Abstract
Background
The epidemic of overweight/obesity affects youth with type 1 diabetes mellitus (T1DM) and their families. In youth with T1DM and their parents, we examined weight status with reported and expected energy intake and with youth hemoglobin A1c (HbA1c).
Methods
In 243 youth (48% female, 13 ± 3 years) and their parents (84% female, 45 ± 6 years), we assessed body mass index (BMI), prevalence of overweight/obesity, reported energy intake (REI), and youth glycemic control (HbA1c). The REI was compared with predicted daily energy requirements (DER; based on age, weight, sex, and physical activity).
Results
Youth had diabetes duration of 6.3 ± 3.4 years and HbA1c of 8.5% ± 1.3%; 69% used insulin pump therapy. Overweight and obesity affected 23% and 11% of youth and 30% and 24% of parents, respectively. Youth and parent BMI (r = 0.38; p < .001) and weight status (overweight/obese; p < .001) were significantly associated. The ratio of REI:DER was significantly lower in overweight/obese compared with underweight/normal weight parents (1.0 ± 0.4 versus 1.2 ± 0.5; p = .001) but did not differ among youth by weight status. Both youth and parent BMI were positively correlated with youth HbA1c (r = 0.14, p = .02; r = 0.16, p = .01, respectively). Hemoglobin A1c tended to be higher in obese than in overweight and normal weight youth (mean ± standard deviation [SD] 8.4 ± 1.4, 8.4 ± 1.3, and 8.8 ± 1.0, respectively; p = .06) and was significantly higher in youth whose parents were obese versus overweight or underweight/normal weight (mean ± SD 8.2 ± 1.2, 8.5 ± 1.4, and 8.9 ± 1.5, respectively; p < .001).
Conclusions
Similar to the general population, overweight and obesity are prevalent among families of youth with T1DM. Weight status appears to influence self-REI in parents and glycemic control in youth with T1DM, suggesting the need for family-based dietary interventions.
Keywords: daily energy intake, hemoglobin A1c, obese, overweight, type 1 diabetes mellitus
Introduction
Currently, approximately 68% of American adults and 33% of children are overweight or obese.1 Further, childhood weight status typically tracks into adulthood because of environmental factors, genetic factors, or a combination of both.2–3 Obtaining accurate data on dietary intake is important with respect to understanding weight status for both clinical and research purposes. Notably, substantial data support the relationship between higher body mass index (BMI) and underreporting of dietary intakes in the general population in both adult and pediatric age groups.4–17 As parent weight status is a significant predictor of child weight status, there are likely associations between parent and youth reports of daily energy intake.18–20 While overweight and obesity are generally related to type 2 diabetes mellitus in both pediatric and adult populations, the associations between youth and parent weight status and investigations of daily energy intake in youth with type 1 diabetes mellitus (T1DM) and their parents require additional study.
Studies have shown similar rates of overweight and obesity in youth with T1DM as in the general population.21 The epidemic of overweight and obesity is particularly concerning in youth with T1DM, as higher BMI may be associated with poorer glycemic control.22 Previous research has suggested that adults with diabetes are more likely to misreport energy intake. A study of 2631 adults found diabetes mellitus to be a risk factor for underreporting of energy intake.13 Additionally, in a study of 1697 youth, ages 12–19 years, with T1DM or type 2 diabetes mellitus, underreporting was more frequent in participants with higher percentage body fat.23
Diabetes treatment, in particular in association with intensive insulin therapy for T1DM, requires attention to both the quality and quantity of foods and is especially related to carbohydrate intake, which greatly contributes to glycemic excursions.24 This detailed emphasis on diet may uniquely impact the youth or parent reporting of intakes in youth with T1DM. In addition, one might expect youth with T1DM to prefer to eat diets similar to their peers. While research in the general population has shown significant associations between youth and parent weight status and between weight status and underreporting of energy intake for both youth and adults, it is unclear whether these associations between weight status and reporting of energy intake would pertain to families of youth with T1DM, a group generally instructed in the careful quantification of energy intake.
The current study examined the associations of parent and child weight status and compared the ratio of reported daily energy intakes to predicted energy requirements by weight status in youth with T1DM and their parents. We further examined the associations of parent and child weight status with youth glycemic control. We hypothesized that weight status in parents and youth would be associated and that youth weight status would be associated with glycemic control.
Methods
Study Population
Youth with T1DM, along with a parent or guardian, were recruited during routine clinic visits to a pediatric diabetes center to participate in a cross-sectional study of dietary intake. Eligible youth were 8–18 years old, with a diagnosis of T1DM of greater than 1 year. Due to the focus on general nutrition and diabetes management, patients with any additional chronic gastrointestinal disease, such as celiac or inflammatory bowel disease, were ineligible for participation. Of 455 eligible families that were approached, 302 (66%) agreed to participate. The 153 youth who declined participation had similar ages and durations of diabetes as those who agreed. The main reasons that families declined participation were related to lack of time or interest. From the 302 participants, 11 subjects were eliminated because they had a sibling with longer diabetes duration also enrolled in the study, and an additional 48 were eliminated because of incomplete youth or parent dietary data. A final sample of 243 families provided complete data for analysis.
Data Collection
Data were collected through electronic medical record review, parent–youth interview, youth surveys, and parent surveys. Youth height and weight were measured in-clinic, while parent height and weight were collected through self-report. Youth BMI was calculated according to age and sex using Centers for Disease Control and Prevention normative data. Weight status was based on Centers for Disease Control and Prevention cutoffs of BMI for youth (underweight/normal weight <1.04, overweight 1.04 to <1.65, and obese ≥1.65) and BMI for parents (underweight/normal weight <25, overweight 25 to <30, and obese ≥30). Glycemic control was measured as hemoglobin A1c (HbA1c; Tosoh, San Francisco, CA; reference range 4–6%). Youth diet was measured using 3-day diet records. Youth with T1DM and their parents were instructed in recording detailed dietary information during three consecutive days (typically two weekdays and one weekend day). A systematic review of dietary assessment methods shows parent report of youth diet to be valid when compared with doubly labeled water.25 Youth dietary data were analyzed using Nutrition Data System for Research (NCC, Minneapolis, MN) by trained staff. Parent dietary data were collected using the whole-grain food frequency questionnaire (FFQ), which assessed usual diet over the past month26 and has been shown to be correlated with intakes measured from diet records.27 This method has been validated against FFQ data and was analyzed by the Channing Lab (Boston, MA).
Calculations of daily energy requirements (DER) for youth utilized the Schofield equation,28,29 which accounted for age, sex, height, weight, and physical activity. Level of physical activity for youth was estimated using interview items based on the behavioral risk factor surveillance system.30 For parents, DER were estimated by multiplying the Mifflin–St. Jeor formula for basal metabolic rate, which accounted for age, sex, height, and weight, by a physical activity factor.31–33 For all parents, a constant activity factor of 1.3 was used to represent light physical activity, based on an average of a sedentary and a low-activity lifestyle from the 2006 Dietary Reference Intakes.34 Reported energy intake (REI) for youth derived from the 3-day diet records and for parents derived from the FFQ were compared with DER. The ratio of REI to DER (REI:DER) was used to classify intake as presumably underreported (<0.8), adequately reported (0.8 to 1.2), and overreported (>1.2). The adequacy of reporting was based on previous nutrition literature utilizing ±20%.35 Study protocol was approved by the institutional review board, and all parents and youth gave informed consent or assent, respectively.
Statistical Analyses
Descriptive analyses of participant characteristics included means, standard deviations (SDs), medians, interquartile ranges, and frequency counts. All analyses accounted for non-normality of continuous variables as indicated. Associations between youth and parent weight (BMI) and weight status were tested using Pearson correlation and McNemar chi-square, respectively. Examination of associations between youth and parent REI:DER used Spearman correlation. Comparisons between weight (BMI) and weight status with REI:DER in youth and parents utilized Spearman correlation and Wilcoxon rank sum or Kruskal–Wallis tests, respectively. Associations between weight (BMI) and weight status with glycemic control were also determined using Spearman correlation and Wilcoxon rank sum tests. All statistical analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC).
Results
Study Population
Participant characteristics are summarized in Table 1. Youth (49% female) with T1DM had a mean age of 13.2 ± 2.8 years, diabetes duration of 6.3 ± 3.4 years, and HbA1c of 8.5% ± 1.3%. Only 28% of youth met American Diabetes Association goals for HbA1c.33 The majority (84%) of parent participants were mothers, with a mean age of 45.0 ± 5.6 years, and the majority (85%) of youth were from two-parent families.
Table 1.
Participant Characteristicsa
| Youth (n = 243) | Parents (n = 243) | |
| Age (years) | 13.2 ± 2.8 | 45.0 ± 5.6 |
| Sex (% female) | 49 | 84 |
| BMI | 0.6 ± 0.8 | 26.8 ± 5.8 |
| Race (% non-Hispanic white) | 92 | — |
| Diabetes duration (years) | 6.3 ± 3.4 | — |
| Insulin regimen (% pump therapy) | 69 | — |
| Insulin dose (U/kg/day) | 0.9 ± 0.2 | — |
| Blood glucose checks/day | 5.5 ± 2.2 | — |
| HbA1cb (%) | 8.5 ± 1.3 | — |
Values are mean ± SD or percentage.
American Diabetes Association goals for HbA1c: <8% for children aged 6–12 years.
Weight Status
Youth had a mean BMI of 0.6 ± 0.8 SD score, and parents had a mean BMI of 26.8 ± 5.8 kg/m2. Youth BMI and their parent’s BMI were positively correlated (r = .36; p < .001). Youth and parent categorical weight status were similarly associated (McNemar S = 34.3; dissipation factor = 34; p < .001). The proportion of overweight and obese youth increased as parental weight status increased (see Table 2). Twenty percent of youth among underweight or normal weight parents were overweight or obese compared with 44% among overweight or obese parents.
Table 2.
Weight Status
| Parents (n = 243) | Row totals (100%) |
|||||
| Underweight 5 (2%) | Normal weight 105 (43%) |
Overweight 75 (31%) | Obese 58 (24%) |
|||
| Youtha (n = 243) |
Underweight (%) | 1 (20%) | 2 (2%) | 0 (0%) | 0 (0%) | 3 (1%) |
| Normal weight (%) | 3 (60%) | 82 (78%) | 47 (63%) | 27 (47%) | 159 (65%) | |
| Overweight (%) | 1 (20%) | 18 (17%) | 20 (27%) | 17 (29%) | 56 (23%) | |
| Obese (%) | 0 (0%) | 3 (3%) | 8 (11%) | 14 (24%) | 25 (10%) | |
| Column totals | 5 (100%) | 105 (100%) | 75 (100%) | 58 (100%) | 243 (100%) | |
Values are numbers of youth (column percentage).
Energy Intake
On average, youth had a daily REI of 2002 ± 525 kcal and had a calculated DER of 2079 ± 720 kcal; parents had a daily REI of 1950 ± 725 kcal and a calculated DER of 1877 ± 329. The mean ratios of REI:DER were 1.03 ± 0.33 for youth (median, 1.01; interquartile range, 0.80, 1.26) and 1.04 ± 0.44 for parents (median, 1.01; interquartile range, 0.76, 1.31). Among youth, 31% reported intake <0.8 DER, 37% reported an intake of 0.8–1.2 DER, and 32% reported intake >1.2 DER. Among parents, 25% reported intake <0.8 DER, 47% reported an intake of 0.8–1.2 DER, and 29% reported intake >1.2 DER. The ratios of REI:DER of youth and parents were not correlated (Spearman r = 0.0203; p = .86).
Next, we assessed the associations of weight (BMI) and weight status with the ratio of REI:DER in both youth and parents. In youth, BMI and REI:DER were not correlated (Spearman r = -0.07; p = .25). Similarly, the ratios of REI:DER were comparable between underweight/normal weight and overweight/obese youth (see Figure 1). However, parent BMI and REI:DER were significantly correlated (Spearman r =-0.27; p < .0001) and the ratios of REI:DER differed significantly between underweight/normal weight parents and overweight/obese parents, with overweight/obese parents reporting lower energy intakes relative to DER (ratio of REI:DER = 1.2 ±0.5 in underweight/normal weight parents versus 1.0 ±0.4 in overweight/obese parents; p = .002; see Figure 1).
Figure 1.
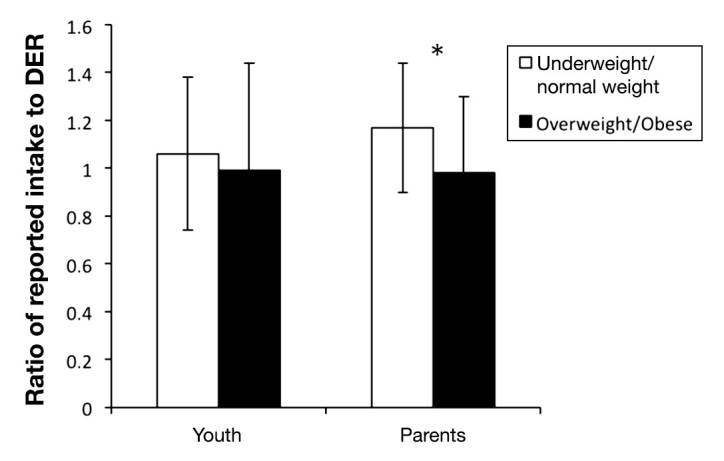
Ratio of reported intake (REI) to DER by weight status in youth with T1DM and their parents. The ratios of REI:DER were comparable between underweight/normal weight and overweight/obese youth. However, overweight/obese parents reported significantly lower energy intakes relative to DER (ratio of REI:DER = 1.2 ± 0.5 in underweight/normal weight parents versus 1.0 ± 0.4 in overweight/obese parents; p = .002. Analyses conducted using Wilcoxon rank sum test.
Weight Status and Glycemic Control
Youth BMI and HbA1c were modestly correlated (Spearman r = 0.14; p = .02), and the association of youth weight status with HbA1c approached significance (Kruskal–Wallis test; p = .06); mean HbA1c was 8.4% ± 1.4%for underweight/normal weight youth, 8.4% ± 1.3% for overweight youth, and 8.8% ± 1.0% for obese youth. Youth HbA1c was also correlated with parent BMI (Spearman r = 0.16; p = .01). Youth HbA1c was also significantly associated with parent weight status. The mean HbA1c was 8.2% ± 1.2% for youth with underweight/normal weight parents, 8.5% ± 1.4% for youth with overweight parents, and 8.9% ± 1.5% for youth with obese parents (p = .01; see Figure 2). Hemoglobin A1c and ratio of REI:DER was not correlated in parents (Spearman r = 0.02; p = .71) and, although weakly correlated in youth, is not at a clinically significant level (Spearman r = -0.15; p = 0.02).
Figure 2.
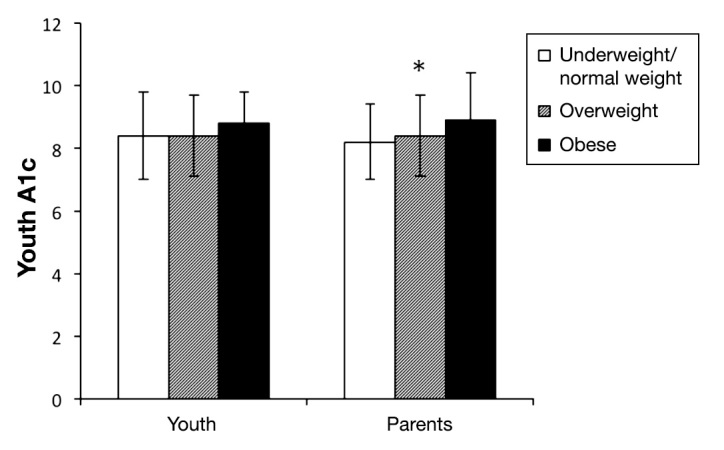
Youth HbA1c according to youth and parent weight status. The mean HbA1c was 8.4% ± 1.4% for underweight/normal weight, 8.4% ± 1.3% for overweight, and 8.8% ± 1.0% for obese youth (p = .06). The mean HbA1c was 8.2% ± 1.2% for youth with underweight/normal weight parents, 8.5% ± 1.4% for youth with overweight parents, and 8.9% ± 1.5% for youth with obese parents (p = .01; Kruskal–Wallis test).
Discussion
Our sample of youth with T1DM had a prevalence of childhood overweight and obesity of 33%, similar to that of the general population. Fifty-five percent of the parents of these youth were overweight or obese, which is somewhat lower than the 68% observed in the general population,1,2 potentially due to differences in the age and racial/ethnic characteristics of our population. Specifically, adults in our sample were younger and predominantly white. The significant association that we observed between the weight status of youth with T1DM and their parents is consistent with findings in the general population.17–19 This, along with previous research suggesting an increased incidence of overweight and obesity in youth with T1DM, highlights the need for family-focused lifestyle interventions to target optimal weight as part of the diabetes management programs for youth with T1DM.
Previous research has indicated that underreporting of daily energy intake is more common in overweight or obese as compared with normal weight or underweight adults.3–13 Our observation that overweight/obese parents had a lower ratio of REI:DER than underweight/normal weight parents is consistent with this literature on reporting accuracy in adults. Due to the nature of the FFQ used in this study, which only offers rank order and does not yield actual calculations of true daily caloric intake, we are only able to make conclusions about relative reporting accuracy between groups. In addition, because physical activity was assumed to be sedentary/light for all parent participants, our observations cannot account for likely differences in physical activity levels by weight status among the adults. In other words, we were unable to determine if underweight/normal weight participants were more physically active than overweight/obese parents, which could account for their relative overreporting of intake compared with predicted requirements. Specifically, the estimates of DER would have been higher, for example, if the physical activity level of the underweight/normal weight adults was higher, such as an activity factor of 1.5 rather than the assigned 1.3, thereby increasing the estimated energy requirements and decreasing the ratio of REI:DER.
Our finding regarding a non-significant relationship between weight status and the ratio of REI:DER in youth is not consistent with some previous research in the general population. A study of German youth, ages 1–18 years, found that, for females, higher BMI was related to underreporting, but these investigators did not find the same relationship in males.13 However, a Canadian study of 9th and 10th graders found that higher BMI was associated with underreporting in both males and females.14 Studies in younger children have also found similar relationships between higher BMI and underreporting using diet records and FFQs.13–16,24 In addition, a study comparing reporting from diet records and total energy expenditure measured by doubly labeled water in overweight adolescents found that underreporting increased in those with higher BMI.36 Differences between these results and the findings of our analyses may be the result of a heightened awareness by parents and youth with T1DM of the youth’s dietary intake due to the dependence of insulin dosing upon their food intake, thus potentially reducing underreporting. In addition, while parents’ self-report of their own caloric intake may be susceptible to social desirability, especially among overweight/obese adults, it is less likely that such influences would impact the reporting of youth dietary intake by parents.
Our demonstration of a significant correlation between youth BMI and youth HbA1c along with the somewhat higher, although nonsignificant, HbA1c in obese compared with normal weight and overweight youth with T1DM is consistent with previously published data.23 Notably, we also found a positive association between youth HbA1c and parent BMI. These findings suggest a link between family weight management and youth glycemic control, particularly among those at the most extremes of weight status, namely, within the obese category. Weight status and dietary practices often track within families. It is possible that family weight and glycemic control could be linked through insulin resistance due to either common environmental influences within families such as low levels of physical activity and less healthful diets or via familial genetic influences. Our findings also offer potential future avenues to improve youth glycemic control through interventions targeted at family weight management.
There are several limitations to our study, including the use of different dietary assessment tools for youth and parents. Although different tools were used, other researchers have found similar associations between weight status and dietary underreporting measured by 24 h recalls, diet records, and FFQs, suggesting that overweight and obese adults in the general population tend to underreport both current and habitual diets.10–15 Thus the estimation of total calorie intake using a FFQ in parents likely does not account for the differences in dietary reporting by weight status. Next, we unfortunately did not collect physical activity data for parents as we did for youth, necessitating uniform assignment of a sedentary level of physical activity for all parent DER calculations. Thus future studies can be improved with determinations of adult physical activity levels. Another limitation of the present study is that the height and weight of parents were obtained through self-report. It is possible that BMI calculated according to self-reported height and weight may underestimate actual BMI in overweight and obese parents and may overestimate BMI in underweight parents,37 adding bias to our results. Such differential self-reporting according to weight status, however, would not change the direction of our findings. For example, a higher, “true” BMI determined from measured height and weight in overweight or obese adults would yield higher estimated energy requirements and an even lower ratio of REI:DER. Future studies should include actual parental measurements.
The main strengths of this study include the relatively large sample size of youth with T1DM and their parents and the attention to dietary data collection for both youth with T1DM and their parents. In addition, the current analyses utilized equations for energy requirements that have been reported to be particularly robust in the general population. The Mifflin–St. Jeor equation has been found to be the most stable equation in healthy normal weight and overweight adults.32,33
Conclusion
In the collection of dietary data, it is important to be aware of the possibility of underreporting among those who are overweight or obese. These analyses demonstrate the presence of an inverse relationship between BMI and REI:DER in parents of youth with T1DM. Importantly, we did not find a relationship between the weight status of youth with T1DM and REI:DER, which may be due to the tight linkage of diabetes management and diet in T1DM. We also confirmed positive associations between the weight status of parents and their children with T1DM in addition to significant associations between both youth and parent weight status with the glycemic control of the youth with T1DM. These findings suggest opportunities to intervene at the level of the family in order to optimize both weight status and glycemic control for youth with T1DM.
Acknowledgments
Additional support came from the Katherine Adler Astrove Youth Education Fund, the Maria Griffin Drury Fund, and the Eleanor Chesterman Beatson Program at the Joslin Diabetes Center.
Glossary
- (BMI)
body mass index
- (DER)
daily energy requirement
- (FFQ)
food frequency questionnaire
- (HbA1c)
hemoglobin A1c
- (REI)
reported energy intake
- (SD)
standard deviation
- (T1DM)
type 1 diabetes mellitus
Funding
This research was supported by the intramural research program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, contract number HHSN267200703434C. Additional support came from the Katherine Adler Astrove Youth Education Fund and the Maria Griffin Drury Fund at the Joslin Diabetes Center.
References
- 1.Centers for Disease Control and Prevention. Overweight and obesity: data and statistics. http://www.cdc.gov/obesity/data/ [Google Scholar]
- 2.Juonala M, Juhola J, Magnussen CG, Würtz P, Viikari JS, Thomson R, Seppälä I, Hernesniemi J, Kähönen M, Lehtimäki T, Hurme M, Telama R, Mikkilä V, Eklund C, Räsänen L, Hintsanen M, Keltikangas-Järvinen L, Kivimäki M, Raitakari OT. Childhood environmental and genetic predictors of adulthood obesity: the cardiovascular risk in young finns study. J Clin Endocrinol Metab. 2011;96(9):E1542–9. doi: 10.1210/jc.2011-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- 4.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65(4 Suppl):1203S–1209S. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari P, Slimani N, Ciampi A, Trichopoulou A, Naska A, Lauria C, Veglia F, Bueno-de-Mesquita HB, Ocké MC, Brustad M, Braaten T, José Tormo M, Amiano P, Mattisson I, Johansson G, Welch A, Davey G, Overvad K, Tjønneland A, Clavel-Chapelon F, Thiebaut A, Linseisen J, Boeing H, Hemon B, Riboli E. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5(6B):1329–45. doi: 10.1079/PHN2002409. [DOI] [PubMed] [Google Scholar]
- 6.Haraldsdóttir J, Sandström B. Detection of underestimated energy intake in young adults. Int J Epidemiol. 1994;23(3):577–82. doi: 10.1093/ije/23.3.577. [DOI] [PubMed] [Google Scholar]
- 7.Johansson L, Solvoll K, Bjorneboe GE, Drevon CA. Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr. 1998;68(2):266–74. doi: 10.1093/ajcn/68.2.266. [DOI] [PubMed] [Google Scholar]
- 8.Johansson G, Wikman A, Ahren AM, Hallmans G, Johansson I. Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr. 2001;4(4):919–27. doi: 10.1079/phn2001124. [DOI] [PubMed] [Google Scholar]
- 9.McGowan MJ, Harrington KE, Kiely M, Robson PJ, Livingstone MB, Gibney MJ. An evaluation of energy intakes and the ratio of energy intake to estimated basal metabolic rate (EI/BMRest) in the North/South Ireland Food Consumption Survey. Public Health Nutr. 2001;4(5A):1043–50. doi: 10.1079/phn2001185. [DOI] [PubMed] [Google Scholar]
- 10.Pikholz C, Swinburn B, Metcalf P. Under-reporting of energy intake in the 1997 National Nutrition Survey. N Z Med J. 2004;117(1202):U1079. [PubMed] [Google Scholar]
- 11.Bedard D, Shatenstein B, Nadon S. Underreporting of energy intake from a self-administered food-frequency questionnaire completed by adults in Montreal. Public Health Nutr. 2004;7(5):675–81. doi: 10.1079/PHN2003578. [DOI] [PubMed] [Google Scholar]
- 12.Lau C, Toft U, Tetens I, Richelsen B, Jørgensen T, Borch-Johnsen K, Glümer C. Association between dietary glycemic index, glycemic load, and body mass index in the Inter99 study: is underreporting a problem? Am J Clin Nutr. 2006;84(3):641–5. doi: 10.1093/ajcn/84.3.641. [DOI] [PubMed] [Google Scholar]
- 13.Yannakoulia M, Panagiotakos DB, Pitsavos C, Bathrellou E, Chrysohoou C, Skoumas Y, Stefanadis C. Low energy reporting related to lifestyle, clinical, and psychosocial factors in a randomly selected population sample of Greek adults: the ATTICA Study. J Am Coll Nutr. 2007;26(4):327–33. doi: 10.1080/07315724.2007.10719619. [DOI] [PubMed] [Google Scholar]
- 14.Sichert-Hellert W, Kersting M, Schoch G. Underreporting of energy intake in 1 to 18 year old German children and adolescents. Z Ernahrungswiss. 1998;37(3):242–51. doi: 10.1007/s003940050023. [DOI] [PubMed] [Google Scholar]
- 15.Vance VA, Woodruff SJ, McCargar LJ, Husted J, Hanning RM. Self-reported dietary energy intake of normal weight, overweight and obese adolescents. Public Health Nutr. 2009;12(2):222–7. doi: 10.1017/S1368980008003108. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Down L, O’Loughlin J, Koski KG, Gray-Donald K. High prevalence of obesity in low income and multiethnic schoolchildren: a diet and physical activity assessment. J Nutr. 1997;127(12):2310–5. doi: 10.1093/jn/127.12.2310. [DOI] [PubMed] [Google Scholar]
- 17.Fisher JO, Johnson RK, Lindquist C, Birch LL, Goran MI. Influence of body composition on the accuracy of reported energy intake in children. Obes Res. 2000;8(8):597–603. doi: 10.1038/oby.2000.77. [DOI] [PubMed] [Google Scholar]
- 18.Burke V, Beilin LJ, Dunbar D. Family lifestyle and parental body mass index as predictors of body mass index in Australian children: a longitudinal study. Int J Obes Relat Metab Disord. 2001;25(2):147–57. doi: 10.1038/sj.ijo.0801538. [DOI] [PubMed] [Google Scholar]
- 19.Maffeis C, Talamini G, Tato L. Influence of diet, physical activity and parents’ obesity on children’s adiposity: a four-year longitudinal study. Int J Obes Relat Metab Disord. 1998;22(8):758–64. doi: 10.1038/sj.ijo.0800655. [DOI] [PubMed] [Google Scholar]
- 20.Guillaume M, Lapidus L, Beckers F, Lambert A, Bjorntorp P. Familial trends of obesity through three generations: the Belgian-Luxembourg child study. Int J Obes Relat Metab Disord. 1995;19(Suppl 3):S5–9. [PubMed] [Google Scholar]
- 21.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, Dabelea D, Hamman R, Waitzfelder B, Kahn HS. SEARCH for Diabetes in Youth Study Group. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11(1):4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Vliet M, Van der Heyden JC, Diamant M, Von Rosenstiel IA, Schindhelm RK, Aanstoot HJ, Veeze HJ. Overweight is highly prevalent in children with type 1 diabetes and associates with cardiometabolic risk. J Pediatr. 2010;156(6):923–9. doi: 10.1016/j.jpeds.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, Pihoker C, Rodriguez BL, Thomas J, Williams D. SEARCH for Diabetes in Youth Study Group. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc. 2006;106(5):689–97. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Patton SR. Adherence to diet in youth with type 1 diabetes. J Am Diet Assoc. 2011;111(4):550–5. doi: 10.1016/j.jada.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110(10):1501–10. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Eny KM, Wolever TM, Fontaine-Bisson B, El-Sohemy A. Genetic variant in the glucose transporter type 2 is associated with higher intakes of sugars in two distinct populations. Physiol Genomics. 2008;33(3):355–60. doi: 10.1152/physiolgenomics.00148.2007. [DOI] [PubMed] [Google Scholar]
- 27.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 28.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 29.Rodriguez G, Moreno LA, Sarria A, Fleta J, Bueno M. Resting energy expenditure in children and adolescents: agreement between calorimetry and prediction equations. Clin Nutr. 2002;21(3):255–60. doi: 10.1054/clnu.2001.0531. [DOI] [PubMed] [Google Scholar]
- 30.Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl HW, 3rd, Macera CA. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;39(8):1267–74. doi: 10.1249/mss.0b013e3180618bbe. [DOI] [PubMed] [Google Scholar]
- 31.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 32.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105(5):775–89. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Seagle HM, Strain GW, Makris A, Reeves RS. American Dietetic Association. Position of the American Dietetic Association: weight management. J Am Diet Assoc. 2009;109(2):330–46. doi: 10.1016/j.jada.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 34.Otten JJ, Hellwig JP, Meyers LD, editors. Washington DC: The National Academies Press; 2006. Dietary reference intakes: the essential guide to nutrient requirements; pp. 84–7. [Google Scholar]
- 35.Mehta SN, Quinn N, Volkening LK, Laffel LM. Impact of carbohydrate counting on glycemic control in children with type 1 diabetes. Diabetes Care. 2009;32(6):1014–6. doi: 10.2337/dc08-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh R, Martin BR, Hickey Y, Teegarden D, Campbell WW, Craig BA, Schoeller DA, Kerr DA, Weaver CM. Comparison of self-reported, measured, metabolizable energy intake with total energy expenditure in overweight teens. Am J Clin Nutr. 2009;89(6):1744–50. doi: 10.3945/ajcn.2008.26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8(4):307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]


