Abstract
According to large randomized trials, results suggest that maintaining normoglycemia postoperatively through tight glycemic control (TGC) and intensive insulin therapy (IIT) can improve surgical outcomes as well as reduce mortality and morbidity in critically ill patients. However, trials examining the effects of TGC have had conflicting results. Systematic reviews and meta-analyses have also led to differing conclusions. The main reason these clinical trials and meta-analyses show negative results for TGC is the high incidence of hypoglycemia induced by IIT. This could not be prevented because there is no reliable technique that can avoid this condition during IIT. The development of accurate, continuous blood glucose monitoring devices and closed-loop systems for computer-assisted blood glucose control in the intensive care unit (ICU) will probably help avoid hypoglycemia in these situations.
The STG closed-loop glycemic control system was introduced to our department to be used and evaluated for strict serum glucose control with no hypoglycemic episodes during IIT in the surgical ICU, to reduce the workload of ICU nurses, and to decrease incidents related to the management of blood glucose levels according to manual conventional venous infusion insulin therapy. The goal of our team was to use the STG closed-loop glycemic control system for perioperative TGC in surgical patients to solve the complications of IIT and reduce risk of hypoglycemia. The challenge at our hospital demonstrated that the STG closed-loop glycemic control system can be expected to achieve TGC with no occurrence of hypoglycemia induced by IIT after surgery.
Keywords: closed-loop glycemic control system, hypoglycemia, intensive care unit, intensive insulin therapy, liver, pancreas, STG, surgery, tight glycemic control
Introduction
Perioperative hyperglycemia in critically ill surgery patients increases the risk of postoperative infection, which is a common surgical complication.1–2 Until 2001, neglecting hyperglycemia was standard intensive care unit (ICU) care, and it was in a landmark paper that Van den Berghe and coauthors3 published the results of a randomized controlled trial of critically ill surgical patients showing that tight glycemic control (TGC) reduced both hospital mortality and morbidity by one-third.4 However, subsequent trials examining the effects of TGC produced conflicting results,5–7 while systematic reviews and meta-analyses also led to differing conclusions.4–8 The negative results for TGC in these clinical trials and meta-analyses largely reflected the high incidence of hypoglycemia (10–17%) induced by intensive insulin therapy (IIT).4,8
In many ICUs, blood glucose (BG) management by TGC with IIT commonly employs the manual conventional use of an intravenous infusion of insulin in saline. However, incidents related to the management of BG levels, such as hypoglycemic attacks and mistakes in the units of insulin administered, account for the majority of the total number of complications.9,10 The development of accurate, continuous BG monitoring devices and closed-loop systems for computer-assisted BG control in the ICU will probably help avoid hypoglycemia and reduce the workload of ICU nursing staffs in these situations.11
This article describes the technical challenges and clinical outcomes of using the STG-22 system (Nikkiso, Tokyo, Japan) for closed-loop glycemic control to avoid hypoglycemia during IIT and to reduce the workload of ICU nursing staffs in the hospital through several different studies as follows: experimental study in earlier work, technical challenges in the clinical setting, and clinical outcomes of using the STG system for closed-loop glycemic control in the hospital.
STG Closed-Loop Glycemic Control System
The STG-22 has been commercially applicable in Japan for more than 20 years and remains the only glycemic control system in the world that uses a closed-loop system (Figure 1).12 Unfortunately, the STG-22 has been used mostly in cases that involve glucose clamp techniques, and only a small number of the institutions use a glycemic control device. However, clinical surgeons and anesthesiologists have a strong need for a closed-loop glycemic control system, especially for patients with unstable BG control in both medical and surgical ICUs.
Figure 1.
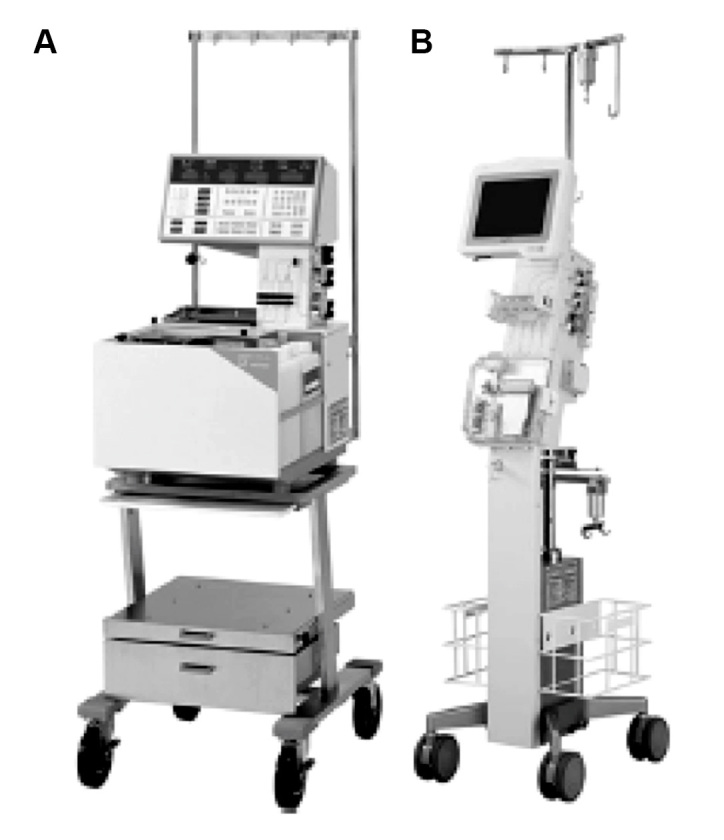
Two bedside-type closed-loop glycemic control systems: (A) STG-22, a conventional closed-loop system; and (B) STG-55, a new, progressive closed-loop system.
The STG-22 system is composed of (1) a glucose sensor that performs glucose detection/monitoring and (2) pumps that infuse the appropriate amount of insulin or glucose.13 The insulin and glucose pumps are computer regulated based on a target BG value predefined prior to system initiation. It would be, of course, of interest to know whether the target can be set for any range depending on the requirements of different hospitals and depending on the intended patient population. Peripheral blood for glucose monitoring was sampled continuously at 2 ml/h during TGC in the surgical ICU (Figure 2). Furthermore, the STG-22 was employed to evaluate the patient’s insulin requirements. However, the current price of the ordinary STG-22 system is high (approximately $138,000), 2 ml/h is a substantial amount of blood required for continuous sampling, and the method involves approximately 1 h of preparation time. Therefore, these disadvantages of the STG-22 will be addressed by the new progressive closed-loop glycemic control system (STG-55) system that will be commercially available in the near future (Figure 1). The STG-55 will be easier to use and will feature improved feasibility and cost-effectiveness in the clinical setting as compared with the STG-22.
Figure 2.
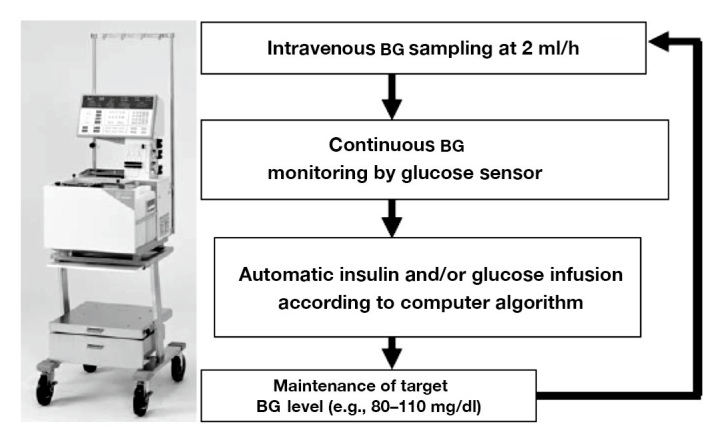
Computer-regulated control for BG concentration. STG automatically infuses insulin and/or glucose to adjust the BG level of the patient in accordance with a target glucose value.
Blood Glucose Control by a Closed-Loop Glycemic Control System in a Pancreatectomized Canine Model
In earlier work at Baylor College of Medicine in 2000, a total pancreatectomy canine model was used to evaluate the safety and feasibility of the STG system.14 Figure 3 shows BG levels controlled by the STG for 3 days after total pancreatectomy in experimental dogs (n = 5). Blood glucose levels remained around 100 mg/dl, coincidental with targeting BG levels of 90–110 mg/dl, even with pancreatogenic diabetes after a total pancreatectomy. In all dogs, the BG concentration was controlled tightly at a mean level of 110 ± 4 mg/dl without the occurrence of hypoglycemia (see Figure 3). These experimental data contributed significantly to establishing a basic method for TGC using a closed-loop glycemic control system in animals that had undergone a total pancreatectomy, which mimics the most severe diabetic condition in human patients. Based on these successful results in an animal model,14 we initiated a clinical practice in 2006 to evaluate the efficacy of TGC by using a closed-loop glycemic control system (STG) in surgical patients.
Figure 3.
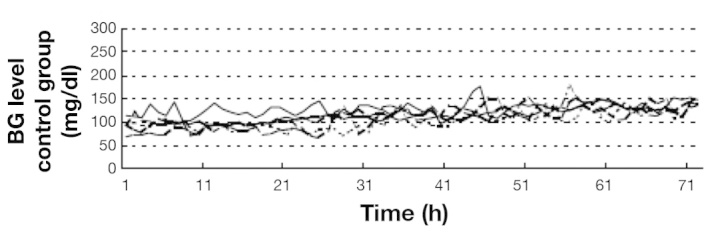
Perioperative continuous BG levels in dogs with total pancreatectomy (n = 5).14 In all dogs, the BG concentration was controlled tightly.14
Technical Challenges of Using the STG System for Closed-Loop Glycemic Control in the Hospital
Accuracy and Reliability of Continuous Blood Glucose Measurement in Post-Surgical Patients
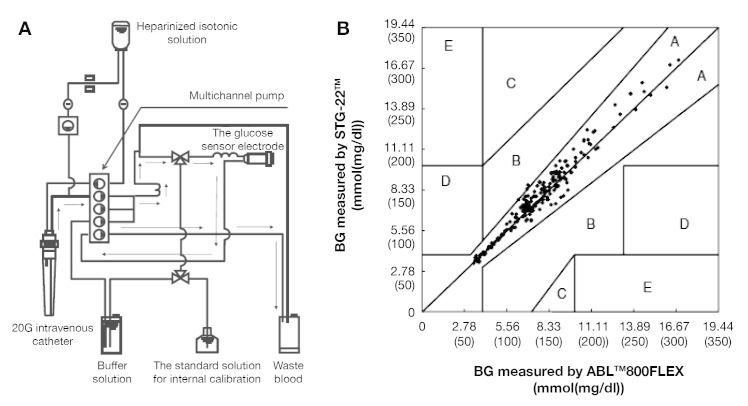
Fifty surgical patients (34 males and 16 females, 70 ± 8 years of age, 160 ± 9 cm in height, and 56 ± 10 kg in weight) were recruited in the study to evaluate the accuracy and reliability of the STG-22, and these patients had undergone hepatectomy (n = 34), pancreaticoduodenectomy (n = 4), total esophagectomy (n = 6), vascular surgery (n = 4), and off-pump coronary artery bypass grafting (n = 4). From the 50 patients, 200 matched sets of data were obtained. Blood glucose levels and insulin infusion rates were automatically recorded every 1 min by the STG-22 that performs continuous BG monitoring using the dual lumen catheter blood sampling technique, a high-quality roller pump (multichannel pump), and a glucose sensor electrode with a glucose oxidase membrane (Yellow Springs Inc., Dayton, OH; Figure 4A). The dual lumen catheter blood sampling technique is a novel method for preventing blood coagulation, and blood is easily withdrawn from a peripheral forearm vein without applying a tourniquet. The technique does not require administration of heparin to the patient, as shown in Figure 4A. Two-point calibration was performed using the standard solution for internal calibration [glucose concentration 0(0) mmol (mg/dl)] and the standard glucose solution [glucose concentration 11.11(200) mmol (mg/dl)] before starting glucose monitoring; internal calibration was automatically performed every 4 h using the standard solution during continuous BG monitoring. Our team compared BG levels measured by the STG-22 and the ABL 800FLEX (Radiometer Medical ApS, Brønshøj, Denmark) recommended by the National Committee for Clinical Laboratory during surgery and ICU stays.15,16 The error grid analysis of BG measurements showed these systems to be effective for continuous monitoring of BG levels in the ICU for 16 h (Figure 4B). For all data, correlation coefficient (R2) was 0.96 (p < .01). In the Clarke error grid, 99.8% of the paired measurements were in zone A and 0.02% of those were in zone B (Figure 4B). The following conclusions were made: the STG can be used for measuring BG levels continuously, measurement results are consistent with intermittent measurement, and the STG is a useful device for monitoring BG levels in the surgical ICU.
Figure 4.
Error grid analysis for BG concentrations measured by the STG-22 compared with those measured by the ABL 800FLEX in the ICU (n = 50).16 The error grid analysis of BG measurements showed these systems to be effective for continuous monitoring of BG levels in the ICU for 16 h (n = 50).16 (A) The whole circuit of the STG. (B) Error grid analysis for BG concentrations measured by STG-22.
Preliminary Study by Using a Closed-Loop Glycemic Control System in the Clinical Setting
Nineteen patients who underwent hepatic resection for primary liver tumor were enrolled in the study. Following surgery, BG was continuously monitored by the STG.2 Glucose levels were controlled using either a manual injection of insulin according to the commonly used sliding scale (sliding-scale group, n = 9) or a programmed infusion of insulin determined by the control algorithm of the STG (STG group, n = 10). The first 9 patients were assigned to the manual insulin group in which BG levels were continuously monitored by the STG and also routinely assessed in capillary finger-stick blood by nursing staff every 6 h using a handheld glucose meter. In this group of patients, BG levels were controlled by the subcutaneous injection of regular human insulin. The dose was determined by the commonly used sliding scale and the target BG level was 150–200 mg/dl to avoid hypoglycemia. The subsequent 10 patients were assigned to the programmed insulin group in which BG levels were monitored and controlled automatically by the intravenous infusion of regular insulin or glucose, according to the control algorithm preprogrammed in the STG. In this study, the targeted BG level was 90–110 mg/dl in the programmed insulin group. For these reasons, it should be conceivable that some of the difference noted was due to the different glucose target ranges chosen for the STG and the conventional sliding scale. The total amount of insulin required for glycemic control in the first 16 h after hepatic resection was compared for patients in both groups. The total amount of insulin used in the first 16 h after hepatic resection in the two groups was measured.
In the sliding-scale group, postoperative BG rose initially and reached a plateau of approximately 250 mg/dl between 4 and 7 h after hepatectomy and then returned toward normal levels by 16 h. In the STG group, BG was steadily lowered, reaching the target zone (90–110 mg/dl) by 12 h post-surgery. Figure 5 shows suppression of this hyper-glycemia following hepatic resection by maintaining TGC with the STG to near normoglycemic levels, even in diabetes patients. Blood glucose levels were significantly different between both groups of patients at 4, 7, 10, and 13 h after hepatectomy. No patient in either group became hypoglycemic during their stay in the surgical ICU. Our team published the first report of a closed-loop glucose-sensing and insulin delivery system such as the STG being used in the postoperative management of hepatectomized patients in a surgical ICU. Postoperative hyperglycemia was observed for up to 16 h in hepatectomized patients. It was suggested that the STG closed-loop glycemic control system is of great clinical value in patients undergoing hepatic resection, as the unit can control the postoperative hyperglycemia induced by surgical stress. In this postoperative hyperglycemic state, the STG safely and quickly achieved glycemic control without the occurrence of hypoglycemia during TGC, indicating its clinical value in the postoperative management of hepatectomized patients.2
Figure 5.
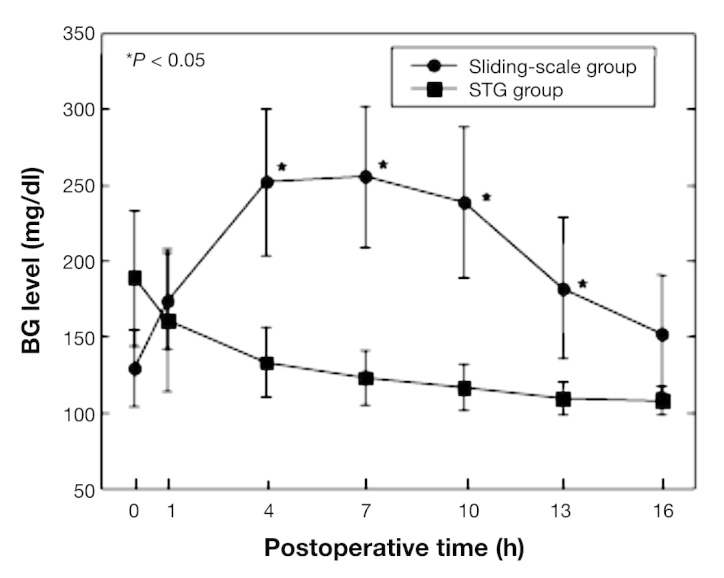
Continuous BG levels in hepatectomy patients under glycemic control using the STG-22 system and sliding-scale method.2 Postoperative BG levels (mg/dl), expressed as means ± standard deviation, in the sliding-scale group (filled circles; n = 9) and STG group (filled squares; n = 10) monitored using the STG-22 system. Asterisks indicate that the BG levels between the patient groups were significantly different at p < .05.2
Clinical Outcomes of Using the STG System for Closed-Loop Glycemic Control in the Hospital
Hyperglycemia induced by surgical stress often dys-regulates liver metabolism and immune function, resulting in impaired postoperative recovery.17,18 The adverse effects of hyperglycemia on the function of white blood cells have been well described. Numerous cellular mechanisms are improved by insulin and better glycemic control, indicating a direct relation between white cell function and elevated BG level.19 Furthermore, several immunologic pathways have been identified by which hyperglycemia affects the host immune system, increasing its susceptibility to infection. There is substantial in vitro and in vivo evidence that short-term hyperglycemia impairs immune function through the following pathways/mechanisms: abnormalities in neutrophil activity, increased expression of intercellular adhesion molecules and E-selectins, the inflammatory cytokine cascade with increasing levels of early pro-inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α, impairment of the microvasculature’s ability to relax in the presence of vasodilating stimuli such as nitric oxide radical, and promotion of adherence and sequestration of neutrophils and monocytes into peripheral tissue.20
Patients with BG levels >200 mg/dl following open-heart surgery develop surgical site infection (SSI) more frequently than patients with lower glucose concentrations.21 In addition, continuous perioperative insulin infusion in diabetes patients undergoing cardiac surgery significantly reduces major infectious morbidity and its associated socioeconomic costs.22 However, the relationship between IIT and SSI is unknown. To address this knowledge gap, our team performed two prospective randomized clinical trials in patients undergoing either pancreatectomy or hepatectomy.23–24
Effect of Intensive Insulin Therapy Using a Closed-Loop Glycemic Control System in Hepatic Resection Patients
Anatomically, the liver is situated downstream from the pancreas. It is a primary site for the metabolism of pancreatic hormones, such as insulin and glucagon, which have a central role in regulating peripheral BG levels. In addition, the liver is positioned downstream from the gut, from which it absorbs a large amount of ingested glucose, and is involved in glycogenolysis and gluconeogenesis. Accordingly, either reduced liver function or removed liver parenchyma following hepatic resection may result in metabolic disturbances of the pancreatic hormones and glucose intolerance.
A prospective randomized trial was conducted in patients undergoing hepatic resection.23 The perioperative BG concentration was continuously monitored using the STG-22 closed-loop system. The clinical parameters analyzed included patient characteristics, indicators of liver status, and operation-related risk factors. We prospectively divided patients into two groups: one for patients whose glucose levels were controlled using a manual injection of insulin according to the commonly used sliding scale (150–200 mg/dl; sliding-scale group [n = 44]) and a second group that received programmed infusions of insulin determined by the control algorithm of the STG system (80–110 mg/dl; STG group [n = 44]). The 44 patients assigned to the sliding-scale group underwent continuous monitoring of BG by the STG b-22 and were routinely checked by nursing staff, and then insulin was given every 2 h. In this group of patients, BG levels were controlled by the subcutaneous injection of regular human insulin; the dose was determined by the commonly used sliding scale, and the target BG level to avoid hypoglycemia was 150–200 mg/dl. Perioperative BG levels in the STG group were near 100 mg/dl, but those in the sliding-scale group were greater than 150 mg/dl (Figure 6A). The incidence of SSI in the STG group was significantly lower than that in the sliding-scale group. The length of hospitalization required for patients in the STG group was significantly shorter than that in the sliding-scale group (Figure 6B). Total hospital costs for patients in the STG group were significantly lower than for those in the sliding-scale group (Figure 6B). This prospective clinical study for liver resection patients demonstrated that IIT using a closed-loop glycemic control system during hepatic resection maintained near-normoglycemia and contributed to a reduction in the incidence of SSI and total hospital costs due to shortened hospitalization.23
Figure 6.
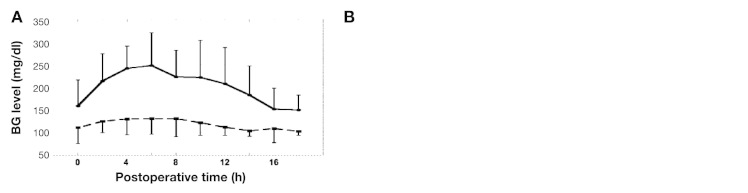
Effect of IIT using the STG in hepatic resection patients. (A) Mean BG levels were adequately controlled by a closed-loop glycemic control system.23 (B) The incidence of SSI in the STG group was significantly lower than that in the sliding-scale group. The length of hospitalization required for patients in the STG group was significantly shorter than that in the sliding-scale group. Total hospital costs for patients in the STG group were significantly lower than for those in the sliding-scale group.12 Values are mean ± standard deviation.
Continuous Postoperative Blood Glucose Monitoring and Control by the STG System in Patients Undergoing Pancreatic Resection
Under normal conditions, BG homeostasis is regulated by hepatic and/or pancreatic metabolism.25 The role of the hepatocyte in producing glucose in the fasting and stressed state or for postprandial glucose uptake is critical for metabolic homeostasis.25 These functions depend largely on three circulating glucoregulatory hormones that are secreted by the pancreas: insulin, glucagon, and pancreatic polypeptide. After pancreatectomy, insufficiency or deficiency of these hormones causes glucose intolerance, a form of secondary diabetes mellitus termed pancreatogenic diabetes.26 The appropriate method for glycemic control in pancreatogenic diabetes after pancreatectomy has yet to be established because of the instability of BG levels, especially in patients after total pancreatectomy.
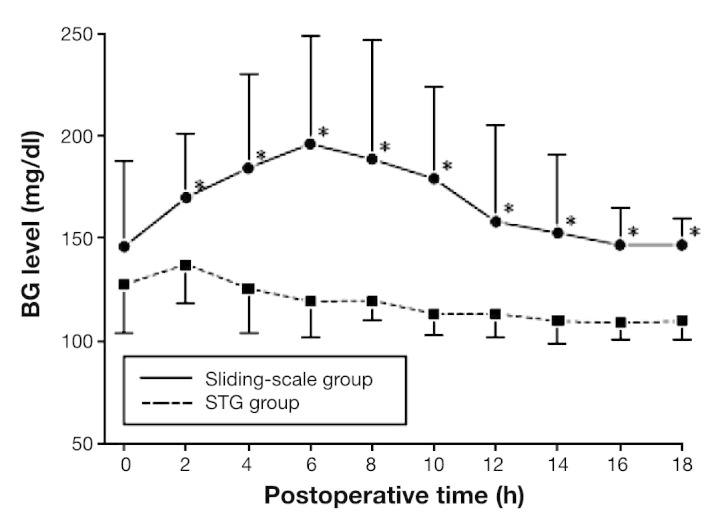
Patients who underwent pancreatic resection for pancreatic neoplasm were prospectively randomized.24 This study recruited 32 patients undergoing elective pancreatic resection for pancreatic disease. Perioperative BG levels were continuously monitored using the STG-22. We prospectively divided patients into two groups: one for patients whose glucose levels were controlled using a manual injection of insulin according to the commonly used sliding scale (sliding-scale group, n = 15) and another that received programmed infusions of insulin determined by the control algorithm of the artificial pancreas (STG group, n = 17). In the sliding-scale group, postoperative BG levels rose initially before reaching a plateau of approximately 200 mg/dl between 4 and 6 h after pancreatectomy. The levels remained high for 18 h postoperatively. In the STG group, BG levels reduced steadily, reaching the target zone (80–110 mg/dl) by 6 h post-surgery (Figure 7). Sliding-scale group patients required 8 ± 6 IU of insulin per patient, according to the routine sliding scale (range 0–20 IU; n = 13). In contrast, STG group patients needed 107 ± 109 IU of insulin (range 21–390 IU; n = 17) per patient for intensive glycemic control by a closed-loop glycemic control system during the first 18 h after pancreatic resection. Also, neither group showed hypoglycemia. Perioperative use of a closed-loop glycemic control system to control pancreatogenic diabetes after pancreatic resection is an easy and effective way to maintain near-normal BG levels. This prospective clinical study for liver resection patients revealed that STG promises to perform insulin treatment for patients with pancreatogenic diabetes after pancreatic resection.24
Figure 7.
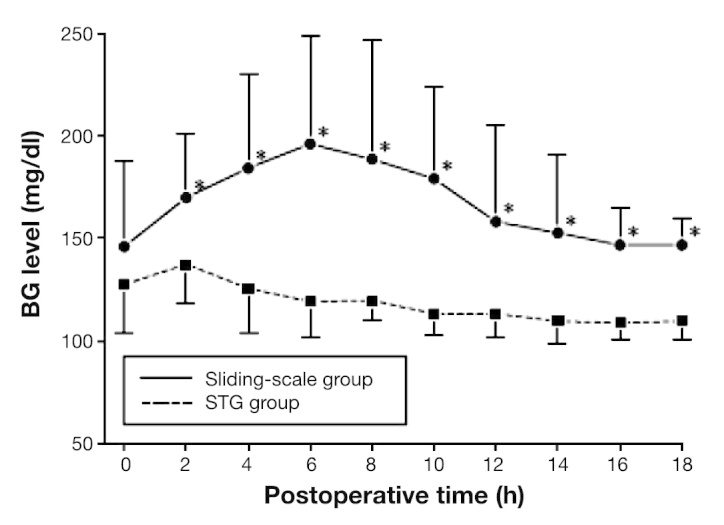
Postoperative BG levels in the sliding-scale and STG groups during the first 18 h after pancreatic resection.24 Asterisks indicate that the BG levels between the patient groups were significantly different at p < .05.24
Our results suggest that perioperative TGC by a closed-loop glycemic control system in both hepatectomized and pancreatectomized patients could be a safe and effective method for lowering the SSI rates without increasing the risk of hypoglycemia and its associated problems. However, prospective study should be performed to assess perioperative glycemic control strategies for reducing SSI by comparing IIT with a target BG range of 80 to 110 mg/dl and intermediate insulin therapy with a target BG range of 140 to 180 mg/dl using a closed-loop glycemic control system,3–8 because, in previous studies, the benefits for IIT with STG could be demonstrated for either hepatectomized or pancreatectomized patients, compared with the ordinary sliding-scale methods.23,24
Workload of Intensive Care Unit Nursing Staff and Iatrogenic Medical Events
Patient safety, a key component of hospital performance, is a focus of increasing attention at all levels of the health care system, most notably when designing health care policies and hospital quality assurance programs. Iatrogenic events are major contributors to mortality morbidity hospital stay prolongation and health care costs. In ICUs, the complexity of care and severity of illnesses result in a high risk of iatrogenic events.10 Blood glucose management is one of the important therapies in the ICU. However, BG management using the sliding-scale method of an intravenous infusion of insulin in saline increases the workload of ICU nurses.
Our team examined the hypothesis that, compared with conventional methods, BG management using the STG reduces the workload of ICU nurses and has a positive impact on awareness regarding the management of blood glucose. In this retrospective study,10 the patients who underwent elective surgery and were treated at the ICU postoperatively were separated into the following two groups: (1) BG was maintained using the STG (STG group) and (2) BG was maintained using the sliding-scale method (sliding-scale group). In addition, a questionnaire was developed for an awareness survey of ICU nurses. As a result, the frequency of blood sampling and number of double checks were significantly lower in the STG group. Furthermore, the time needed for glucose management per admission was significantly shorter in the STG group. Use of STG-22 for glucose management in the ICU increased the degree of attention given by nurses to glucose management and contributed to an improved sense of security (Figure 8). This retrospective study suggested that using the STG in the ICU reduces the workload of ICU nurses compared with using the sliding-scale method. It also contributed to the reduction of the ICU nurses’ anxiety related to the management of blood glucose.10
Figure 8.
Workload date of nursing staff and medical errors.10 Using the STG in the ICU reduces the workload of ICU nurses compared with using the sliding-scale method.10 Data are mean ± standard deviation. Ad, admission; number of Dr. calls, number of calls made to the physician; time needed for management of BG, time needed for the management of BG per admission.
Conclusion
Our review evaluated the STG-22 closed-loop glycemic control system for perioperative TGC in surgical patients following safety and efficacy guidelines. The results indicate a reduced risk of hypoglycemia and a decreased incidence of SSI after hepatectomy or pancreatectomy.
Acknowledgments
This work would not have been possible without the support and suggestions of K. Hanazaki, H. Maeda, K. Ichikawa, M. Munekage, T. Yatabe, K. Yamashita, and M. Yokoyama (Center for Innovative and Translational Medicine, Regenerative Medicine Group, Kochi University, Japan).
Glossary
- (BG)
blood glucose
- (ICU)
intensive care unit
- (IIT)
intensive insulin therapy
- (SSI)
surgical site infection
- (TGC)
tight glycemic control
Funding
This work was supported by the Kochi organization for medical reformation and renewal grants.
References
- 1.Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Yatabe T, Maeda H, Kobayashi M, Hanazaki K. Risk factors and predictors for surgical site infection after hepatic resection. J Hosp Infect. 2009;73(1):47–53. doi: 10.1016/j.jhin.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Okabayashi T, Hanazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, Dabanaka K, Kobayashi M, Yamashita K. Continuous post-operative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci. 2008;53(5):1405–10. doi: 10.1007/s10620-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 4.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300(8):933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 5.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 6.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 8.Langley J, Adams G. Insulin-based regimens decrease mortality rates in critically ill patients: a systematic review. Diabetes Metab Res Rev. 2007;23(3):184–92. doi: 10.1002/dmrr.696. [DOI] [PubMed] [Google Scholar]
- 9.Mibu K, Yatabe T, Hanazaki K. Blood glucose control using an artificial pancreas reduces the workload of ICU nurses. J Artif Organs. 2012;15(1):71–6. doi: 10.1007/s10047-011-0611-7. [DOI] [PubMed] [Google Scholar]
- 10.Garrouste-Orgeas M, Timsit JF, Vesin A, Schwebel C, Arnodo P, Lefrant JY, Souweine B, Tabah A, Charpentier J, Gontier O, Fieux F, Mourvillier B, Troché G, Reignier J, Dumay MF, Azoulay E, Reignier B, Carlet J, Soufir L, OUTCOMEREA Study Group Selected medical errors in the intensive care unit: results of the IATROREF study: parts I and II. Am J Respir Crit Care Med. 2010;181(2):134–42. doi: 10.1164/rccm.200812-1820OC. [DOI] [PubMed] [Google Scholar]
- 11.Van den Berghe G. Insulin therapy in the intensive care unit should be targeted to maintain blood glucose between 4.4 mmol/l and 6.1 mmol/l. Diabetologia. 2008;51(6):911–5. doi: 10.1007/s00125-007-0878-7. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto Y, Okabayashi T, Hanazaki K. Progressive artificial endocrine pancreas: The era of novel perioperative blood glucose control for surgery. Surg Today. 2011;41(10):1344–51. doi: 10.1007/s00595-011-4537-8. [DOI] [PubMed] [Google Scholar]
- 13.Hanazaki K, Nosé Y, Brunicardi FC. Artificial endocrine pancreas. J Am Coll Surg. 2001;193(3):310–22. doi: 10.1016/s1072-7515(01)01014-6. [DOI] [PubMed] [Google Scholar]
- 14.Kono T, Hanazaki K, Yazawa K, Ashizawa S, Fisher WE, Wang XP, Nosé Y, Brunicardi FC. Pancreatic polypeptide administration reduces insulin requirements of artificial pancreas in pancreatectomized dogs. Artif Organs. 2005;29(1):83–7. doi: 10.1111/j.1525-1594.2004.29008.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. The accuracy of a continuous blood glucose monitor during surgery. Anesth Analg. 2008;106(1):160–3. doi: 10.1213/01.ane.0000296461.26492.3c. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53(1):66–71. doi: 10.1111/j.1399-6576.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 17.Huo TI, Lui WY, Huang YH, Chau GY, Wu JC, Lee PC, Chang FY, Lee SD. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003;98(10):2293–8. doi: 10.1111/j.1572-0241.2003.07688.x. [DOI] [PubMed] [Google Scholar]
- 18.Little SA, Jarnagin WR, DeMatteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6(1):88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 19.Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, Miyazaki M. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68(3):230–3. doi: 10.1016/j.jhin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Blondet JJ, Beilman G. Glycemic control and prevention of perioperative infection. Curr Opin Crit Care. 2007;13(4):421–7. doi: 10.1097/MCC.0b013e32826388a1. [DOI] [PubMed] [Google Scholar]
- 21.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–61. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 22.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–60. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 23.Okabayashi T, Nishimori I, Maeda H, Yamashita K, Yatabe T, Hanazaki K. Effect of intensive insulin therapy using a closed-loop glycemic control system in hepatic resection patients: a prospective randomized clinical trial. Diabetes Care. 2009;32(8):1425–7. doi: 10.2337/dc08-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Maeda H, Yatabe T, Kohsaki T, Kobayashi M, Hanazaki K. Continuous postoperative blood glucose monitoring and control by artificial pancreas in patients having pancreatic resection: a prospective randomized clinical trial. Arch Surg. 2009;144(10):933–7. doi: 10.1001/archsurg.2009.176. [DOI] [PubMed] [Google Scholar]
- 25.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116(7):1767–75. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganda OP. Secondary forms of diabetes. In: Kahn CR, Weir GC, editors. Joslin’s diabetes mellitus. 13th ed. Malvern: Lea & Febiger; 1994. [Google Scholar]