.16 On addition of glucose, the mean lifetime of GBP–badan increases by a maximum of 2 ns at saturating glucose concentration (Figure 3), with the fraction of the long lifetime component increasing and the fraction of the short lifetime component decreasing. It is therefore likely that the 0.5 ns lifetime represents the open form of GBP (no glucose bound) where badan is exposed to a polar environment, whereas the 1.3 ns lifetime represents the closed form (glucose bound) with badan buried in a hydrophobic environment as the lobes of the protein close around the glucose (Figure 1).
To investigate the stability of fluorescence lifetime measurements over time, we studied the fluorescence decay at zero glucose and saturating glucose concentration at time 0 and 5 hours later. We found the decay curves were superimposable at 0 and 5 hours, whether at zero glucose concentration or with added glucose (Figure 4), indicating that the lifetime of GBP-badan at a given glucose concentration was constant over time.
Figure 4.
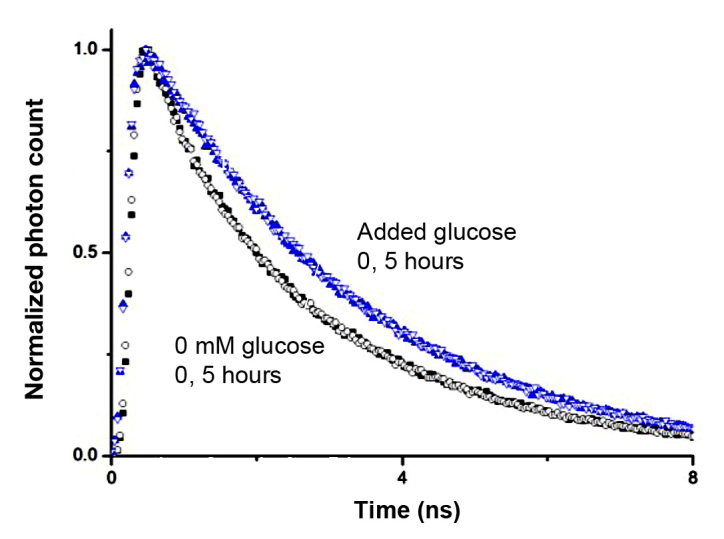
Stability of GBP–badan fluorescence lifetime over time. Lifetime decays were superimposable 0 and 5 h later for both 0 and saturating glucose concentration. Closed symbols represent time 0, and open symbols represent time +5 h.
One notable problem with native GBP and GBP (H152C)–badan is that the binding constant is in the micromolar range (Kd 2–3 µM), with a maximal fluorescence increase at approximately 20 µM glucose. A glucose sensor that is used for clinical purposes needs to operate in the millimolar range because blood glucose levels in diabetes patients are commonly recorded from approximately 1–30 mM. We therefore investigated whether the Kd of GBP could be increased by engineering a series of mutants of the protein with amino acid substitutions chosen from the X-ray crystallographic structure of GBP as locations likely to influence glucose binding.17 Of the five mutants we synthesized (H152C/D14E/F16A, H152C/A213R, H152C/L238S, H152C/A213R/L238S), the triple mutant H152C/A213R/L238S had the highest Kd (11 mM), a 200% maximal increase in fluorescence and an operating range of 1–100 mM glucose, in buffer or serum (Figure 5). Although the different mutants displayed variable maximal increases in fluorescence intensity with glucose addition, the extent of fluorophore labeling was similar for all mutants at 0.9–1.0 mole badan/mole GBP.
Figure 5.
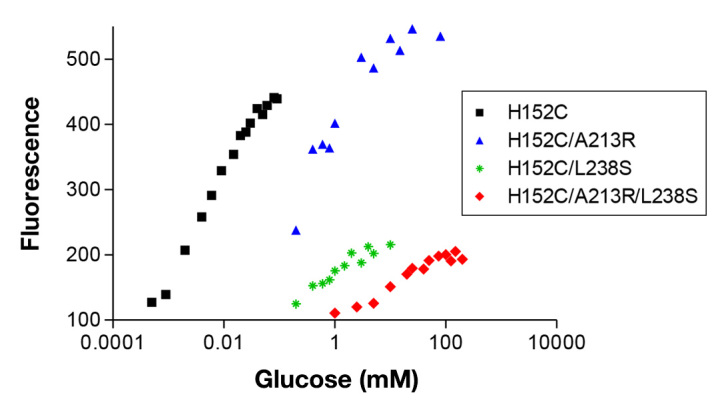
Glucose-induced fluorescence changes for GBP and mutants intended to increase the Kd (representative data). The triple mutant has a Kd in the pathophysiological range. Fluorescence at zero glucose normalized to 100.
As with the GBP (H152C)–badan, the mean lifetime of the triple mutant also increases markedly on glucose addition (~70% at saturating glucose levels), and the decay curve was best fitted by a biexponentional model with 0.9 and 3.1 ns components. Again, the fractional contribution of the long-lifetime component increased with increasing glucose concentration, and the contribution of the short lifetime component decreased.17
Changes in the conformational structure of GBP can be confirmed by time-resolved fluorescence anisotropy decay measurements,8 which record the correlation time φ of GBP–badan when undergoing Brownian rotation. The hydrodynamic diameter d of GBP is determined from the Stokes–Einstein relation φ = ηπd3/6KT, where η is viscosity, K is the Boltzmann constant, and T is temperature. Table 1 shows an overall decrease in d from ~6.4 nm with increasing glucose concentration, the diameter being consistent with what is known from X-ray diffraction (Protein Data Bank, www.pdb.org). Note that d is an average over both open and closed conformations of GBP (see Figure 1), the relative abundance of these changing with glucose concentration.
Table 1.
Rotational Correlation Time Φ and Hydrodynamic Diameter d of Mutant Glucose/Galactose-Binding Protein–Badan in the Presence of Glucosea
| Glucose (mM) | φ(ns) | d (nm) |
| 0 | 33.4 | 6.4 |
| 10 | 38.3 | 6.6 |
| 20 | 28.0 | 6.0 |
| 30 | 26.6 | 5.9 |
| 40 | 29.5 | 6.1 |
| 50 | 24.8 | 5.8 |
| 75 | 23.4 | 5.7 |
| 100 | 23.1 | 5.6 |
| 150 | 22.7 | 5.6 |
| 200 | 21.6 | 5.5 |
| 250 | 20.5 | 5.4 |
A second rotational correlation time of ~0.1 ns used in the analysis, in addition to the Brownian rotation described by φ, describes the wobbling of badan when bound to GBP. The error in d is ~±0.1 nm.
Fiber Optic Glucose and Smart Tattoo Sensors Using Glucose/Galactose-Binding Protein–Badan
We have two broad strategies for glucose monitoring using GBP. The first is immobilization of fluorophore-labeled GBP at the tip of a fiber optic probe that is reimplanted at up to 7-day intervals in the subcutaneous tissue of patients, thereby measuring interstitial glucose concentrations. Fluorescence excitation and recording of emitted fluorescence will be via a handheld device with miniature light source and fluorometer. The second approach is to create a noninvasive CGM system by incorporation of fluorophore-labeled GBP within microcapsules or nanocapsules impregnated into the dermis or subcutaneous tissue, with excitation and recording of fluorescence from the skin surface via a handheld meter. This type of glucose sensor is sometimes known as a “smart tattoo.”31–33
Immobilization of GBP at a fiber optic in a way that retains the sensing element at the probe without interfering with the functionality of the protein is a significant problem. We have found that one of the best ways of immobilizing GBP–badan is to link the protein to Ni-nitrolotriacetic acid (NTA)-functionalized agarose or polystyrene beads via the oligohistidine tag that we engineered at the C terminus of GBP (Figure 6). Ni-nitrolotriacetic acid binds strongly to two histidine residues and is commonly used to purify proteins by affinity chromatography, but we found that GBP–badan immobilized to NTA beads retains both the fluorescence intensity and lifetime response to glucose.18 The labeled beads can be visualized by fluorescence lifetime imaging microscopy (FLIM), where the glucose-induced lifetime increase associated with the GBP–badan is visualized by contrast or as a color change (blue for short and red for long lifetimes).18
Figure 6.
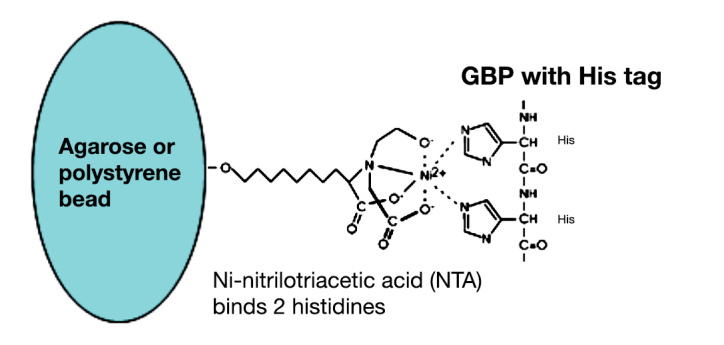
Immobilization of GBP–badan on NTA-functionalized agarose or poly(styrene) beads via binding to the oligohistidine tag on the protein.
For in vitro testing, we loaded the beads with linked GBP–badan into a porous chamber at the end of a multimode optical fiber with pulsed laser excitation, high-speed photodiode detection, and TCSPC for lifetime measurement. We found that the fluorescence lifetime increase with glucose addition was reversible on glucose withdrawal, and good working day stability was obtained. Animal studies have now commenced using a mouse model with implantation of the fiber optic into the peritoneum or subcutaneous tissue (Figure 7), with reversible tracking of blood glucose increases and decreases. The somewhat smaller percentage change in sensor signal in vivo compared with GBP–badan in solution may be due to changed responses when the protein is immobilized or due to dampened glucose changes in the interstitial space. The response times for the prototype sensor are not yet satisfactory for clinical use and likely due to slowed glucose diffusion into the sensing chamber; optimizing the immobilization may be helpful, as GBP–badan has an almost instantaneous response to glucose in solution.
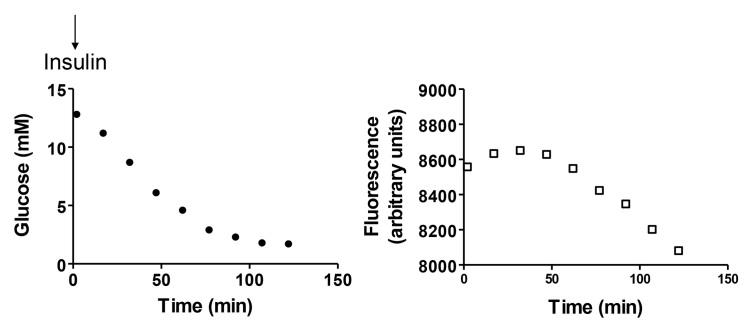
Figure 7.
In vivo testing of a fiber optic glucose sensor based on GBP–badan. The sensing probe was inserted into the peritoneal cavity of an isoflurane-anesthetized mouse. Insulin (0.1 ml of 1 mg/ml human regular) was given subcutaneously as shown. Blood samples were obtained from the tail vein and measured by a reference method.
We developed a GBP-sensing technology suitable for smart tattoos based on incorporating the protein into multilayer, nano-thickness capsules applied by the layer-by-layer technique.16 Glucose/galactose-binding protein–badan was initially adsorbed to a template of 5 µm calcium carbonate microparticles, followed by six alternating bilayers of positively and negatively charged polymers, poly-L-lysine and heparin, which bind by electrostatic attraction. All buffers contained poly(ethylene glycol) (PEG), which we have found to increase the protein loading into the microcapsules. Changes in the fluorescence lifetime associated with the GBP–badan capsules were studied with FLIM, where we found glucose addition caused an increase in the long-lifetime state.
Discussion
A major challenge for in vivo glucose sensing based on fluorescence lifetime measurements is miniaturization of the instrumentation, essentially for wearable use by the patient. The two main methods for lifetime measurement are classed as time domain, where the fluorophore is excited with a short optical pulse and the exponential decay of the fluorescence intensity is observed by a method such as TCSPC, or frequency domain, where the exciting light is modulated and the decay is calculated from the resultant modulated emission and its phase shift and reduction in modulation depth.8 We have used TCSPC in our research studies to date because of its advantages in determining unknown kinetics.34 Time-correlated single-photon counting essentially records the arrival time of single photons that constitute the fluorescence decay following the excitation pulse and has advantages of wide dynamic range, independence from fluctuations of the light source and photobleaching, picosecond resolution, good signal-to-noise ratio, and well-defined Poisson statistics. However, a drawback of traditional TCSPC is the present benchtop dimensions of the instrumentation and the difficulty in reducing it to a wearable size. Although compact and portable application-specific integrated circuit versions of TCSPC have been demonstrated,34 it seems likely that either a “few-point” determination of the average lifetime in the time domain or a phase shift determination in the frequency domain is adequate for a clinical fluorescence lifetime-based CGM system.
The smart tattoo notion for an implanted glucose sensor is generally based on the knowledge that the tissues are transparent to NIR light so that a glucose receptor linked to a NIR fluorophore can be excited and interrogated from outside the body. The maximum excitation and emission wavelengths for badan are approximately 400/550 nm and thus not suitable for such a system, though we note that one report shows that a glucose sensor using fluorescent hydrogel fibers with similar excitation/emission properties was successfully used in vivo when implanted in the dermis of the mouse ear.35 Possibly, therefore, badan-based sensors will have sufficient efficacy as a smart tattoo if impregnated very close to the skin surface in patients.
More likely, badan could be replaced by a NIR, environmentally sensitive fluorophore. The most studied example of such a dye is the lipophilic phenoxazine fluorophore Nile red,36,37 though it does not in itself have a functional group that would allow covalent linking to GBP. Thomas and coauthors28 synthesized two thiol-reactive derivatives of Nile red that, when bound to GBP mutants, gave a glucose-induced increase in fluorescence intensity at around 640–650 nm. Single-walled carbon nanotubes (SWCN) fluoresce in the NIR range, and Yoon and coauthors38 demonstrated that GBP can be attached to carboxylated poly(vinyl alcohol) when coated onto SWCN; addition of glucose resulted in concentration-dependent fluorescence quenching, though the signal was relatively small. Interestingly, although these authors apparently used the native protein with micromolar Kd rather than a mutation of GBP with high Kd, glucose response was observed between 2 and 50 mM glucose. A limited number of other solvent-sensitive, NIR fluorophores that can be used to probe conformational changes in proteins have been described. Toutchkine and coauthors,39 for example, described water-soluble merocyanine dyes that do not aggregate (which merocyanine dyes normally tend to do), are highly fluorescent at long wavelengths, and can be used for probing protein conformation. These newer NIR fluorophores have not been systematically evaluated for GBP-linked glucose sensing, as far as we are aware. For all NIR dyes, photobleaching will be a significant issue to be addressed. Indeed, badan is also vulnerable to photobleaching, although this is ameliorated in the lifetime approach. Indeed, badan fluorescence also displays photo-induced changes. During steady-state fluorescence measurements on GBP–badan, we frequently observed a reduction in fluorescence intensity following an initial scan that then levels off to a constant intensity. However, the superimposable fluorescence decay curves of Figure 4 over 5 h clearly demonstrates how the decay time can indeed overcome such photobleaching effects.
Other significant challenges for smart tattoos based on multilayer nano-encapsulated sensors, such as those we describe here, include ensuring stability of the sensing constructs in the in vivo environment and nontoxicity. These issues need to be systematically investigated before clinical testing.
In addition to our work with GBP, summarized earlier, two other groups have reported studies with GBP glucose-sensing technology configured as a fiber optic probe intended for in vivo use, though their reports were based on fluorescence intensity and neither has described fluorescence lifetime measurements. Weidemaier and coauthors40 covalently attached mutants of GBP (W183C and another with unspecified amino acid substitutions in addition to W183C) to a PEG dimethyl methacrylate:methacrylic acid hydrogel immobilized at the tip of a fiber optic. A ratiometric method was used to measure responses, with excitation at 420 nm and recording at 465 and 560 nm. The probe was implanted in the subcutaneous tissue of pigs, and blood glucose and sensor responses were compared for up to 7 days. The mean sensor error at day 7 was 16.4% ± 5.0% using a single daily reference blood glucose test to calibrate the device. Siegrist and coauthors41 used a mutant of GBP labeled with the environmentally sensitive fluorophore, N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide (MDCC), which has excitation/emission maxima at 425/485 nm. GBP–MDCC was covalently linked to acrylic acid and then incorporated into a polyacrylamide hydrogel.
Conclusion
Fluorescence glucose sensors using GBP would seem to promise several advantages over conventional methods of in vivo CGM systems. The fluorescence lifetime approach, in particular, offers a disruptive solution that is particularly attractive since compact and user-friendly instrumentation, satisfying both the technical requirements and ergonomics, is now well within reach. Full in vivo testing of both intensity- and lifetime-based sensors is now required in order for a full assessment of the technology and for any necessary improvements to be realized.
Acknowledgments
The authors thank Dr. Dalibor Pánek for discussion on the photophysics of badan.
Glossary
- (CGM)
continuous glucose monitoring
- (FLIM)
fluorescence lifetime imaging microscopy
- (FRET)
fluorescence resonance energy transfer
- (GBP)
glucose/galactose-binding protein
- (MDCC)
N-[2-(1-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide
- (NIR)
near infrared
- (NTA)
Ni-nitrolotriacetic acid
- (PEG)
poly(ethylene glycol)
- (RCT)
randomized controlled trial
- (SWCN)
single-walled carbon nanotubes
- (TCSPC)
time-correlated single-photon counting
Funding
The authors wish to acknowledge the Engineering and Physical Sciences Research Council for grant support in a Science and Innovation Award and the European Foundation for the Study of Diabetes and the Diabetes Foundation for additional funding.
References
- 1.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 2.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, Chase P, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Huang ES, Kollman C, Kowalski AJ, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer SA, Wilson DM, Wolpert H, Wysocki T, Xing D. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–83. doi: 10.2337/dc09-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795–800. doi: 10.2337/dc10-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385–95. doi: 10.1038/nrendo.2011.32. [DOI] [PubMed] [Google Scholar]
- 6.Ciudin A, Hernandez C, Simo R. Non-invasive methods of glucose measurement: current status and future perspectives. Curr Diabetes Rev. 2012;8(1):48–54. doi: 10.2174/157339912798829197. [DOI] [PubMed] [Google Scholar]
- 7.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJ. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20(12):2555–65. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lakowicz JR. 2nd ed. New York: Kluwer Academic/Plenum; 1999. Principles of fluorescence spectroscopy. [Google Scholar]
- 9.McCartney LJ, Pickup JC, Rolinski OJ, Birch DJS. Near-infrared fluorescence lifetime assay for serum glucose based on allophycocyanin-labeled concanavalin A. Anal Biochem. 2001;292(2):216–21. doi: 10.1006/abio.2001.5060. [DOI] [PubMed] [Google Scholar]
- 10.Meadows D, Schulz JS. Fiber optic biosensors based on fluorescence resonance energy transfer. Talanta. 1988;35(2):145–50. doi: 10.1016/0039-9140(88)80053-5. [DOI] [PubMed] [Google Scholar]
- 11.D’Auria S, Herman P, Rossi M, Lakowicz JR. The fluorescence emission of the apo-glucose oxidase from Aspergillus niger as probe to estimate glucose concentrations. Biochem Biophys Res Commun. 1999;263(2):550–3. doi: 10.1006/bbrc.1999.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain F, Birch DJ, Pickup JC. Glucose sensing based on the intrinsic fluorescence of sol-gel immobilized yeast hexokinase. Anal Biochem. 2005;339(1):137–43. doi: 10.1016/j.ab.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 13.James TD, Sandanayake KR, Shinkai S. Saccharide sensing with molecular receptors based on boronic acid. Angew Chem Intl Ed Engl. 1996;35:1910–22. [Google Scholar]
- 14.DiCesare N, Lakowicz JR. Evaluation of two synthetic glucose probes for fluorescence-lifetime-based sensing. Anal Biochem. 2001;294(2):154–60. doi: 10.1006/abio.2001.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan F, Gnudi L, Pickup JC. Fluorescence-based sensing of glucose using engineered glucose/galactose-binding protein: a comparison of fluorescence resonance energy transfer and environmentally sensitive dye labelling strategies. Biochem Biophys Res Commun. 2008;365(1):102–6. doi: 10.1016/j.bbrc.2007.10.129. [DOI] [PubMed] [Google Scholar]
- 16.Saxl T, Khan F, Matthews DR, Zhi ZL, Rolinski O, Ameer-Beg S, Pickup J. Fluorescence lifetime spectroscopy and imaging of nano-engineered glucose sensor microcapsules based on glucose/galactose-binding protein. Biosens Bioelectron. 2009;24(11):3229–34. doi: 10.1016/j.bios.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Saxl TE, Pickup JC. Fluorescence intensity- and lifetime-based glucose sensing using an engineered high-Kd mutant of glucose/galactose-binding protein. Anal Biochem. 2010;399(1):39–43. doi: 10.1016/j.ab.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Saxl T, Khan F, Ferla M, Birch D, Pickup J. A fluorescence lifetime-based fibre-optic glucose sensor using glucose/galactose-binding protein. Analyst. 2011;136(5):968–72. doi: 10.1039/c0an00430h. [DOI] [PubMed] [Google Scholar]
- 19.Dwyer MA, Hellinga HW. Periplasmic binding proteins: a versatile superfamily for protein engineering. Curr Opin Struct Biol. 2004;14(4):495–504. doi: 10.1016/j.sbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Borrok MJ, Kiessling LL, Forest KT. Conformational changes of glucose/galactose-binding protein illuminated by open, unliganded, and ultra-high-resolution ligand-bound structures. Protein Sci. 2007;16(6):1032–41. doi: 10.1110/ps.062707807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffrey CJ. Engineering periplasmic ligand binding proteins as glucose nanosensors. Nano Rev. 2011;2 doi: 10.3402/nano.v2i0.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbán J, Sanz-Vicente I, Ortega E, del Barrio M, de Marcos S. Reagentless fluorescent biosensors based on proteins for continuous monitoring systems. Anal Bioanal Chem. 2012;402(10):3039–54. doi: 10.1007/s00216-012-5715-2. [DOI] [PubMed] [Google Scholar]
- 23.Ye K, Schultz JS. Genetic engineering of an allosterically based glucose indicator protein for continuous glucose monitoring by fluorescence resonance energy transfer. Anal Chem. 2003;75(14):3451–9. doi: 10.1021/ac034022q. [DOI] [PubMed] [Google Scholar]
- 24.Fehr M, Lalonde S, Lager I, Wolff MW, Frommer WB. In vivo imaging of the dynamics of glucose uptake in the cytosol of COS-7 cells by fluorescent nanosensors. J Biol Chem. 2003;278(21):19127–33. doi: 10.1074/jbc.M301333200. [DOI] [PubMed] [Google Scholar]
- 25.Veetil JV, Jin S, Ye K. A glucose sensor protein for continuous glucose monitoring. Biosens Bioelectron. 2010;26(4):1650–5. doi: 10.1016/j.bios.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marvin JS, Hellinga HW. Engineering biosensors by introducing fluorescence allosteric signal transduction: construction of a novel glucose sensor. J Am Chem Soc. 1998;120:7–11. [Google Scholar]
- 27.Tolosa L, Gryczynski I, Eichhorn LR, Dattelbaum JD, Castellano FN, Rao G, Lakowicz JR. Glucose sensor for low-cost lifetime-based sensing using a genetically engineered protein. Anal Biochem. 1999;267(1):114–20. doi: 10.1006/abio.1998.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas KJ, Sherman DB, Amiss TJ, Andaluz SA, Pitner JB. A long-wavelength fluorescent glucose biosensor based on bioconjugates of galactose/glucose binding protein and Nile Red derivatives. Diabetes Technol Ther. 2006;8(3):261–8. doi: 10.1089/dia.2006.8.261. [DOI] [PubMed] [Google Scholar]
- 29.Salins LL, Ware RA, Ensor CM, Daunert S. A novel reagentless sensing system for measuring glucose based on the galactose/glucose-binding protein. Anal Biochem. 2001;294(1):19–26. doi: 10.1006/abio.2001.5131. [DOI] [PubMed] [Google Scholar]
- 30.Ge X, Tolosa L, Rao G. Dual-labeled glucose binding protein for ratiometric measurements of glucose. Anal Chem. 2004;76(5):1403–10. doi: 10.1021/ac035063p. [DOI] [PubMed] [Google Scholar]
- 31.Pickup JC, Zhi ZL, Khan F, Saxl T, Birch DJ. Nanomedicine and its potential in diabetes research and practice. Diabetes Metab Res Rev. 2008;24(8):604–10. doi: 10.1002/dmrr.893. [DOI] [PubMed] [Google Scholar]
- 32.Ritter D, McShane M. Microcapsules as optical biosensors. J Mater Chem. 2010;20:8189–93. [Google Scholar]
- 33.Cash KJ, Clark HA. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol Med. 2010;16(12):584–93. doi: 10.1016/j.molmed.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birch DJ. Fluorescence detections and directions. Meas Sci Technol. 2011;23:052002. [Google Scholar]
- 35.Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci U S A. 2011;108(33):13399–403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sackett DL, Wolff J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal Biochem. 1987;167(2):228–34. doi: 10.1016/0003-2697(87)90157-6. [DOI] [PubMed] [Google Scholar]
- 37.Sackett DL, Knutson JR, Wolff J. Hydrophobic surfaces of tubulin probed by time-resolved and steady-state fluorescence of Nile red. J Biol Chem. 1990;265(25):14899–906. [PubMed] [Google Scholar]
- 38.Yoon H, Ahn JH, Barone PW, Yum K, Sharma R, Boghossian AA, Han JH, Strano MS. Periplasmic binding proteins as optical modulators of single-walled carbon nanotube fluorescence: amplifying a nanoscale actuator. Angew Chem Int Ed Engl. 2011;50(8):1828–31. doi: 10.1002/anie.201006167. [DOI] [PubMed] [Google Scholar]
- 39.Toutchkine A, Kraynov V, Hahn K. Solvent-sensitive dyes to report protein conformational changes in living cells. J Am Chem Soc. 2003;125(14):4132–45. doi: 10.1021/ja0290882. [DOI] [PubMed] [Google Scholar]
- 40.Weidemaier K, Lastovich A, Keith S, Pitner JB, Sistare M, Jacobson R, Kurisko D. Multi-day pre-clinical demonstration of glucose/galactose binding protein-based fiber optic sensor. Biosens Bioelectron. 2011;26(10):4117–23. doi: 10.1016/j.bios.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Siegrist J, Kazarian T, Ensor C, Joel S, Madou M, Wang P, Daunert S. Continuous glucose sensor using novel genetically engineered binding polypeptides towards in vivo applications. Sens Actu B Chem. 2010;149(1):51–8. [Google Scholar]