Abstract
The papillomavirus E1 replicative helicase is essential for replication and maintenance of extrachromosomal viral genomes in infected cells. We previously found that the bovine papillomavirus E1 protein is a substrate of the ubiquitin-dependent proteolytic pathway. Here we show that E1 is targeted for degradation by the anaphase-promoting complex (APC). Inhibition of APC activity by the specific inhibitor Emi1 or point mutations in the D-box and KEN-box motifs of E1 stabilize the protein and increase viral DNA replication in both a cell-free system and in living cells. These findings involve APC as the ubiquitin ligase that controls E1 levels to maintain a constant low copy number of the viral genome during latent infection.
Papillomaviruses are a family of small DNA tumor viruses that induce persistent epithelial lesions. A latent infection is established in the dividing cells of the basal layer of these lesions, where the 8-kb double-stranded circular genome is replicated and maintained at a low copy number as an autonomous nuclear plasmid (10). Viral DNA persistence is a significant risk factor for the development of proliferative lesions and their progression to cancer (24). The function of the E1 gene product has been shown to be absolutely necessary for autonomous replication and maintenance of latent replication (12, 22). E1 is the viral initiator of replication that provides the functions of origin recognition and DNA strand separation through its ATP-dependent helicase activity (7, 19).
We reported previously that a major regulatory restriction to viral replication can be imposed by regulated ubiquitination and degradation of the bovine papillomavirus (BPV) E1 protein (14). Results obtained in a cell-free system derived from Xenopus egg extracts allowed us to show that cyclin E-Cdk2, the major Cdk activity responsible for initiation of DNA replication, contributes to E1 stabilization and subsequent activation of the viral origin of replication. Importantly, we also found that protection of E1 is reversed as a consequence of replication (4, 14). We next became interested in identifying the ubiquitin ligase involved in E1 recognition. E1 shares functional homology with the replication initiation factor Cdc6, reported to be an anaphase-promoting complex (APC) substrate: Cdc6 is the initiation factor that recruits cyclin E-Cdk2 to chromosomal origins of replication, and recently cyclin E has been proposed to be involved in stabilization of Cdc6 (1, 5). This led us to investigate the involvement of APC in the ubiquitin-mediated degradation of E1. APC is a large multisubunit complex that promotes the specific and programmed degradation of several proteins to initiate chromosome segregation and exit from mitosis and that prevents unscheduled DNA replication during G1. Although it is present throughout the cell cycle, specific binding of activators, Cdc20 and Cdh1, which appear to function by recruiting substrates to the APC, results in a peak of APCCdc20 activity in mitosis and APCCdh1 activity in late mitosis and G1 (6). Recent studies have identified Emi1 as a specific inhibitor of APC activity, periodically expressed at the G1/S-phase transition and destroyed in mitosis. Emi1 has been shown to bind and inhibit substrate binding to Cdc20 as well as to Cdh1 (8, 18).
Here we report that Emi1 prevents BPV E1 degradation and increases viral replication both in vitro and in vivo. By using different assays we also show that BPV E1 contains a functional KEN box and a destruction box (D box) which serve as recognition motifs for the APC machinery. These findings support the involvement of the APC in E1 regulation and maintenance of latent viral replication. This also indicates that the molecular mechanisms of this regulation overlap mechanisms that control host cell DNA replication.
Cancellation of APC activity stabilizes E1 and enhances viral DNA synthesis in vitro.
Ubiquitin-mediated E1 degradation was initially detected in an E1-dependent replication assay developed with Xenopus membrane-free egg extracts arrested in interphase high-speed supernatant (HSS) (14). We have taken advantage of this assay to investigate the effect of depleting APC ubiquitin ligase on both the stability of E1 and the level of E1-dependent replication. An E1-containing reticulocyte lysate was used as a source of E1. 35S-labeled E1 molecules are stable in interphase HSS, as are in vitro-translated cyclin B or other known APC substrates. Efficient E1 degradation was, however, triggered by addition of BPV-ori plasmids that allow E1-dependent replication events (Fig. 1A). To determine whether or not E1 degradation depended on the APC, HSS were immunodepleted of the APC complex with antibodies directed against the Cdc27 core subunit of the APC. While a gradual loss of E1 was detected in a control-depleted extract, E1 molecules remained stable in Cdc27-depleted extract during the first 30 min of replication. Furthermore, removal of the APC from the extracts specifically enhanced E1-dependent replication by 2.8-fold compared to that of control extracts, indicating that the lack of E1 degradation causes an increase in replication (Fig. 1A). To further support the responsibility of the APC in E1 degradation, we tested the effect of Emi1 addition on E1 stability and activity. Emi1 was translated in egg extracts and was added to HSS prior to the addition of E1 and DNA template. Addition of Emi1 in the replication assay inhibited the destruction of E1 and, furthermore, increased E1-dependent replication to the same extent as APC depletion (Fig. 1B). Thus, Emi1 addition has the same consequence as APC removal from replication extracts. These data argue for an involvement of APC in the E1 destruction process triggered by replication.
FIG. 1.
Immunodepletion of the APC or addition of Emi1 stabilizes E1 and enhances E1-dependent replication. (A) 35S-labeled E1 protein and pSKori+ DNA were added to HSS pretreated with control antibody (PI) or an anti-CDC27 antibody (Cdc27). The samples were warmed to 25°C, and aliquots (2 μl) were removed at 0, 10, and 30 min and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography (upper panel, left). Nondepleted (lane 1), Cdc27-depleted (lane 2), and mock-depleted (lane 3) extracts were assayed for Cdc27 by immunoblotting (lower panel, left). Half of each reaction mixture was supplemented with (32P)dCTP and was used in the BPV1 origin replication assay. After a 30-min incubation, DNA products from nondepleted (lane 1), control-depleted (lanes 2 and 5), or Cdc27-depleted (lanes 3 and 4) extracts supplemented with either E1 or control buffer were purified, subjected to agarose gel electrophoresis, and detected by incorporation of [32P]dCTP. FI and FII designate the migration positions of supercoiled monomer circle and nicked monomer circle markers, respectively. The amount of replicated DNA was quantified from the late replicative intermediates (RI) marked with an asterisk (right panel). (B) Emi1 was translated in fresh interphase egg extracts by adding mRNA encoding this protein. One hour later an Emi1-containing extract (+) or an extract devoid of Emi1 mRNA (−) was combined with HSS (1:4 ratio). After 20 min, 35S-labeled E1 protein and pSKori+ DNA were added and samples were removed for both protein analysis (left panel) and DNA analysis (right panel) as described for panel A. Samples to be analyzed for DNA synthesis were incubated 15 min (lanes 2 and 3) or 30 min (lanes 1, 3, and 5). A reaction without E1 was used in lane 1. The amount of replicated DNA was quantified from the late replicative intermediates (RI) marked with an asterisk.
Emi1 prevents E1 destruction in vivo.
We next tested whether Emi1 can protect E1 from degradation in living cells. Overexpression of Emi1 in human cells was shown to promote S-phase entry and to specifically block the degradation of APCCdh1 targets, such as cyclin A and B (8). The viral protein E1 has never been detected in papillomavirus-transformed cells and is also difficult to detect following transfection of an E1 expression vector, unless cells are cultured in the presence of a proteasome inhibitor. Previous studies have also demonstrated that detection of polyubiquitinated E1 molecules in cells is facilitated by the presence of another viral protein, E2, and BPV origin-containing plasmid that allow in vivo replication (14). E2 is dispensable in cell-free systems (2) but is absolutely necessary in vivo (16, 22), a requirement explained by the fact that E2 mediates the recruitment of E1 in nuclear replication foci as well as the attachment of viral genomes to cellular chromosomes, ensuring their segregation in mitosis (9, 20, 21).
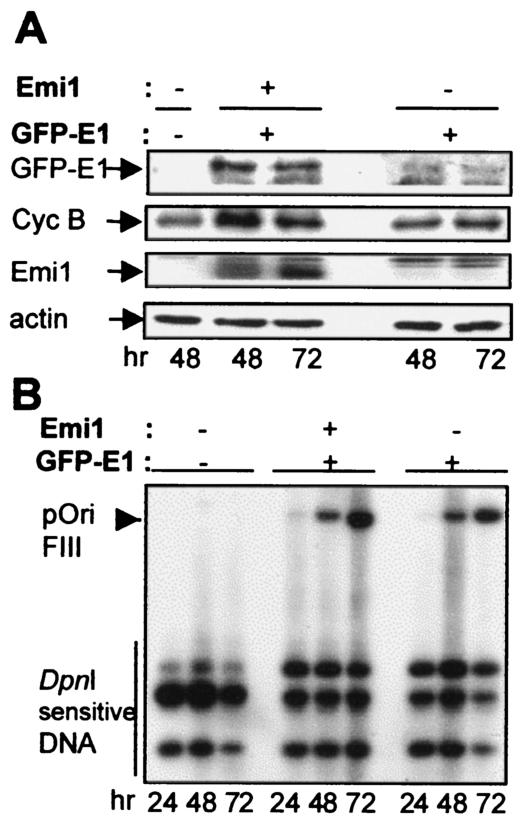
We transiently cotransfected vectors expressing Emi1, a recombinant green fluorescent protein (GFP)-E1 protein and E2 plus a BPV-ori plasmid in 293 cells. Total cell extracts were prepared at 48 and 72 h after transfection and were analyzed by immunoblotting with an antibody to GFP and an antibody to cyclin B. Immunoblots showed that Emi1 strongly increased the levels of both E1 and cyclin B (Fig. 2A), supporting the hypothesis that E1 degradation is mediated by the APC pathway in vivo. Furthermore, overexpression of Emi1 in the transient replication assay also stimulated the level of viral DNA replication 2.7-fold (Fig. 2B), indicating that inhibition of the APC is sufficient to enhance the level of E1-dependent replication events.
FIG. 2.
Emi1 prevents degradation of E1 and stimulates E1-dependent replication in living cells. (A) 293 cells were transfected with pEGFP-E1, pCGE2, and pSKori+ in the presence or absence of pCS2-Emi1. Lanes marked with a minus had the GFP-E1 or the Emi1 vectors replaced with an equal amount of the empty parental expression vector. At the indicated hours posttransfection, cells were harvested and total cell lysates were immunoblotted for GFP-E1 with an antibody to GFP, cyclin B, Emi1, and actin as a loading control. (B) Low-molecular-weight DNA was extracted from parallel cultures transfected as for panel A, digested with EcoRI and DpnI, and analyzed by Southern blotting. Filters were probed with radiolabeled pSKori+ plasmid to detect the presence of DpnI-resistant replication products. pOri FIII indicates the band generated after linearization of pSKori+ with EcoRI.
Mutation of a specific D box of E1 has the same consequence as APC depletion or inhibition in vitro.
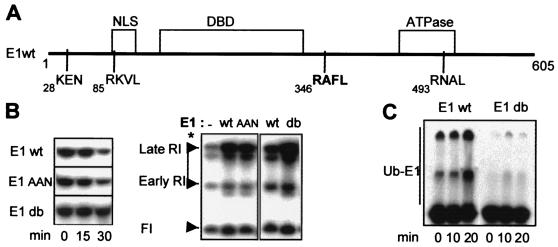
The APC targets proteins contain two types of destruction sequences: the D box (RxxL, where x is any amino acid) and the KEN box (15). Examination of the E1 sequence revealed one putative KEN box located near the N terminus of E1 and three putative D-box motifs (Fig. 3A). We generated an E1 KEN-box mutant (E1AAN) and several E1 D-box mutants in which each RxxL sequence was mutated to AxxA and tested the degradation and activity of the 35S-labeled mutated forms in the in vitro replication assay carried out in Xenopus HSS. The KEN-box E1 mutant was found to behave like wild-type E1 (E1wt) protein (Fig. 3B), which is not surprising, because the KEN-box sequence is exclusively recognized by APCCdh1, and Cdh1 has not been detected in embryonic cell cycles that lack a G1 phase (11). Concerning E1 D-box mutants, mutant R493A/L496A was totally impaired for replication, whereas mutant R85A/L88A behaved like the wild-type protein. Finally, the double mutant with alanines at residues 346 and 349 was found to remain stable throughout replication, while enhancing the level of BPV-ori plasmid replication threefold compared to that of the reaction carried out with E1wt (Fig. 3B). Furthermore, in contrast to E1wt, this E1 mutant, called E1db, was not ubiquitinated during the course of the replication assay with Xenopus HSS (Fig. 3C), indicating that this sequence plays a role in targeting E1 for ubiquitin-mediated degradation.
FIG. 3.
A D-box-like motif is a required determinant for the degradation of E1 in Xenopus egg extracts. (A) Schematic drawing of BPV-E1 showing the domains and positions of putative KEN-box and D-box motifs. (B) 35S-radiolabeled E1wt, E1AAN (K28A/E29A), or E1db (R346A/L349A) was added to HSS together with pSKori+ DNA, and aliquots (2 μl), removed at 0, 10, and 30 min, were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography (right panel). Half of each reaction mixture was supplemented with [32P]dCTP and was used in the BPV1 origin replication assay. After a 30-min incubation, DNA products were purified and analyzed directly by agarose gel electrophoresis. The amount of replicated DNA was quantified from the late replicative intermediates (RI) marked with an asterisk (left panel). (C) 35S-labeled E1wt or E1db was incubated with pSKori+ DNA in HSS. Overexposure of the autoradiogram shows the appearance of high-molecular-weight labeled ubiquitin-E1 conjugates (Ub-E1) in the replication assay carried out with E1wt.
The KEN box of E1 is functional in living cells.
To next determine whether the mutations in the E1 D box or KEN box alter E1 stability and activity in vivo, 293 cells were transfected with either the GFP-E1wt, the GFP-E1db mutant, or the GFP-E1AAN construct, together with the E2 expression vector and BPV origin-containing plasmids. The relative abundance of the E1db mutant 48 h following transfection was much greater than that of the E1 wild type, indicating that the two point mutations in the D box stabilize E1 in living cells. In addition, mutation of the KEN box partially stabilized E1, indicating that the KEN box is also involved in E1 turnover (Fig. 4A). Surprisingly, E1db was totally impaired for replication in living cells, while in contrast, E1AAN was found to support higher levels of viral DNA synthesis with an increase of replicated BPV-ori plasmids of 2-fold at the 24-h time point and 2.5-fold at the 48-h time point compared to that of E1wt (Fig. 4B). Similarly, the double mutant (KEN and D box) was not active in vivo (data not shown). Because E1db is not defective for replication functions in vitro, we examined whether E1db had any other defects which could account for its inability to replicate in vivo. In addition to interacting with BPV E2, which is only required in vivo, E1 was shown to interact with Ubc9, the enzyme that conjugates the ubiquitin-like protein SUMO to target proteins. The D box is found in the sequence of E1 required for Ubc9 interaction, mapped to amino acids 315 to 459 (17). We found that E1db remained competent for interacting with BPV E2 but not with Ubc9 (F. Mechali and C. Bonne-Andrea, unpublished observations). Because reduction in Ubc9 binding was shown to correlate with defective viral replication in vivo, it is possible that the loss of replication capacity of E1db is due to a defect in sumoylation.
FIG. 4.
The degradation of E1 is mediated by the APC-Cdh1 complex in living cells. (A) 293 cells were transfected with the wild type or mutant GFP-E1 expression vectors together with pCGE2 and pSKori+. Cells were harvested at the indicated hours posttransfection, and cell lysates were assayed by Western blotting for GFP-E1 with an antibody to GFP and actin as a loading control. (B) Low-molecular-weight DNA was extracted from cultures transfected as described for panel A, and plasmid replication was assayed as described in the legend to Fig. 2B. (C) The stability of 35S-radiolabeled E1wt or E1AAN was monitored in interphase egg extracts with or without Cdh1 mRNA as indicated. 35S-radiolabeled cyclin B was added to each reaction as an internal control. Samples were removed at different times and were analyzed by autoradiography.
However, the results obtained with the E1 KEN-box mutant nevertheless indicate that E1 degradation occurs in an APC-Cdh1 manner in vivo. To directly confirm the involvement of the KEN-box motif in E1 degradation, we used the APCCdh1-dependent degradation assay reconstituted with interphase Xenopus egg extracts supplemented with mRNA encoding Xenopus Cdh1, also called Fizzy related (3). While 35S-labeled E1wt and 35S-labeled cyclin B, used here as an internal control, were stable in interphase extracts, both proteins were efficiently degraded in Cdh1-containing extracts. In contrast to E1wt, E1AAN remained stable in Cdh1-containing extracts, demonstrating that alanine substitution of the E1 KEN box completely protects the protein against APCCdh1 degradation (Fig. 4C). Thus, the KEN box of E1 directs its recognition through Cdh1.
The present data reveal that ubiquitin-mediated degradation of BPV E1 involves the ubiquitin ligase complex APC. The results obtained with the cell-free system show that cancellation of APC activity as well as mutation of a D box specifically blocks replication-dependent E1 degradation, thereby causing additional replication events. Since E1 is stable in the absence of BPV-ori plasmids, these results raise the intriguing possibility that a specific function of APC targets E1 molecules engaged in replication. Cdc20 is the only APC regulator so far characterized for the Xenopus egg (11). However, all the experiments aimed at inhibiting this activity in the cell-free system were unsuccessful in that E1 stability and activity were not affected. Our results also demonstrate that an APC-Cdh1 activity is not involved, because the E1 KEN-box mutant that is resistant to APCCdh1 degradation is destroyed as a consequence of replication in egg extracts. Given that this APC activity is inhibited by Emi1 and that this inhibitor acts through substrate recognition factors, such as Cdc20 and Cdh1, one possible interpretation of these results is that replication-dependent E1 degradation is mediated by another, as-yet unidentified, substrate recognition factor. This hypothesis is supported by the recent identification of various CDH1 homologs in vertebrates, suggesting that APC associates with diverse substrate-specific adaptator protein (23).
The data obtained from transfected human 293 cells demonstrate that E1 degradation is also mediated by the APC pathway in living cells. In addition, E1 degradation appears to depend not only on a D box but also on a KEN box. Whether E1 is degraded in S phase during a somatic cell cycle remains to be determined. So far, the low level of GFP-E1 full-length protein as well as the accumulation of truncated GFP-E1 products has impeded precise determination of the timing of E1 degradation during transient replication in transfected cells.
Although the inability of the E1 D-box mutant to drive viral replication in an intact nucleus has not allowed us to determine the biological consequences of the D-box mutation, our data show that stabilization of E1 by mutation of the KEN box or Emi1 overexpression give rise to an increase of viral DNA replication in vivo. This indicates that APC-mediated E1 degradation controls the copy number of BPV-ori plasmids. Our previous studies showed that E1, stably associated to cyclin E, is protected from degradation, coupling E1 stability to the periodic accumulation of cyclin E-Cdk2 at the G1/S-phase transition and the onset of DNA synthesis (4, 14). Thus, E1 steady-state levels are most likely regulated by an appropriate balance between these two key cell cycle regulators.
A constant event, correlated with malignant transformation, is the integration of viral DNA into the host genome (24). It is not known whether integration precedes malignant transformation or if it is one of its consequences. However, autonomous replication of papillomavirus genomes depends on E1 function, and the viral initiator was shown to be one of the factors required to specifically prevent integration (12). Thus, E1 elimination caused by any unbalance of cyclin E and/or APC regulators would abrogate autonomous replication and cause integration. Conversely, a drop of E1 levels might have deleterious effects by compromising the regulation of the endogenous cellular targets of cyclin E and the APC. This is particularly relevant, because APC regulators are low-abundance, rate-limiting components. The finding that HPV18 E1 is the most frequently recovered cyclin E-interacting protein from a HeLa cell cDNA library has demonstrated that E1 is well produced in a cell line derived from a cervical carcinoma containing integrated papillomavirus DNA (13). Thus, further studies are required to investigate whether this viral initiator affects the host cell cycle and contributes to the oncogenic potential of papillomaviruses.
Acknowledgments
We thank M. Morris and B. Hipskind for critical reading of the manuscript and C. Bernis for scientific advice.
This work was supported by a grant from the Association pour la Recherche sur le Cancer to C.B.-A. and by the Ligue Nationale Contre le Cancer (Equipe Labellisée). A.C. is a postdoctoral fellow supported by the Ligue Nationale Contre le Cancer.
REFERENCES
- 1.Bermejo, R., N. Vilaboa, and C. Cales. 2002. Regulation of CDC6, Geminin and Cdt1 in human cells that undergo polyploidization. Mol. Biol. Cell. 13:3989-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonne-Andrea, C., S. Santucci, and P. Clertant. 1995. Bovine papillomavirus E1 protein can, by itself, efficiently drive multiple rounds of DNA synthesis in vitro. J. Virol. 69:3201-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro, A., Y. Arlot-Bonnemains, S. Vigneron, J. C. Labbe, C. Prigent, and T. Lorca. 2002. APC/Fizzy-related targets Aurora-A kinase for proteolysis. EMBO Rep. 3:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cueille, N., R. Nougarede, F. Mechali, M. Philippe, and C. Bonne-Andrea. 1998. Functional interaction between the bovine papillomavirus type 1 replicative helicase E1 and cyclin E-Cdk2. J. Virol. 72:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furstenthal, L., B. K. Kaiser, C. Swanson, and P. K. Jackson. 2001. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J. Cell Biol. 152:1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper, J. W., J. L. Burton, and M. J. Solomon. 2002. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16:2179-2206. [DOI] [PubMed] [Google Scholar]
- 7.Holt, S. E., G. Schuller, and V. G. Wilson. 1994. DNA binding specificity of the bovine papillomavirus E1 protein is determined by sequences contained within an 18-base-pair inverted repeat element at the origin of replication. J. Virol. 68:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu, J. H., J. D. R. Reimann, C. S. Sorensen, J. Lukas, and P. K. Jackson. 2002. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nat. Cell Biol. 4:358-366. [DOI] [PubMed] [Google Scholar]
- 9.Ilves, I., S. Kivi, and M. Ustav. 1999. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which is mediated by the viral E2 protein and its binding sites. J. Virol. 73:4404-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert, P. F., C. Baker, and P. M. Howley. 1988. The genetics of bovine papillomavirus type 1. Annu. Rev. Genet. 22:235-258. [DOI] [PubMed] [Google Scholar]
- 11.Lorca, T., A. Castro, A.-M. Martinez, S. Vigneron, N. Morin, S. Sigrist, C. Lehner, M. Dorée, and J.-C. Labbé. 1998. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J. 17:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lusky, M., and M. Botchan. 1985. Genetic analysis of bovine papillomavirus type I trans-acting replication factors. J. Virol. 53:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma, T., N. Zou, B. Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malcles, M.-H., N. Cueille, F. Mechali, O. Coux, and C. Bonne-Andrea. 2002. Regulation of bovine papillomavirus replicative helicase E1 by the ubiquitin-proteasome pathway. J. Virol. 76:11350-11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfleger, C. M., E. Lee, and M. W. Kirschner. 2001. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15:2396-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piirsoo, M., E. Ustav, T. Mandel, A. Stenlund, and M. Ustav. 1996. Cis and trans requirements for stable episomal maintenance of the BPV-1 replicator. EMBO J. 15:1-11. [PMC free article] [PubMed] [Google Scholar]
- 17.Rangasamy, D., and V. G. Wilson. 2000. Bovine papillomavirus E1 protein is sumoylated by the host cell Ubc9 protein. J. Biol. Chem. 275:30487-30495. [DOI] [PubMed] [Google Scholar]
- 18.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105:645-655. [DOI] [PubMed] [Google Scholar]
- 19.Sedman, J., and A. Stenlund. 1998. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J. Virol. 72:6893-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skiadopoulos, M., and A. McBride. 1998. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J. Virol. 72:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ustav, M., and A. Stenlund. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan, Y., and M. W. Kirschner. 2001. Identification of multiple CDH1 homologues in vertebrates conferring different substrate specificities. Proc. Natl. Acad. Sci. USA 98:13066-13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nature 2:342-350. [DOI] [PubMed] [Google Scholar]